Like a Bolt from the Blue: Phthalocyanines in Biomedical Optics
Abstract
:1. Introduction
- • Is composed of a undefined mixture of hematoporphyrin derivatives (HpD);
- • Induces a long-lasting skin photosensitization (2 to 3 months post injection);
- • Has a low extinction coefficient at wavelengths for optimal tissue penetration;
- • Displays a limited selectivity for the target tissue.
- • Single and chemically pure compound;
- • Stability and good solubility in pharmaceutically acceptable formulations and in biological media;
- • Low tendency to aggregate;
- • High singlet oxygen quantum yield;
- • Photostability;
- • Fluorescence;
- • Low phototoxicity towards healthy tissue;
- • No dark toxicity;
- • Fast clearance from the healthy parts of the body and specific retention in diseased tissues;
- • Strong absorbance in NIR region and minimal absorbance between 400 and 600 nm.
| Photosensitizers | Trade Name | Absorption Wavelength | Potential Indications |
|---|---|---|---|
| HpD, Porfimer sodium | Photofrin, Photogem, Photosan, Hemporfin | 630 nm | Cervical, brain, oesophageal, breast, head and neck, lung, bladder, superficial gastric cancers, Bowen's disease, cutaneous Kaposi's sarcoma |
| m-THPC, Temoporfin | Foscan | 652 nm | Oesophageal, prostate and pancreatic cancer, advanced head and neck tumors |
| Verteporfin | Visudyne | 689 nm | Basal and squamous cell carcinomas |
| HPPH, 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-alpha | Photochlor | 665 nm | Basal cell carcinoma, Oesophageal cancers, Head and Neck tumors |
| Palladium-bacteria-pheophorbide | Tookad | 763 nm | Prostate cancer |
| 5-ALA,5-aminolevulinic acid | Levulan | 630 nm | Skin tumors, head and neck, gynaecological tumors and basal cell carcinomas |
| 375-400 nm | Brain, head and neck and bladder cancer photodetection | ||
| 5-ALA-methylester | Metvix | 635 nm | Basal cell carcinoma |
| 5-ALA benzylester | Benzvix | 635 nm | Gastrointestinal tumors |
| 5-ALA hexylester | Hexvix | 375-400 nm | Photodectection of bladder cancer |
| Lutetium (III)-texaphyrin or Motexafin-lutetium | Lutex, Lutrin, Antrin, Optrin | 732 nm | Prostate, cervical, breast, brain cancer, melanoma |
| SnET2, Tin (IV) ethyl etiopurpurin | Purlytin, Photrex | 659 nm | Kaposi's sarcoma, cutaneous metastatic adenocarcinomas, prostate, brain, lung cancers, basal cell carcinomas |
| NPe6, mono-L-aspartyl chlorin e6, talaporfin sodium | Talaporfin, Laserphyrin | 664 nm | Solid tumors, lung cancer, cutaneous malignancies |
| BOPP, boronated protoporphyrin | BOPP | 630 nm | Malignant gliomas |
| Zinc phthalocyanine | CGP55847 | 670 nm | Squamous cell carcinoma of upper aerodigestive tract |
| Silicon phthalocyanine | Pc 4 | 675 nm | Cutaneous and subcutaneous lesions from diverse solid tumor origins |
| Mixture of sulfonated aluminium phthalocyanine derivatives | Photosens | 675 nm | Skin, breast, lung, oropharingeal, breast, larynx, head and neck cancers, Sarcoma M1, epibulbal and choroidal tumors, eyes and eyelids tumors, cervical cancer |
| ATMPn, Acetoxy-tetrakis(β-methoxyethyl)-porphycene | NA | 600-750 nm | Skin cancer |
| TH9402, dibromorhodamine methyl ester | NA | 515 nm | Breast, myeloma, non-melanoma skin cancer |
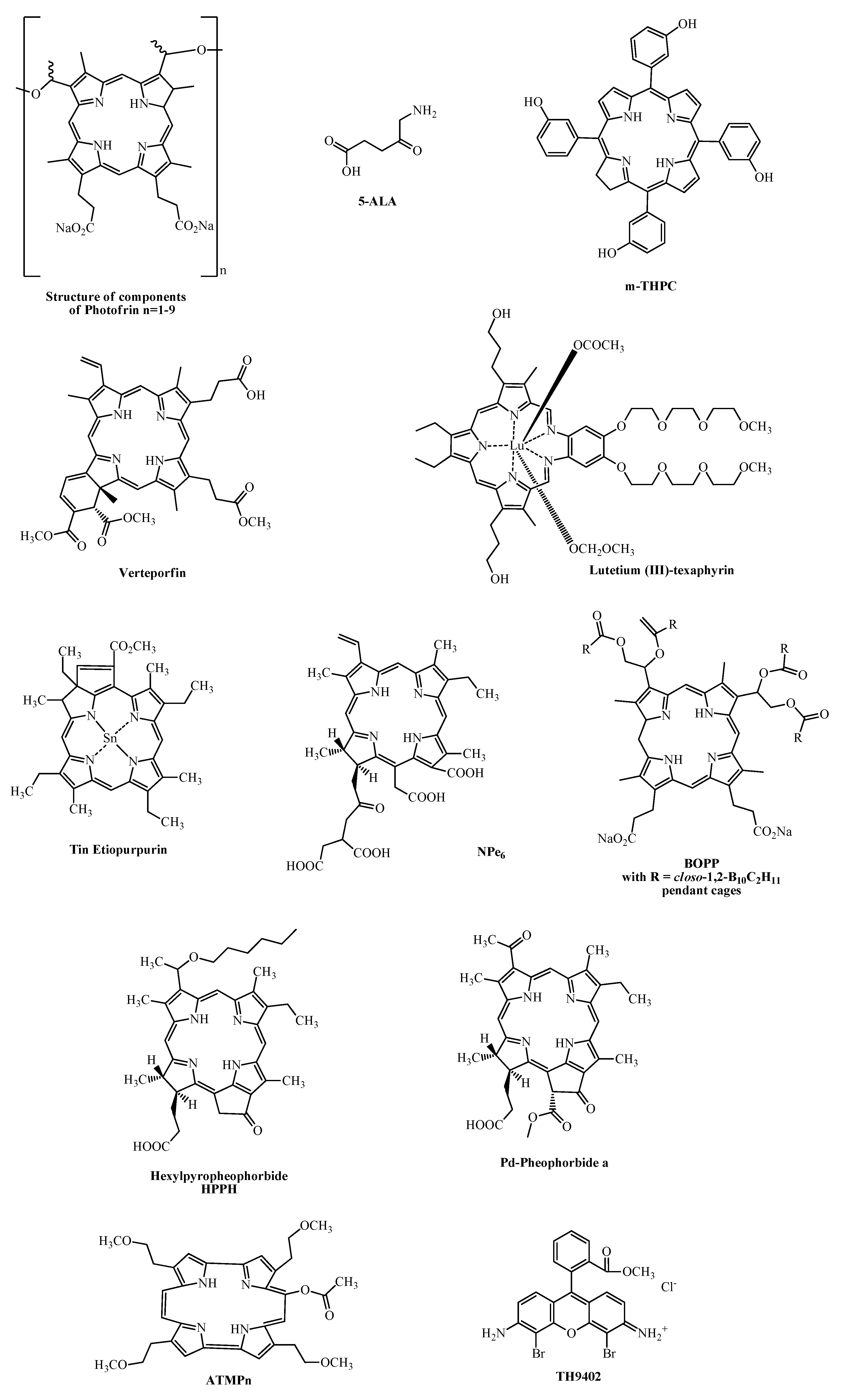
2. Phthalocyanines

2.1. Aluminium Based Phthalocyanines
2.1.1. Aluminium Sulfonated Phthalocyanines—SAR
2.1.2. Influence of the Degree of Sulfonation
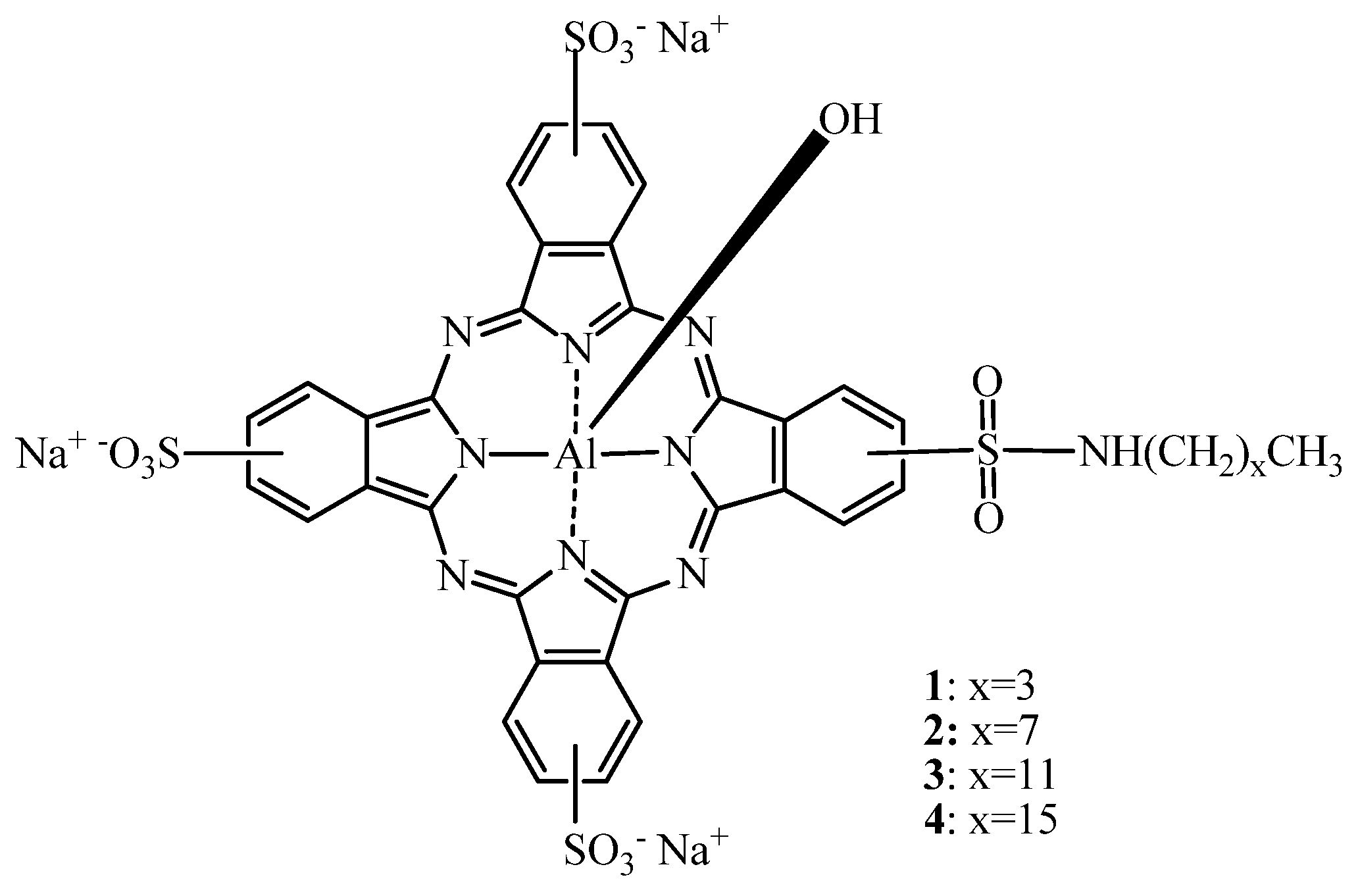
2.1.3. Photosens®
2.1.4. AlPcS-Conjugation to Tumor Targeting Moieties

2.1.5. AlPcS4-Formulation using Targeted Delivery Systems
2.1.6. AlClPc—SAR
2.1.7. AlClPc-Formulation
2.2. Zinc Based Phthalocyanines
2.2.1. ZnPc—SAR
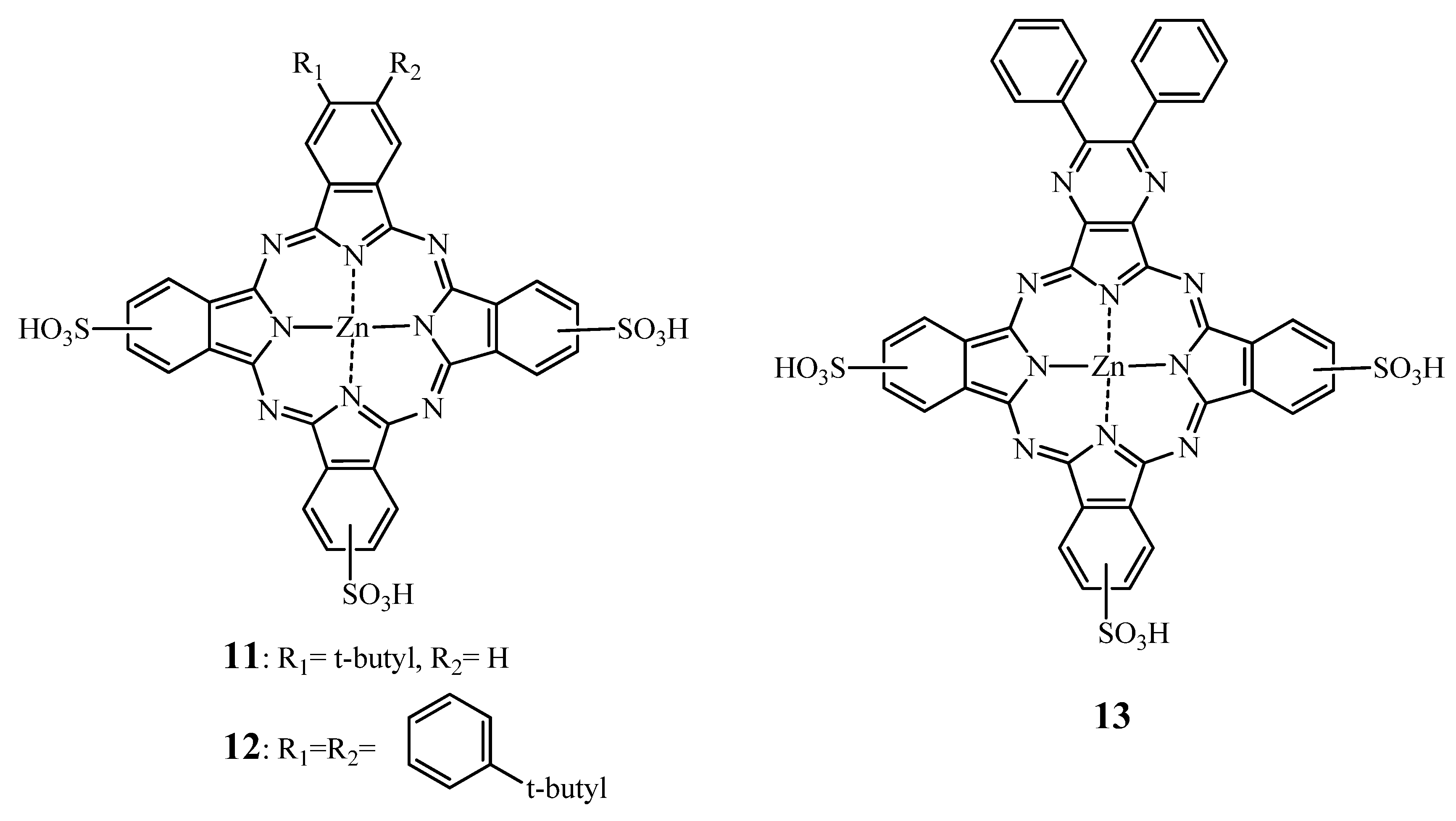
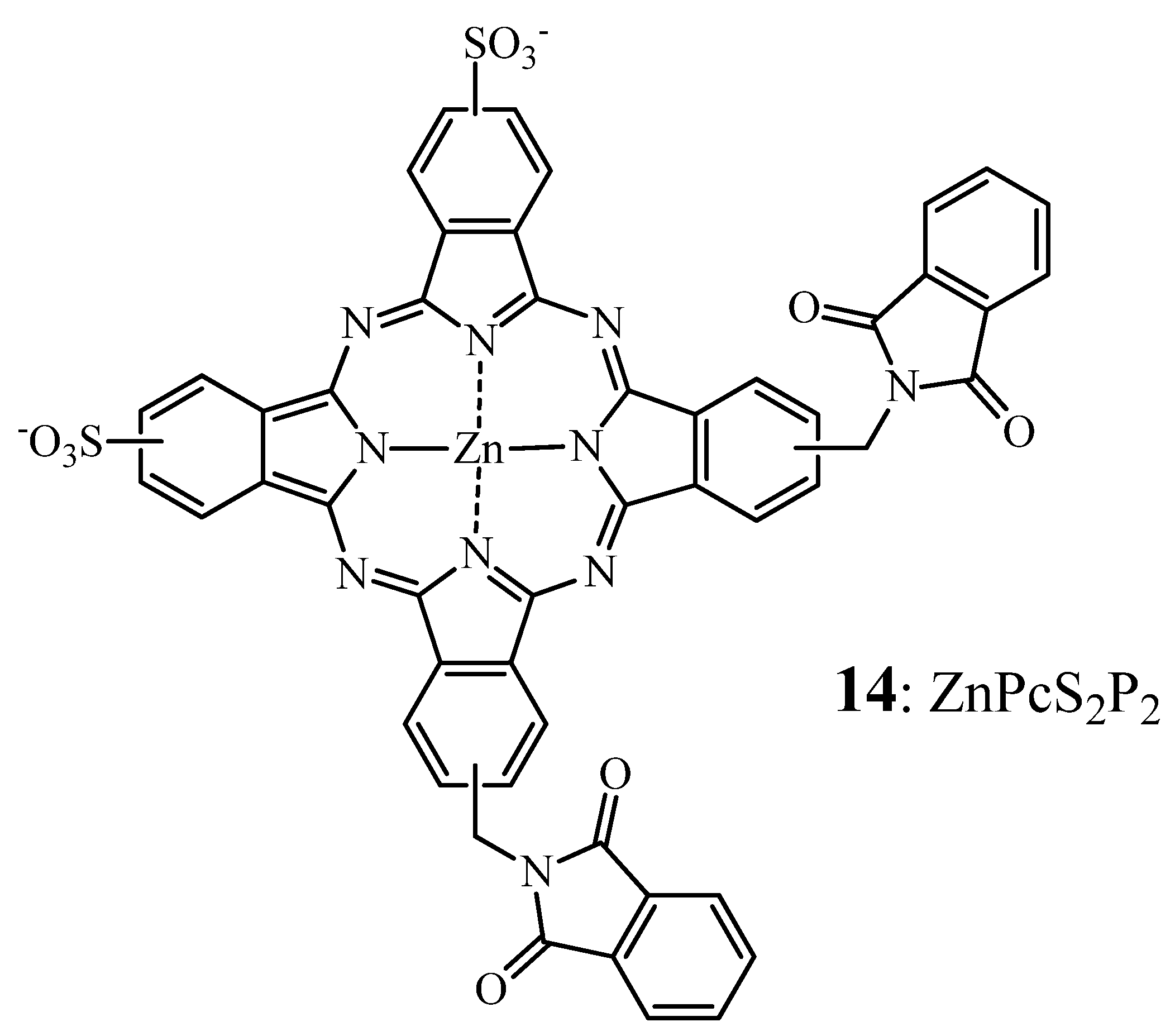
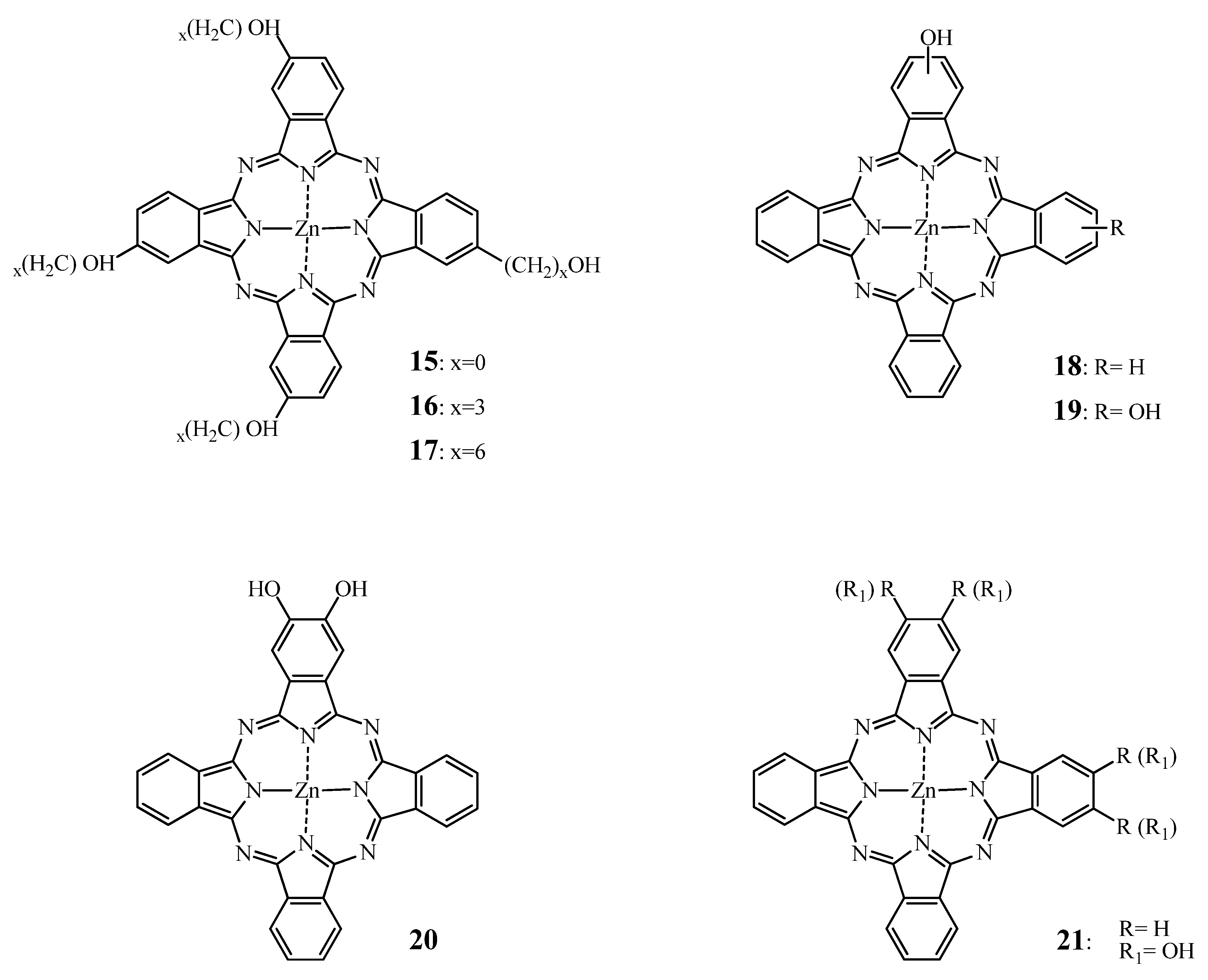


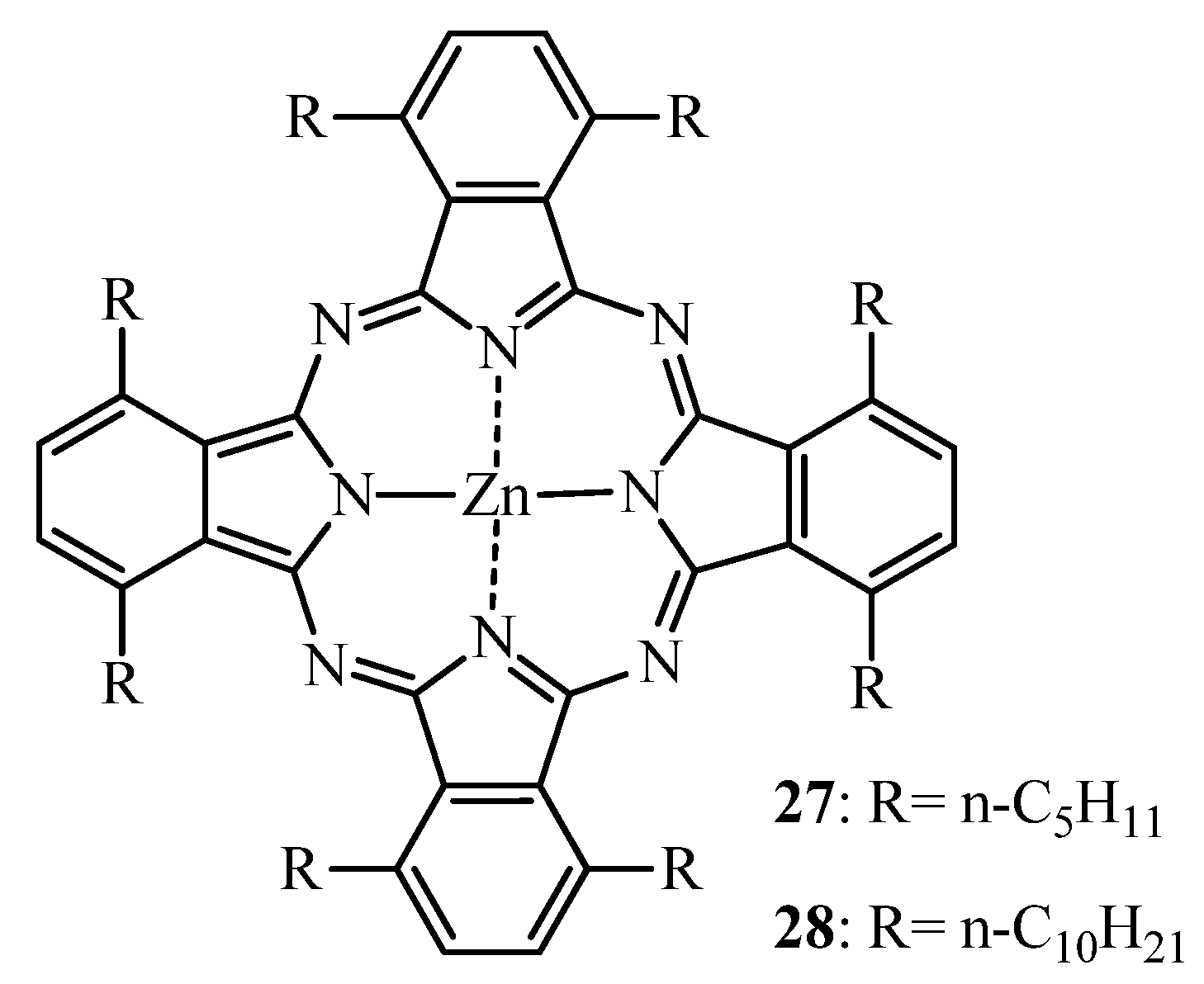
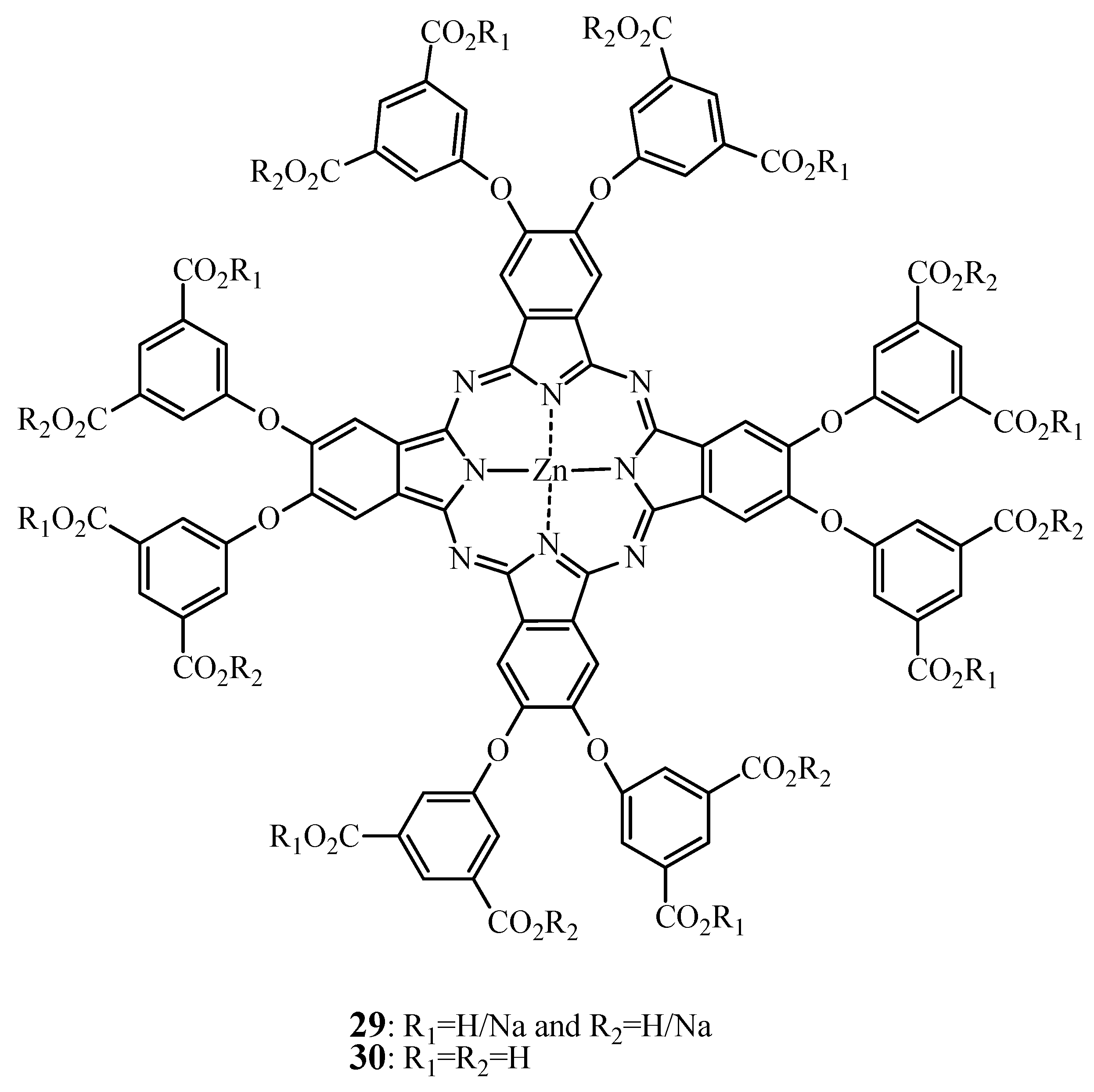
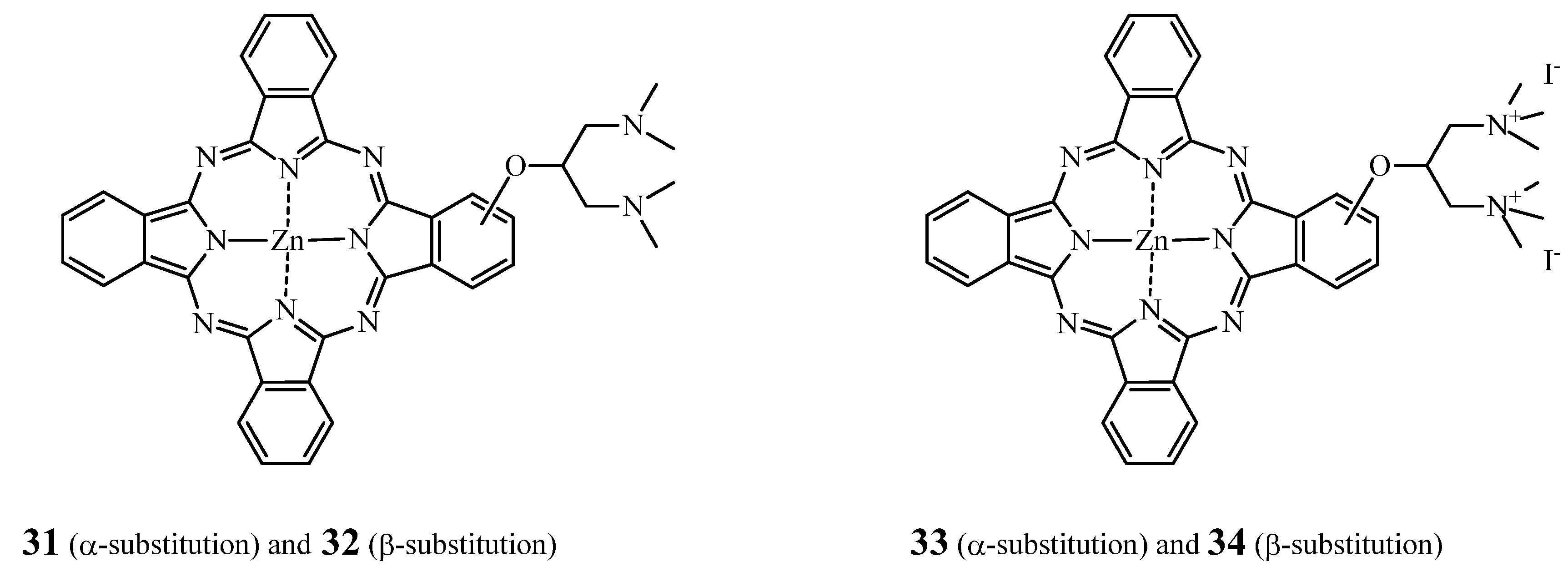

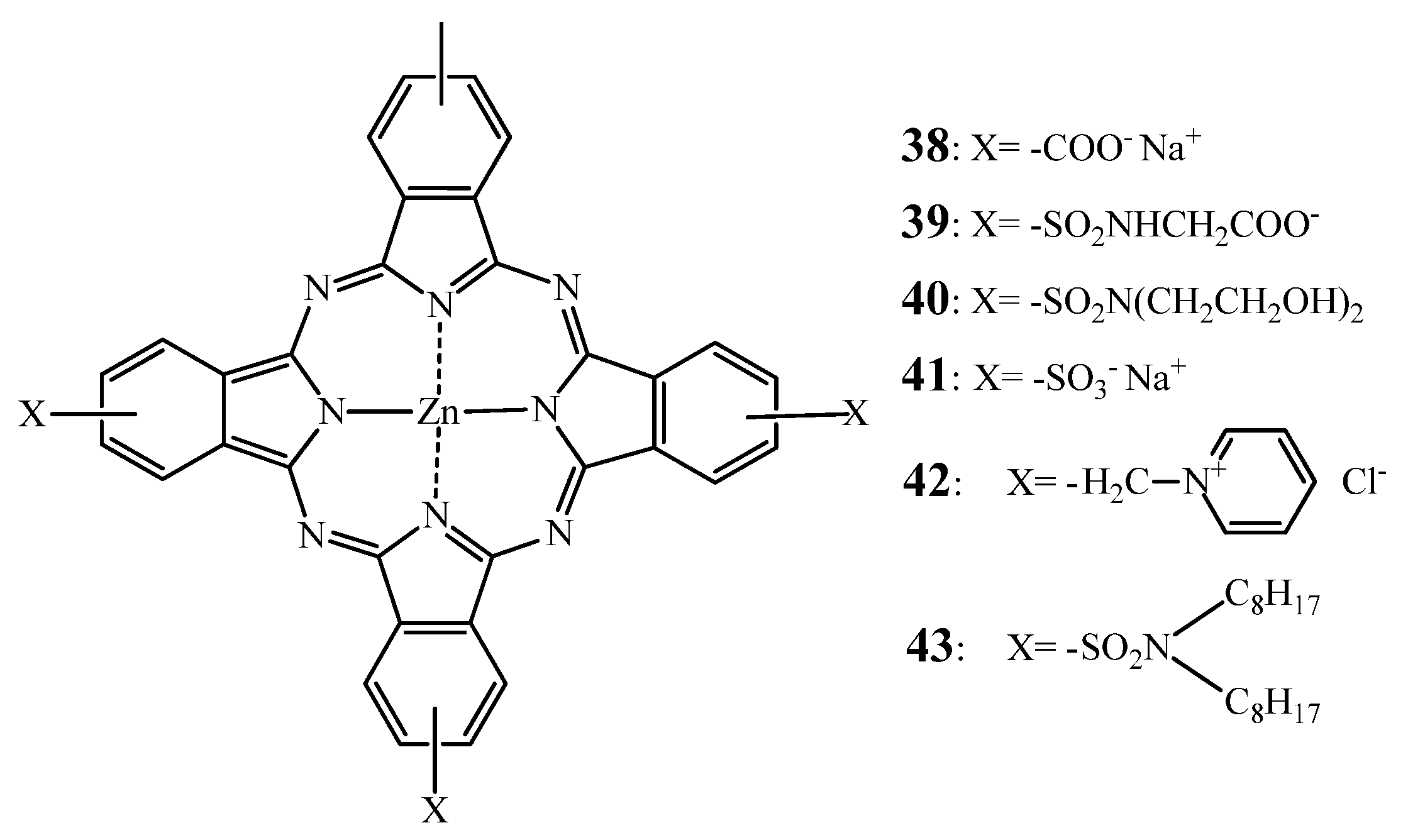

2.2.2. Tumor Targeting with Zinc Based Pc-Conjugates
2.2.3. Formulation of ZnPcs
2.3. Silicon Based Phthalocyanines: Pc 4 and its Analogues
2.3.1. SiPc-SAR
2.3.2. SiPc Formulations
3. Applications of Pcs in Fluorescence Imaging
4. Conclusions
Acknowledgements
References and Notes
- McKeown, N.B. The synthesis of symmetrical phthalocyanines. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 61–124. [Google Scholar]
- Thomas, A.L. Phthalocyanine Research and Applications; CRC Press: Baton Rouge, FL, USA, 1990; p. 2. [Google Scholar]
- McKeown, N.B. An introduction to the phthalocyanines. In Phthalocyanine Materials: Synthesis, Structure and Function; McKeown, N.B., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 1–10. [Google Scholar]
- Geng, Y.Y.; Gu, D.H.; Wu, Y.Q.; Gan, F.X. High speed recording property of phthalocyanine thin film for compact disc recordable. Proc. SPIE Int. Soc. Opt. Eng. 2003, 63, 63–66. [Google Scholar]
- Petritsch, K.; Friend, R.H.; Lux, A.; Rozenberg, G.; Moratti, S.C.; Holmes, A.B. Liquid crystalline phthalocyanines in organic solar cells. Synth. Met. 1999, 102, 1776–1777. [Google Scholar]
- Nyokong, T.; Vilakazi, S. Phthalocyanines and related complexes as electrocatalysts for the detection of nitric oxide. Talanta 2003, 61, 27–35. [Google Scholar] [CrossRef]
- Ali, H.; van Lier, J.E. Porphyrins and phthalocyanines as photosensitizers and radiosensitizers. In Handbook of Porphyrin Science with Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medecine; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific: Singapore, 2010; pp. 1–119. [Google Scholar]
- Alonso, C.; Boyle, R.W. Bioconjugates of porphyrins and related molecules for photodynamic Therapy. In Handbook of Porphyrin Science with Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medecine; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific: Singapore, 2010; pp. 121–190. [Google Scholar]
- Ethirajan, M.; Patel, N.J.; Pandey, R.K. Porphyrin-based multifunctional agents for tumor-imaging and photodynamic therapy (PDT). In Handbook of Porphyrin Science with Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medecine; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific: Singapore, 2010; pp. 249–323. [Google Scholar]
- Jux, N.; Röder, B. Targeting strategies for tetrapyrrole-based photodynamic therapy. In Handbook of Porphyrin Science with Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medecine; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific: Singapore, 2010; pp. 325–401. [Google Scholar]
- Nyokong, T.; Antunes, E. Photochemical and photophysical properties of metallophthalocyanines. In Handbook of Porphyrin Science with Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medecine; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific: Singapore, 2010; pp. 247–349. [Google Scholar]
- Fukuda, T.; Kobayashi, N. UV-visible absorption spectroscopic properties of phthalocyanines and related macrocycles. In Handbook of Porphyrin Science with Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medecine; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific: Singapore, 2010; pp. 1–645. [Google Scholar]
- Dietze, A.; Peng, Q.; Selbo, P.K.; Kaalhus, O.; Muller, C.; Bown, S.; Berg, K. Enhanced photodynamic destruction of a transplantable fibrosarcoma using photochemical internalisation of gelonin. Br. J. Cancer 2005, 92, 2004–2009. [Google Scholar]
- Selbo, P.K.; Sivam, G.; Fodstad, O.; Sandvig, K.; Berg, K. In vivo documentation of photochemical internalization, a novel approach to site specific cancer therapy. Int. J. Cancer 2001, 92, 761–766. [Google Scholar] [CrossRef]
- Adigbli, D.K.; Wilson, D.G.; Farooqui, N.; Sousi, E.; Risley, P.; Taylor, I.; Macrobert, A.J.; Loizidou, M. Photochemical internalisation of chemotherapy potentiates killing of multidrug-resistant breast and bladder cancer cells. Br. J. Cancer 2007, 97, 502–512. [Google Scholar]
- Norum, O.J.; Selbo, P.K.; Weyergang, A.; Giercksky, K.E.; Berg, K. Photochemical internalization (PCI) in cancer therapy: From bench towards bedside medicine. J. Photochem. Photobiol. B 2009, 96, 83–92. [Google Scholar]
- Norum, O.J.; Bruland, O.S.; Gorunova, L.; Berg, K. Photochemical internalization of bleomycin before external-beam radiotherapy improves locoregional control in a human sarcoma model. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 878–885. [Google Scholar]
- Norum, O.J.; Giercksky, K.E.; Berg, K. Photochemical internalization as an adjunct to marginal surgery in a human sarcoma model. Photochem. Photobiol. Sci. 2009, 8, 758–762. [Google Scholar]
- Prasmickaite, L.; Hogset, A.; Selbo, P.K.; Engesaeter, B.O.; Hellum, M.; Berg, K. Photochemical disruption of endocytic vesicles before delivery of drugs: A new strategy for cancer therapy. Br. J. Cancer 2002, 86, 652–657. [Google Scholar]
- Hogset, A.; Prasmickaite, L.; Selbo, P.K.; Hellum, M.; Engesaeter, B.O.; Bonsted, A.; Berg, K. Photochemical internalisation in drug and gene delivery. Adv. Drug Deliv. Rev. 2004, 56, 95–115. [Google Scholar]
- Ben-Hur, E.; Rosenthal, I. The phthalocyanines: A new class of mammalian cells photosensitizers with a potential for cancer phototherapy. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1985, 47, 145–147. [Google Scholar] [CrossRef]
- Brasseur, N.; Ali, H.; Autenrieth, D.; Langlois, R.; van Lier, J.E. Biological activities of phthalocyanines-III. Photoinactivation of V-79 Chinese hamster cells by tetrasulfophthalocyanines. Photochem. Photobiol. 1985, 42, 515–521. [Google Scholar] [CrossRef]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar]
- Sharman, W.M.; Allen, C.M.; van Lier, J.E. Photodynamic therapeutics: Basic principles and clinical applications. Drug Discov. Today 1999, 4, 507–517. [Google Scholar]
- Allen, C.M.; Langlois, R.; Sharman, W.M.; La, M.C.; van Lier, J.E. Photodynamic properties of amphiphilic derivatives of aluminum tetrasulfophthalocyanine. Photochem. Photobiol. 2002, 76, 208–216. [Google Scholar]
- Brown, S.B.; Brown, J.E.; Vernon, D.I. Photosensitising drugs—Their potential in oncology. Expert Opin. Invest. Drugs 1999, 8, 1967–1979. [Google Scholar]
- Allison, R.R. Photosenitizers in clinical PDT. Photodiag. Photod. Ther. 2004, 1, 27–42. [Google Scholar]
- Lange, N. Controlled drug delivery in photodynamic therapy and fluorescence-based diagnosis of cancer. In Handbook of Biomedical Fluorescence; Mary-Ann, M., Brian, W.P., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 563–635. [Google Scholar]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy and the development of metal-based photosensitisers. Met. Based Drugs 2008, 2008, 1–24. [Google Scholar]
- Ethirajan, M.; Saenz, C.; Gupta, A.; Dobhal, M.P.; Pandey, R.K. Photosensitizers for photodynamic therapy and imaging. In Advances in Photodynamic Therapy: Basic, Translational, and Clinical; Michael, R.H., Pawel, M., Eds.; Artech House: Boston, MA, USA, 2008; pp. 13–39. [Google Scholar]
- Stolik, S.; Delgado, J.A.; Perez, A.; Anasagasti, L. Measurement of the penetration depths of red and near infrared light in human "ex vivo" tissues. J. Photochem. Photobiol. B 2000, 57, 90–93. [Google Scholar] [CrossRef]
- Sevick-Muraca, E.M.; Godavarty, A.; Houston, J.P.; Thompson, A.B.; Roy, R. Near-infrared imaging with fluorescent contrast agents. In Handbook of Biomedical Fluorescence; Mycek, M.-A., Pogue, B.W., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 445–527. [Google Scholar]
- MacDonald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyr. Phthalocya. 2001, 5, 105–129. [Google Scholar]
- Lang, K.; Mosinger, J.; Wagnerova, D.M. Photophysical properties of porphyrinoid sensitizers non-covalently bound to host molecules, models for photodynamic therapy. Coord. Chem. Rev. 2004, 248, 321–350. [Google Scholar]
- Jori, G. Far-red-absorbing photosensitizers—Their use in the photodynamic therapy of tumors. J. Photochem. Photobiol. A 1992, 62, 371–378. [Google Scholar]
- Haddad, R.; Blumenfeld, A.; Siegal, A.; Kaplan, O.; Cohen, M.; Skornick, Y.; Kashtan, H. In vitro and in vivo effects of photodynamic therapy on murine malignant melanoma. Ann. Surg. Oncol. 1998, 5, 241–247. [Google Scholar] [CrossRef]
- Boyle, R.W.; Dolphin, D. Structure and biodistribution relationships of photodynamic sensitizers. Photochem. Photobiol. 1996, 64, 469–485. [Google Scholar]
- Bonnett, R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 1995, 24, 19–33. [Google Scholar]
- Brasseur, N.; Menard, I.; Forget, A.; El Jastimi, R.; Hamel, R.; Molfino, N.A.; van Lier, J.E. Eradication of multiple myeloma and breast cancer cells by TH9402-mediated photodynamic therapy: Implication for clinical ex vivo purging of autologous stem cell transplants. Photochem. Photobiol. 2000, 72, 780–787. [Google Scholar] [CrossRef]
- Brown, S.B.; Brown, E.A.; Walker, I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar]
- Ferreira, J.; Menezes, P.F.C.; Kurachi, C.; Sibata, C.H.; Allison, R.R.; Bagnato, V.S. Comparative study of photodegradation of three hematoporphyrin derivative: Photofrin (R), Photogem (R) and Photosan. Laser Phys. Lett. 2007, 4, 743–748. [Google Scholar]
- Fickweiler, S.; Abels, C.; Karrer, S.; Baumler, W.; Landthaler, M.; Hofstadter, F.; Szeimies, R.M. Photosensitization of human skin cell lines by ATMPn (9-acetoxy-2,7,12,17-tetrakis-(beta-methoxyethyl)-porphycene) in vitro: Mechanism of action. J. Photochem. Photobiol. B 1999, 48, 27–35. [Google Scholar] [CrossRef]
- Jori, G. Tumour photosensitizers: Approaches to enhance the selectivity and efficiency of photodynamic therapy. J. Photochem. Photobiol. B 1996, 36, 87–93. [Google Scholar]
- Karrer, S.; Abels, C.; Szeimies, R.M.; Baumler, W.; Dellian, M.; Hohenleutner, U.; Goetz, A.E.; Landthaler, M. Topical application of a first porphycene dye for photodynamic therapy—Penetration studies in human perilesional skin and basal cell carcinoma. Arch. Dermatol. Res. 1997, 289, 132–137. [Google Scholar]
- Mathai, S.; Bird, D.K.; Stylli, S.S.; Smith, T.A.; Ghiggino, K.P. Two-photon absorption cross-sections and time-resolved fluorescence imaging using porphyrin photosensitisers. Photochem. Photobiol. Sci. 2007, 6, 1019–1026. [Google Scholar]
- Mody, T.D. Pharmaceutical development and medical applications of porphyrin-type macrocycles. J. Porphyr. Phthalocya. 2000, 4, 362–367. [Google Scholar]
- Nyman, E.S.; Hynninen, P.H. Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B 2004, 73, 1–28. [Google Scholar]
- Ochsner, M. Light scattering of human skin: A comparison between zinc(II)-phthalocyanine and Photofrin II(R). J. Photochem. Photobiol. B 1996, 32, 3–9. [Google Scholar]
- Palumbo, G. Photodynamic therapy and cancer: A brief sightseeing tour. Expert Opin. Drug Del 2007, 4, 131–148. [Google Scholar] [CrossRef]
- Rosenthal, M.A.; Kavar, B.; Hill, J.S.; Morgan, D.J.; Nation, R.L.; Stylli, S.S.; Basser, R.L.; Uren, S.; Geldard, H.; Green, M.D.; et al. Phase I and pharmacokinetic study of photodynamic therapy for high-grade gliomas using a novel boronated porphyrin. J. Clin. Oncol. 2001, 19, 519–524. [Google Scholar]
- Usuda, J.; Kato, H.; Okunaka, T.; Furukawa, K.; Tsutsui, H.; Yamada, K.; Suga, Y.; Honda, H.; Nagatsuka, Y.; Ohira, T.; et al. Photodynamic therapy (PDT) for lung cancers. J. Thorac. Oncol. 2006, 1, 489–493. [Google Scholar] [CrossRef]
- Ogunsipe, A.; Nyokong, T. Photophysicochemical consequences of bovine serum albumin binding to non-transition metal phthalocyanine sulfonates. Photochem. Photobiol. Sci. 2005, 4, 510–516. [Google Scholar] [CrossRef]
- Huang, J.D.; Wang, S.; Lo, P.C.; Fong, W.P.; Ko, W.H.; Ng, D.K.P. Halogenated silicon(iv) phthalocyanines with axial poly(ethylene glycol) chains. Synthesis, spectroscopic properties, complexation with bovine serum albumin and in vitro photodynamic activities. New J. Chem. 2004, 28, 348–354. [Google Scholar] [CrossRef]
- Chen, X.L.; Li, D.H.; Zhu, Q.Z.; Yang, H.H.; Zheng, H.; Wang, Z.H.; Xu, J.G. Determination of proteins at nanogram levels by a resonance light-scattering technique with tetra-substituted sulphonated aluminum phthalocyanine. Talanta 2001, 53, 1205–1210. [Google Scholar] [CrossRef]
- Vrouenraets, M.B.; Visser, G.W.; Stigter, M.; Oppelaar, H.; Snow, G.B.; van Dongen, G.A. Targeting of aluminum (III) phthalocyanine tetrasulfonate by use of internalizing monoclonal antibodies: Improved efficacy in photodynamic therapy. Cancer Res. 2001, 61, 1970–1975. [Google Scholar]
- Peng, X.; Draney, D.R.; Volcheck, W.M.; Bashford, G.R.; Lamb, D.T.; Zhang, Y.; Johnson, C.M. Phthalocyanine dye as an extremely photostable and highly fluorescent near-infrared labeling reagent. Proc. SPIE Int. Soc. Opt. Eng. 2006, 6097, 60970. [Google Scholar]
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef]
- Duan, W.; Smith, K.; Savoie, H.; Greenman, J.; Boyle, R.W. Near IR emitting isothiocyanato-substituted fluorophores: Their synthesis and bioconjugation to monoclonal antibodies. Org. Biomol. Chem. 2005, 3, 2384–2386. [Google Scholar]
- Carcenac, M.; Dorvillius, M.; Garambois, V.; Glaussel, F.; Larroque, C.; Langlois, R.; Hynes, N.E.; van Lier, J.E.; Pelegrin, A. Internalisation enhances photo-induced cytotoxicity of monoclonal antibody-phthalocyanine conjugates. Br. J. Cancer 2001, 85, 1787–1793. [Google Scholar]
- Devlin, R.; Studholme, R.M.; Dandliker, W.B.; Fahy, E.; Blumeyer, K.; Ghosh, S.S. Homogeneous detection of nucleic acids by transient-state polarized fluorescence. Clin. Chem. 1993, 39, 1939–1943. [Google Scholar]
- Hammer, R.P.; Owens, C.V.; Hwang, S.H.; Sayes, C.M.; Soper, S.A. Asymmetrical, water-soluble phthalocyanine dyes for covalent labeling of oligonucleotides. Bioconjug. Chem. 2002, 13, 1244–1252. [Google Scholar] [CrossRef]
- Koval, V.V.; Chernonosov, A.A.; Abramova, T.V.; Ivanova, T.M.; Fedorova, O.S.; Derkacheva, V.M.; Lukyanets, E.A. Photosensitized and catalytic oxidation of DNA by metallophthalocyanine-oligonucleotide conjugates. Nucleos. Nucleot. Nucl. 2001, 20, 1259–1262. [Google Scholar]
- Nesterova, I.V.; Verdree, V.T.; Pakhomov, S.; Strickler, K.L.; Allen, M.W.; Hammer, R.P.; Soper, S.A. Metallo-phthalocyanine near-IR fluorophores: Oligonucleotide conjugates and their applications in PCR assays. Bioconjug. Chem. 2007, 18, 2159–2168. [Google Scholar]
- Walker, G.T.; Fraiser, M.S.; Schram, J.L.; Little, M.C.; Nadeau, J.G.; Malinowski, D.P. Strand displacement amplification—An isothermal, in vitro DNA amplification technique. Nucleic Acids Res. 1992, 20, 1691–1696. [Google Scholar] [CrossRef]
- Rio, Y.; Rodriguez-Morgade, M.S.; Torres, T. Modulating the electronic properties of porphyrinoids: A voyage from the violet to the infrared regions of the electromagnetic spectrum. Org. Biomol. Chem. 2008, 6, 1877–1894. [Google Scholar]
- Ali, H.; van Lier, J.E. Metal complexes as photo- and radiosensitizers. Chem. Rev. 1999, 99, 2379–2450. [Google Scholar]
- Claessens, C.G.; Blau, W.J.; Cook, M.; Hanack, M.; Nolte, R.J.M.; Torres, T.; Whörle, D. Phthalocyanines and phthalocyanine analogues: The quest for applicable optical properties. Monatsh. Chem./Chem. Monthly 2001, 132, 3–11. [Google Scholar]
- Nyokong, T.; Isago, H. The renaissance in optical spectroscopy of phthalocyanines and other tetraazaporphyrins. J. Porphyr. Phthalocya. 2004, 8, 1083–1090. [Google Scholar] [CrossRef]
- Foley, M.S.; Beeby, A.; Parker, A.W.; Bishop, S.M.; Phillips, D. Excited triplet state photophysics of the sulphonated aluminium phthalocyanines bound to human serum albumin. J. Photochem. Photobiol. B 1997, 38, 10–17. [Google Scholar]
- Feofanov, A.; Grichine, A.; Karmakova, T.; Kazachkina, N.; Pecherskih, E.; Yakubovskaya, R.; Luk'yanets, E.; Derkacheva, V.; Egret-Charlier, M.; Vigny, P. Chelation with metal is not essential for antitumor photodynamic activity of sulfonated phthalocyanines. Photochem. Photobiol. 2002, 75, 527–533. [Google Scholar]
- Karmakova, T.; Feofanov, A.; Nazarova, A.; Grichine, A.; Yakubovskaya, R.; Luk'yanets, E.; Maurizot, J.C.; Vigny, P. Distribution of metal-free sulfonated phthalocyanine in subcutaneously transplanted murine tumors. J. Photochem. Photobiol. B 2004, 75, 81–87. [Google Scholar]
- Kolarova, H.; Nevrelova, P.; Bajgar, R.; Jirova, D.; Kejlova, K.; Strnad, M. In vitro photodynamic therapy on melanoma cell lines with phthalocyanine. Toxicol. In vitro 2007, 21, 249–253. [Google Scholar] [CrossRef]
- Ward, A.J.; Matthews, E.K. Cytotoxic, nuclear, and growth inhibitory effects of photodynamic drugs on pancreatic carcinoma cells. Cancer Lett. 1996, 102, 39–47. [Google Scholar] [CrossRef]
- Glassberg, E.; Lewandowski, L.; Lask, G.; Uitto, J. Laser-induced photodynamic therapy with aluminum phthalocyanine tetrasulfonate as the photosensitizer: Differential phototoxicity in normal and malignant human cells in vitro. J. Invest. Dermatol. 1990, 94, 604–610. [Google Scholar]
- Tralau, C.J.; Macrobert, A.J.; Coleridge-Smith, P.D.; Barr, H.; Bown, S.G. Photodynamic therapy with phthalocyanine sensitisation: Quantitative studies in a transplantable rat fibrosarcoma. Br. J. Cancer 1987, 55, 389–395. [Google Scholar]
- Chan, W.S.; Marshall, J.F.; Svensen, R.; Bedwell, J.; Hart, I.R. Effect of sulfonation on the cell and tissue distribution of the photosensitizer aluminum phthalocyanine. Cancer Res. 1990, 50, 4533–4538. [Google Scholar]
- Chan, W.S.; West, C.M.; Moore, J.V.; Hart, I.R. Photocytotoxic efficacy of sulphonated species of aluminium phthalocyanine against cell monolayers, multicellular spheroids and in vivo tumours. Br. J. Cancer 1991, 64, 827–832. [Google Scholar] [CrossRef]
- Chan, W.S.; Brasseur, N.; La, M.C.; Ouellet, R.; van Lier, J.E. Efficacy and mechanism of aluminium phthalocyanine and its sulphonated derivatives mediated photodynamic therapy on murine tumours. Eur. J. Cancer 1997, 33, 1855–1859. [Google Scholar]
- Stylli, S.S.; Hill, J.; Sawyer, W.; Kaye, A. Aluminium phthalocyanine mediated photodynamic therapy in experimental malignant glioma. J. Clin. Neurosci. 1995, 2, 146–151. [Google Scholar]
- Stylli, S.S.; Hill, J.S.; Sawyer, W.H.; Kaye, A.H. Phthalocyanine photosensitizers for the treatment of brain tumours. J. Clin. Neurosci. 1995, 2, 64–72. [Google Scholar]
- Sandeman, D.R.; Bradford, R.; Buxton, P.; Bown, S.G.; Thomas, D.G. Selective necrosis of malignant gliomas in mice using photodynamic therapy. Br. J. Cancer 1987, 55, 647–649. [Google Scholar]
- Peng, Q.; Moan, J. Correlation of distribution of sulphonated aluminium phthalocyanines with their photodynamic effect in tumour and skin of mice bearing CaD2 mammary carcinoma. Br. J. Cancer 1995, 72, 565–574. [Google Scholar]
- Paquette, B.; Ali, H.; Langlois, R.; van Lier, J.E. Biological activities of phthalocyanines-VIII. Cellular distribution in V-79 Chinese hamster cells and phototoxicity of selectively sulfonated aluminum phthalocyanines. Photochem. Photobiol. 1988, 47, 215–220. [Google Scholar] [CrossRef]
- Mathews, M.S.; Chighvinadze, D.; Gach, H.M.; Uzal, F.A.; Madsen, S.J.; Hirschberg, H. Cerebral edema following photodynamic therapy using endogenous and exogenous photosensitizers in normal brain. Lasers Surg. Med. 2011, 43, 892–900. [Google Scholar] [CrossRef]
- Gupta, S.; Dwarakanath, B.S.; Chaudhury, N.K.; Mishra, A.K.; Muralidhar, K.; Jain, V. In vitro and in vivo targeted delivery of photosensitizers to the tumor cells for enhanced photodynamic effects. J. Cancer Res. Ther. 2011, 7, 314–324. [Google Scholar] [CrossRef]
- Lagoda, T.S.; Kaplan, M.A.; Krivosheev, I.; Zhavoronkov, L.P.; Bokova, M.B. The optimization of a plan for the photodynamic therapy of sarcoma M1 using photosens. Vopr. Onkol. 2000, 46, 327–331. [Google Scholar]
- Budzinskaia, M.V.; Likhvantseva, V.G.; Shevxchik, S.A.; Loshchenov, V.B.; Kuz'min, S.G.; Vorozhtsov, G.N. Experimental assessment of the capacities of use of photosense. Communication 2. Photodynamic therapy for epibulbar and choroid tumors. Vestn. Oftalmol. 2005, 121, 17–19. [Google Scholar]
- Likhvantseva, V.G.; Osipova, E.A.; Petrenko, M.A.; Merzliakova, O.I.; Kuz'min, S.G.; Vorozhtsov, G.N. Analysis of changes in the accumulation of the photosensitizer Photosens, its elimination kinetics and distribution in the tissues of the eye and eyelids in health and in some tumorous processes. Vestn. Oftalmol. 2008, 124, 38–44. [Google Scholar]
- Apolikhin, O.I. Adjuvant photodynamic therapy (PDT) with photosensitizer photosens for superficial bladder cancer. Experimental investigations to treat prostate cancer by PDT with photosens. Soc. Photo-Opt. Instrum. 2007, 8, 45. [Google Scholar]
- Trushina, O.I.; Novikova, E.G.; Sokolov, V.V.; Filonenko, E.V.; Chissov, V.I.; Vorozhtsov, G.N. Photodynamic therapy of virus-associated precancer and early stages cancer of cervix uteri. Photodiagn. Photodyn. Ther. 2008, 5, 256–259. [Google Scholar]
- Carcenac, M.; Larroque, C.; Langlois, R.; van Lier, J.E.; Artus, J.C.; Pelegrin, A. Preparation, phototoxicity and biodistribution studies of anti-carcinoembryonic antigen monoclonal antibody-phthalocyanine conjugates. Photochem. Photobiol. 1999, 70, 930–936. [Google Scholar]
- Vrouenraets, M.B.; Visser, G.W.; Stigter, M.; Oppelaar, H.; Snow, G.B.; van Dongen, G.A. Comparison of aluminium (III) phthalocyanine tetrasulfonate- and meta-tetrahydroxyphenylchlorin-monoclonal antibody conjugates for their efficacy in photodynamic therapy in vitro. Int. J. Cancer 2002, 98, 793–798. [Google Scholar] [CrossRef]
- Vrouenraets, M.B.; Visser, G.W.; Loup, C.; Meunier, B.; Stigter, M.; Oppelaar, H.; Stewart, F.A.; Snow, G.B.; van Dongen, G.A. Targeting of a hydrophilic photosensitizer by use of internalizing monoclonal antibodies: A new possibility for use in photodynamic therapy. Int. J. Cancer 2000, 88, 108–114. [Google Scholar]
- Gueddari, N.; Favre, G.; Hachem, H.; Marek, E.; Le, G.F.; Soula, G. Evidence for up-regulated low density lipoprotein receptor in human lung adenocarcinoma cell line A549. Biochimie 1993, 75, 811–819. [Google Scholar] [CrossRef]
- Urizzi, P.; Allen, C.M.; Langlois, R.; Ouellet, R.; La Madeleine, C.; van Lier, J.E. Low-density lipoprotein-bound aluminum sulfophthalocyanine: Targeting tumor cells for photodynamic therapy. J. Porphyr. Phthalocya. 2001, 5, 154–160. [Google Scholar] [CrossRef]
- Xiao, D.; Wang, J.; Hampton, L.L.; Weber, H.C. The human gastrin-releasing peptide receptor gene structure, its tissue expression and promoter. Gene 2001, 264, 95–103. [Google Scholar]
- Yegen, B.C. Bombesin-like peptides: Candidates as diagnostic and therapeutic tools. Curr. Pharm. Des. 2003, 9, 1013–1022. [Google Scholar]
- Dubuc, C.; Langlois, R.; Benard, F.; Cauchon, N.; Klarskov, K.; Tone, P.; van Lier, J.E. Targeting gastrin-releasing peptide receptors of prostate cancer cells for photodynamic therapy with a phthalocyanine-bombesin conjugate. Bioorg. Med. Chem. Lett. 2008, 18, 2424–2427. [Google Scholar]
- Gijsens, A.; Derycke, A.; Missiaen, L.; De, V.D.; Huwyler, J.; Eberle, A.; De, W.P. Targeting of the photocytotoxic compound AlPcS4 to Hela cells by transferrin conjugated PEG-liposomes. Int. J. Cancer 2002, 101, 78–85. [Google Scholar]
- Derycke, A.S.; Kamuhabwa, A.; Gijsens, A.; Roskams, T.; De, V.D.; Kasran, A.; Huwyler, J.; Missiaen, L.; de Witte, P.A. Transferrin-conjugated liposome targeting of photosensitizer AlPcS4 to rat bladder carcinoma cells. J. Natl. Cancer Inst. 2004, 96, 1620–1630. [Google Scholar]
- Bridges, K.R.; Smith, B.R. Discordance between transferrin receptor expression and susceptibility to lysis by natural-killer cells. J. Clin. Invest. 1985, 76, 913–918. [Google Scholar]
- Qualls, M.M.; Thompson, D.H. Chloroaluminum phthalocyanine tetrasulfonate delivered via acid-labile diplasmenylcholine-folate liposomes: Intracellular localization and synergistic phototoxicity. Int. J. Cancer 2001, 93, 384–392. [Google Scholar]
- Morgan, J.; Gray, A.G.; Huehns, E.R. Specific targeting and toxicity of sulphonated aluminium phthalocyanine photosensitised liposomes directed to cells by monoclonal antibody in vitro. Br. J. Cancer 1989, 59, 366–370. [Google Scholar] [CrossRef]
- Ben-Hur, E.; Rosenthal, I. Photosensitization of Chinese hamster cells by water-soluble phthalocyanines. Photochem. Photobiol. 1986, 43, 615–619. [Google Scholar]
- Decreau, R.; Richard, M.J.; Julliard, M. Photodynamic therapy against achromic M6 melanocytes: Phototoxicity of lipophilic axially substituted aluminum phthalocyanines and hexadecahalogenated zinc phthalocyanines. J. Porphyr. Phthalocya. 2001, 5, 390–396. [Google Scholar]
- Brasseur, N.; Ouellet, R.; La, M.C.; van Lier, J.E. Water-soluble aluminium phthalocyanine-polymer conjugates for PDT: Photodynamic activities and pharmacokinetics in tumour-bearing mice. Br. J. Cancer 1999, 80, 1533–1541. [Google Scholar]
- Dye, D.; Watkins, J. Suspected anaphylactic reaction to cremophor El. Brit. Med. J. 1980, 280, 1353. [Google Scholar]
- Torchilin, V.P. Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Biopolymers 2008, 90, 604–610. [Google Scholar]
- van Nostrum, C.F. Polymeric micelles to deliver photosensitizers for photodynamic therapy. Adv. Drug Delivery Rev. 2004, 56, 9–16. [Google Scholar]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer-chemotherapy—Mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar]
- Taillefer, J.; Jones, M.C.; Brasseur, N.; van Lier, J.E.; Leroux, J.C. Preparation and characterization of pH-responsive polymeric micelles for the delivery of photosensitizing anticancer drugs. J. Pharm. Sci. 2000, 89, 52–62. [Google Scholar]
- Taillefer, J.; Brasseur, N.; van Lier, J.E.; Lenaerts, V.; Le, G.D.; Leroux, J.C. In-vitro and in-vivo evaluation of pH-responsive polymeric micelles in a photodynamic cancer therapy model. J. Pharm. Pharmacol. 2001, 53, 155–166. [Google Scholar]
- Le, G.D.; Taillefer, J.; van Lier, J.E.; Lenaerts, V.; Leroux, J.C. Optimizing pH-responsive polymeric micelles for drug delivery in a cancer photodynamic therapy model. J. Drug Target 2002, 10, 429–437. [Google Scholar]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Delivery Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef]
- Kyriazi, M.; Alexandratou, E.; Yova, D.; Rallis, M.; Trebst, T. Topical photodynamic therapy of murine non-melanoma skin carcinomas with aluminum phthalocyanine chloride and a diode laser: Pharmacokinetics, tumor response and cosmetic outcomes. Photodermatol. Photoimmunol. Photomed. 2008, 24, 87–94. [Google Scholar]
- Robinson, D.J.; de Bruijn, H.S.; van der Veen, N.; Stringer, M.R.; Brown, S.B.; Star, W.M. Fluorescence photobleaching of ALA-induced protoporphyrin IX during photodynamic therapy of normal hairless mouse skin: The effect of light dose and irradiance and the resulting biological effect. Photochem. Photobiol. 1998, 67, 140–149. [Google Scholar] [CrossRef]
- Kruijt, B.; van der Ploeg-van den, Heuvel; de Bruijn, H.S.; Sterenborg, H.J.; Amelink, A.; Robinson, D.J. Monitoring interstitial m-THPC-PDT in vivo using fluorescence and reflectance spectroscopy. Lasers Surg. Med. 2009, 41, 653–664. [Google Scholar] [CrossRef]
- Wohrle, D.; Hirth, A.; Bogdahn-Rai, T.; Schnurpfeil, G.; Shopova, M. Photodynamic therapy of cancer: second and third generations of photosensitizers. Russ. Chem. Bull. 1998, 47, 807–816. [Google Scholar]
- Kudrevich, S.; Brasseur, N.; La, M.C.; Gilbert, S.; van Lier, J.E. Syntheses and photodynamic activities of novel trisulfonated zinc phthalocyanine derivatives. J. Med. Chem. 1997, 40, 3897–3904. [Google Scholar]
- Cauchon, N.; Tian, H.; Langlois, R.; La, M.C.; Martin, S.; Ali, H.; Hunting, D.; van Lier, J.E. Structure-photodynamic activity relationships of substituted zinc trisulfophthalocyanines. Bioconjug. Chem. 2005, 16, 80–89. [Google Scholar]
- Potter, W.R.; Henderson, B.W.; Bellnier, D.A.; Pandey, R.K.; Vaughan, L.A.; Weishaupt, K.R.; Dougherty, T.J. Parabolic quantitative structure-activity relationships and photodynamic therapy: Application of a three-compartment model with clearance to the in vivo quantitative structure-activity relationships of a congeneric series of pyropheophorbide derivatives used as photosensitizers for photodynamic therapy. Photochem. Photobiol. 1999, 70, 781–788. [Google Scholar]
- Liu, E.S.; Dai, Z.F.; Huang, J.D.; Chen, N.S.; Huang, J.L.; Huang, Z.Q.; Sun, J.C. Syntheses of metal phthalocyanines and their photoinactivations on cancer cells. Acta Biochim. Bioph. Sin. 1998, 30, 31–34. [Google Scholar]
- Huang, J.L.; Chen, N.S.; Huang, J.D.; Liu, E.S.; Xue, J.P.; Yang, S.L.; Huang, Z.Q.; Sun, J.C. Metal phthalocyanine as photosensitizer for photodynamic therapy (PDT)—Preparation, characterization and anticancer activities of an amphiphilic phthalocyanine ZnPcS2P2. Sci. China Ser. B 2001, 44, 113–122. [Google Scholar]
- Huang, H.F.; Chen, Y.Z.; Wu, Y. Experimental studies of the effects of ZnPcS2P2-based-photodynamic therapy on bone marrow purging. Chin. Med. J. (Engl.) 2005, 118, 105–110. [Google Scholar]
- Zhang, Z.; Jin, H.; Bao, J.; Fang, F.; Wei, J.; Wang, A. Intravenous repeated-dose toxicity study of ZnPcS2P2-based-photodynamic therapy in Wistar rats. Photochem. Photobiol. Sci. 2006, 5, 1006–1017. [Google Scholar] [CrossRef]
- Boyle, R.W.; Leznoff, C.C.; van Lier, J.E. Biological activities of phthalocyanines-XVI. Tetrahydroxy- and tetraalkylhydroxy zinc phthalocyanines. Effect of alkyl chain length on in vitro and in vivo photodynamic activities. Br. J. Cancer 1993, 67, 1177–1181. [Google Scholar] [CrossRef]
- Hu, M.; Brasseur, N.; Yildiz, S.Z.; van Lier, J.E.; Leznoff, C.C. Hydroxyphthalocyanines as potential photodynamic agents for cancer therapy. J. Med. Chem. 1998, 41, 1789–1802. [Google Scholar]
- Winkelman, J.W.; Arad, D.; Kimel, S. Stereochemical factors in the transport and binding of photosensitizers in biological systems and in photodynamic therapy. J. Photochem. Photobiol. B 1993, 18, 181–189. [Google Scholar]
- Fukushima, K.; Tabata, K.; Okura, I. Photochemical properties of water-soluble fluorinated zinc phthalocyanines and their photocytotoxicity against HeLa cells. J. Porphyr. Phthalocya. 1998, 2, 219–222. [Google Scholar]
- Gao, L.; Qian, X.; Zhang, L.; Zhang, Y. Tetra-trifluoroethoxyl zinc phthalocyanine: potential photosensitizer for use in the photodynamic therapy of cancer. J. Photochem. Photobiol. B 2001, 65, 35–38. [Google Scholar]
- O’Hagan, D.; Rzepa, H.S. Some influences of fluorine in bioorganic chemistry. Chem. Commun. 1997, 645–652. [Google Scholar]
- Allemann, E.; Brasseur, N.; Benrezzak, O.; Rousseau, J.; Kudrevich, S.V.; Boyle, R.W.; Leroux, J.C.; Gurny, R.; van Lier, J.E. PEG-coated poly(lactic acid) nanoparticles for the delivery of hexadecafluoro zinc phthalocyanine to EMT-6 mouse mammary tumours. J. Pharm. Pharmacol. 1995, 47, 382–387. [Google Scholar]
- Allemann, E.; Rousseau, J.; Brasseur, N.; Kudrevich, S.V.; Lewis, K.; van Lier, J.E. Photodynamic therapy of tumours with hexadecafluoro zinc phthalocynine formulated in PEG-coated poly(lactic acid) nanoparticles. Int. J. Cancer 1996, 66, 821–824. [Google Scholar]
- Allemann, E.; Brasseur, N.; Kudrevich, S.V.; La, M.C.; van Lier, J.E. Photodynamic activities and biodistribution of fluorinated zinc phthalocyanine derivatives in the murine EMT-6 tumour model. Int. J. Cancer 1997, 72, 289–294. [Google Scholar]
- Boyle, R.W.; Rousseau, J.; Kudrevich, S.V.; Obochi, M.; van Lier, J.E. Hexadecafluorinated zinc phthalocyanine: Photodynamic properties against the EMT-6 tumour in mice and pharmacokinetics using 65Zn as a radiotracer. Br. J. Cancer 1996, 73, 49–53. [Google Scholar]
- Liu, J.Y.; Lo, P.C.; Fong, W.P.; Ng, D.K. Effects of the number and position of the substituents on the in vitro photodynamic activities of glucosylated zinc(II) phthalocyanines. Org. Biomol. Chem. 2009, 7, 1583–1591. [Google Scholar] [CrossRef]
- Choi, C.F.; Huang, J.D.; Lo, P.C.; Fong, W.P.; Ng, D.K. Glycosylated zinc(II) phthalocyanines as efficient photosensitisers for photodynamic therapy. Synthesis, photophysical properties and in vitro photodynamic activity. Org. Biomol. Chem. 2008, 6, 2173–2181. [Google Scholar] [CrossRef]
- Airley, R.E.; Mobasheri, A. Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: Novel pathways and targets for anticancer therapeutics. Chemotherapy 2007, 53, 233–256. [Google Scholar]
- Medina, R.A.; Owen, G.I. Glucose transporters: Expression, regulation and cancer. Biol. Res. 2002, 35, 9–26. [Google Scholar]
- Ometto, C.; Fabris, C.; Milanesi, C.; Jori, G.; Cook, M.J.; Russell, D.A. Tumour-localising and -photosensitizing properties of a novel zinc(II) octadecylphthalocyanine. Br. J. Cancer 1996, 74, 1891–1899. [Google Scholar]
- Fabris, C.; Ometto, C.; Milanesi, C.; Jori, G.; Cook, M.J.; Russell, D.A. Tumour-localizing and tumour-photosensitizing properties of zinc(II)-octapentyl-phthalocyanine. J. Photochem. Photobiol. B 1997, 39, 279–284. [Google Scholar]
- Jori, G.; Reddi, E. The role of lipoproteins in the delivery of tumour-targeting photosensitizers. Int. J. Biochem. 1993, 25, 1369–1375. [Google Scholar]
- Polo, L.; Reddi, E.; Garbo, G.M.; Morgan, A.R.; Jori, G. The distribution of the tumour photosensitizers Zn(II)-phthalocyanine and Sn(IV)-etiopurpurin among rabbit plasma proteins. Cancer Lett. 1992, 66, 217–223. [Google Scholar] [CrossRef]
- Liu, W.; Jensen, T.J.; Fronczek, F.R.; Hammer, R.P.; Smith, K.M.; Vicente, M.G. Synthesis and cellular studies of nonaggregated water-soluble phthalocyanines. J. Med. Chem. 2005, 48, 1033–1041. [Google Scholar]
- Nesterova, I.V.; Erdem, S.S.; Pakhomov, S.; Hammer, R.P.; Soper, S.A. Phthalocyanine dimerization-based molecular beacons using near-IR fluorescence. J. Am. Chem. Soc. 2009, 131, 2432–2433. [Google Scholar]
- Tyagi, S.; Kramer, F.R. Molecular beacons: Probes that fluoresce upon hybridization. Nat. Biotechnol. 1996, 14, 303–308. [Google Scholar]
- Lo, P.C.; Zhao, B.; Duan, W.; Fong, W.P.; Ko, W.H.; Ng, D.K. Synthesis and in vitro photodynamic activity of mono-substituted amphiphilic zinc(II) phthalocyanines. Bioorg. Med. Chem. Lett. 2007, 17, 1073–1077. [Google Scholar]
- Jeremy, M.B.; John, L.T.; Lubert, S. Biochemistry, 5th ed; W. H. Freeman and Company: New York, NY, USA, 2002. [Google Scholar]
- Liu, J.Y.; Jiang, X.J.; Fong, W.P.; Ng, D.K. Highly photocytotoxic 1,4-dipegylated zinc(II) phthalocyanines. Effects of the chain length on the in vitro photodynamic activities. Org. Biomol. Chem. 2008, 6, 4560–4566. [Google Scholar] [CrossRef]
- Tuncel, S.; Dumoulin, F.; Gailer, J.; Sooriyaarachchi, M.; Atilla, D.; Durmus, M.; Bouchu, D.; Savoie, H.; Boyle, R.W.; Ahsen, V. A set of highly water-soluble tetraethyleneglycol-substituted Zn(II) phthalocyanines: Synthesis, photochemical and photophysical properties, interaction with plasma proteins and in vitro phototoxicity. Dalton Trans. 2011, 40, 4067–4079. [Google Scholar]
- Bai, M.; Lo, P.C.; Ye, J.; Wu, C.; Fong, W.P.; Ng, D.K. Facile synthesis of pegylated zinc(II) phthalocyanines via transesterification and their in vitro photodynamic activities. Org. Biomol. Chem. 2011, 9, 7028–7032. [Google Scholar] [CrossRef]
- Banfi, S.; Caruso, E.; Buccafurni, L.; Ravizza, R.; Gariboldi, M.; Monti, E. Zinc phthalocyanines-mediated photodynamic therapy induces cell death in adenocarcinoma cells. J. Organomet. Chem. 2007, 692, 1269–1276. [Google Scholar]
- Ball, D.J.; Mayhew, S.; Wood, S.R.; Griffiths, J.; Vernon, D.I.; Brown, S.B. A comparative study of the cellular uptake and photodynamic efficacy of three novel zinc phthalocyanines of differing charge. Photochem. Photobiol. 1999, 69, 390–396. [Google Scholar]
- Cruse-Sawyer, J.E.; Griffiths, J.; Dixon, B.; Brown, S.B. The photodynamic response of two rodent tumour models to four zinc (II)-substituted phthalocyanines. Br. J. Cancer 1998, 77, 965–972. [Google Scholar]
- Sibrian-Vazquez, M.; Ortiz, J.; Nesterova, I.V.; Fernandez-Lazaro, F.; Sastre-Santos, A.; Soper, S.A.; Vicente, M.G. Synthesis and properties of cell-targeted Zn(II)-phthalocyanine-peptide conjugates. Bioconjug. Chem. 2007, 18, 410–420. [Google Scholar]
- Torchilin, V.P. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007, 9, E128–E147. [Google Scholar] [CrossRef]
- Liu, R.; Cannon, J.B.; Paspal, S.Y.L. Liposomes in solubilization. In Water-Insoluble Drug Formulation; Liu, R., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 375–409. [Google Scholar]
- Rodal, G.H.; Rodal, S.K.; Moan, J.; Berg, K. Liposome-bound Zn (II)-phthalocyanine. Mechanisms for cellular uptake and photosensitization. J. Photochem. Photobiol. B 1998, 45, 150–159. [Google Scholar] [CrossRef]
- Valduga, G.; Reddi, E.; Garbisa, S.; Jori, G. Photosensitization of cells with different metastatic potentials by liposome-delivered Zn(II)-phthalocyanine. Int. J. Cancer 1998, 75, 412–417. [Google Scholar]
- Milanesi, C.; Zhou, C.; Biolo, R.; Jori, G. Zn(II)-phthalocyanine as a photodynamic agent for tumours. II. Studies on the mechanism of photosensitised tumour necrosis. Br. J. Cancer 1990, 61, 846–850. [Google Scholar] [CrossRef]
- Ben-Hur, E.; Chan, W.S. Phthalocyanines in photobiology and their medical applications. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 1–35. [Google Scholar]
- Allison, B.A.; Pritchard, P.H.; Richter, A.M.; Levy, J.G. The plasma distribution of benzoporphyrin derivative and the effects of plasma-lipoproteins on its biodistribution. Photochem. Photobiol. 1990, 52, 501–507. [Google Scholar]
- Allison, B.A.; Pritchard, P.H.; Levy, J.G. Evidence for low-density-lipoprotein receptor-mediated uptake of benzoporphyrin derivative. Br. J. Cancer 1994, 69, 833–839. [Google Scholar]
- Polo, L.; Bianco, G.; Reddi, E.; Jori, G. The effect of different liposomal formulations on the interaction of Zn(II)-phthalocyanine with isolated low and high density lipoproteins. Int. J. Biochem. Cell Biol. 1995, 27, 1249–1255. [Google Scholar]
- Reddi, E.; Zhou, C.; Biolo, R.; Menegaldo, E.; Jori, G. Liposome- or LDL-administered Zn (II)-phthalocyanine as a photodynamic agent for tumours. I. Pharmacokinetic properties and phototherapeutic efficiency. Br. J. Cancer 1990, 61, 407–411. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Michels, S.; Indorf, L.; Eggers, R.; Birngruber, R. Mechanism of photodynamic occlusion using liposomal Zn(II)-phtalocyanine. Curr. Eye Res. 2005, 30, 601–612. [Google Scholar]
- Gal, D.; Macdonald, P.C.; Porter, J.C.; Simpson, E.R. Cholesterol-metabolism in cancer-cells in monolayer-culture. III. Low-density lipoprotein metabolism. Int. J. Cancer 1981, 28, 315–319. [Google Scholar] [CrossRef]
- Love, W.G.; Duk, S.; Biolo, R.; Jori, G.; Taylor, P.W. Liposome-mediated delivery of photosensitizers: Localization of zinc (II)-phthalocyanine within implanted tumors after intravenous administration. Photochem. Photobiol. 1996, 63, 656–661. [Google Scholar]
- van Leengoed, H.L.; Cuomo, V.; Versteeg, A.A.; van der Veen, N.; Jori, G.; Star, W.M. In vivo fluorescence and photodynamic activity of zinc phthalocyanine administered in liposomes. Br. J. Cancer 1994, 69, 840–845. [Google Scholar] [CrossRef]
- Schieweck, K.; Capraro, H.G.; Isele, U.; van Hoogevest, P.; Ochsner, M.; Maurer, T.; Batt, E. CGP 55847, liposome-delivered zinc(II) phthalocyanine as phototherapeutic agent for tumours. Proc. SPIE Int. Soc. Opt. Eng. 2078, 107–118. [Google Scholar]
- Gelperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef]
- Betancourt, T.; Byrne, J.D.; Sunaryo, N.; Crowder, S.W.; Kadapakkam, M.; Patel, S.; Casciato, S.; Brannon-Peppas, L. PEGylation strategies for active targeting of PLA/PLGA nanoparticles. J. Biomed. Mater. Res. Part A 2009, 91A, 263–276. [Google Scholar]
- Fattal, E.; Vauthier, C. Nanoparticles as Drug Delivery Systems. In Encyclopedia of Pharmaceutical Technology; Swarbrick, J., Boylan, J.C., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2011; pp. 1864–1882. [Google Scholar]
- Fadel, M.; Kassab, K.; Fadeel, D.A. Zinc phthalocyanine-loaded PLGA biodegradable nanoparticles for photodynamic therapy in tumor-bearing mice. Lasers Med. Sci. 2010, 25, 283–292. [Google Scholar]
- Lu, H.L.; Syu, W.J.; Nishiyama, N.; Kataoka, K.; Lai, P.S. Dendrimer phthalocyanine-encapsulated polymeric micelle-mediated photochemical internalization extends the efficacy of photodynamic therapy and overcomes drug-resistance in vivo. J. Control. Release 2011, 155, 458–464. [Google Scholar] [CrossRef]
- Herlambang, S.; Kumagai, M.; Nomoto, T.; Horie, S.; Fukushima, S.; Oba, M.; Miyazaki, K.; Morimoto, Y.; Nishiyama, N.; Kataoka, K. Disulfide crosslinked polyion complex micelles encapsulating dendrimer phthalocyanine directed to improved efficiency of photodynamic therapy. J. Control. Release 2011, 155, 449–457. [Google Scholar]
- Nishiyama, N.; Nakagishi, Y.; Morimoto, Y.; Lai, P.S.; Miyazaki, K.; Urano, K.; Horie, S.; Kumagai, M.; Fukushima, S.; Cheng, Y.; et al. Enhanced photodynamic cancer treatment by supramolecular nanocarriers charged with dendrimer phthalocyanine. J. Control. Release 2009, 133, 245–251. [Google Scholar] [CrossRef]
- Jang, W.D.; Nakagishi, Y.; Nishiyama, N.; Kawauchi, S.; Morimoto, Y.; Kikuchi, M.; Kataoka, K. Polyion complex micelles for photodynamic therapy: Incorporation of dendritic photosensitizer excitable at long wavelength relevant to improved tissue-penetrating property. J. Control. Release 2006, 113, 73–79. [Google Scholar] [CrossRef]
- Baugh, S.D.; Yang, Z.; Leung, D.K.; Wilson, D.M.; Breslow, R. Cyclodextrin dimers as cleavable carriers of photodynamic sensitizers. J. Am. Chem. Soc. 2001, 123, 12488–12494. [Google Scholar]
- Ruebner, A.; Kirsch, D.; Andrees, S.; Decker, W.; Roeder, B.; Spengler, B.; Kaufmann, R.; Moser, J.G. Dimeric cyclodextrin carriers with high binding affinity to porphyrinoid photosensitizers. J. Inclus. Phenom. Mol. 1997, 27, 69–84. [Google Scholar]
- Rossetti, F.C.; Lopes, L.B.; Carollo, A.R.; Thomazini, J.A.; Tedesco, A.C.; Bentley, M.V. A delivery system to avoid self-aggregation and to improve in vitro and in vivo skin delivery of a phthalocyanine derivative used in the photodynamic therapy. J. Control. Release 2011, 155, 400–408. [Google Scholar] [CrossRef]
- Rodriguez, M.E.; Diz, V.E.; Awruch, J.; Dicelio, L.E. Photophysics of zinc (II) phthalocyanine polymer and gel formulation. Photochem. Photobiol. 2010, 86, 513–519. [Google Scholar] [CrossRef]
- Souza, J.G.; Gelfuso, G.M.; Simao, P.S.; Borges, A.C.; Lopez, R.F. Iontophoretic transport of zinc phthalocyanine tetrasulfonic acid as a tool to improve drug topical delivery. Anti-Cancer Drugs 2011, 22, 783–793. [Google Scholar] [CrossRef]
- He, J.; Larkin, H.E.; Li, Y.S.; Rihter, D.; Zaidi, S.I.; Rodgers, M.A.; Mukhtar, H.; Kenney, M.E.; Oleinick, N.L. The synthesis, photophysical and photobiological properties and in vitro structure-activity relationships of a set of silicon phthalocyanine PDT photosensitizers. Photochem. Photobiol. 1997, 65, 581–586. [Google Scholar] [CrossRef]
- Rodriguez, M.E.; Zhang, P.; Azizuddin, K.; Delos Santos, G.B.; Chiu, S.M.; Xue, L.Y.; Berlin, J.C.; Peng, X.; Wu, H.; Lam, M.; et al. Structural factors and mechanisms underlying the improved photodynamic cell killing with silicon phthalocyanine photosensitizers directed to lysosomes versus mitochondria. Photochem. Photobiol. 2009, 85, 1189–1200. [Google Scholar] [CrossRef]
- Lo, P.C.; Huang, J.D.; Cheng, D.Y.; Chan, E.Y.; Fong, W.P.; Ko, W.H.; Ng, D.K. New amphiphilic silicon(IV) phthalocyanines as efficient photosensitizers for photodynamic therapy: Synthesis, photophysical properties, and in vitro photodynamic activities. Chemistry 2004, 10, 4831–4838. [Google Scholar] [CrossRef]
- Lo, P.C.; Chan, C.M.; Liu, J.Y.; Fong, W.P.; Ng, D.K. Highly photocytotoxic glucosylated silicon(IV) phthalocyanines. Effects of peripheral chloro substitution on the photophysical and photodynamic properties. J. Med. Chem. 2007, 50, 2100–2107. [Google Scholar] [CrossRef]
- Leung, S.C.H.; Lo, P.C.; Ng, D.K.P.; Liu, W.K.; Fung, K.P.; Fong, W.P. Photodynamic activity of BAM-SiPc, an unsymmetrical bisamino silicon(IV) phthalocyanine, in tumour-bearing nude mice. Br. J. Pharmacol. 2008, 154, 4–12. [Google Scholar] [CrossRef]
- Lai, J.C.; Lo, P.C.; Ng, D.K.; Ko, W.H.; Leung, S.C.; Fung, K.P.; Fong, W.P. BAM-SiPc, a novel agent for photodynamic therapy, induces apoptosis in human hepatocarcinoma HepG2 cells by a direct mitochondrial action. Cancer Biol. Ther. 2006, 5, 413–418. [Google Scholar] [CrossRef]
- Barge, J.; Decreau, R.; Julliard, M.; Hubaud, J.C.; Sabatier, A.S.; Grob, J.J.; Verrando, P. Killing efficacy of a new silicon phthalocyanine in human melanoma cells treated with photodynamic therapy by early activation of mitochondrion-mediated apoptosis. Exp. Dermatol. 2004, 13, 33–44. [Google Scholar]
- Anderson, C.Y.; Freye, K.; Tubesing, K.A.; Li, Y.S.; Kenney, M.E.; Mukhtar, H.; Elmets, C.A. A comparative analysis of silicon phthalocyanine photosensitizers for in vivo photodynamic therapy of RIF-1 tumors in C3H mice. Photochem. Photobiol. 1998, 67, 332–336. [Google Scholar] [CrossRef]
- Jiang, X.J.; Yeung, S.L.; Lo, P.C.; Fong, W.P.; Ng, D.K. Phthalocyanine-polyamine conjugates as highly efficient photosensitizers for photodynamic therapy. J. Med. Chem. 2011, 54, 320–330. [Google Scholar]
- Leng, X.; Choi, C.F.; Lo, P.C.; Ng, D.K. Assembling a mixed phthalocyanine-porphyrin array in aqueous media through host-guest interactions. Org. Lett. 2007, 9, 231–234. [Google Scholar] [CrossRef]
- Lau, J.T.; Lo, P.C.; Fong, W.P.; Ng, D.K. Preparation and photodynamic activities of silicon(IV) phthalocyanines substituted with permethylated beta-cyclodextrins. Chemistry 2011, 17, 7569–7577. [Google Scholar] [CrossRef]
- Lau, J.T.; Lo, P.C.; Tsang, Y.M.; Fong, W.P.; Ng, D.K. Unsymmetrical beta-cyclodextrin-conjugated silicon(IV) phthalocyanines as highly potent photosensitisers for photodynamic therapy. Chem. Commun. (Camb.) 2011, 47, 9657–9659. [Google Scholar]
- Chan, C.M.; Lo, P.C.; Yeung, S.L.; Ng, D.K.; Fong, W.P. Photodynamic activity of a glucoconjugated silicon(IV) phthalocyanine on human colon adenocarcinoma. Cancer Biol. Ther. 2010, 10, 126–134. [Google Scholar] [CrossRef]
- Agarwal, M.L.; Clay, M.E.; Harvey, E.J.; Evans, H.H.; Antunez, A.R.; Oleinick, N.L. Photodynamic therapy induces rapid cell death by apoptosis in L5178Y mouse lymphoma cells. Cancer Res. 1991, 51, 5993–5996. [Google Scholar]
- Ahmad, N.; Feyes, D.K.; Agarwal, R.; Mukhtar, H. Photodynamic therapy results in induction of WAF1/CIP1/P21 leading to cell cycle arrest and apoptosis. Proc. Natl. Acad. Sci. USA 1998, 95, 6977–6982. [Google Scholar]
- Colussi, V.C.; Feyes, D.K.; Mulvihill, J.W.; Li, Y.S.; Kenney, M.E.; Elmets, C.A.; Oleinick, N.L.; Mukhtar, H. Phthalocyanine 4 (Pc4) photodynamic therapy of human OVCAR-3 tumor xenografts. Photochem. Photobiol. 1999, 69, 236–241. [Google Scholar]
- Whitacre, C.M.; Feyes, D.K.; Satoh, T.; Grossmann, J.; Mulvihill, J.W.; Mukhtar, H.; Oleinick, N.L. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4 of SW480 human colon cancer xenografts in athymic mice. Clin. Cancer Res. 2000, 6, 2021–2027. [Google Scholar]
- Whitacre, C.M.; Satoh, T.H.; Xue, L.; Gordon, N.H.; Oleinick, N.L. Photodynamic therapy of human breast cancer xenografts lacking caspase-3. Cancer Lett. 2002, 179, 43–49. [Google Scholar]
- Fei, B.; Wang, H.; Meyers, J.D.; Feyes, D.K.; Oleinick, N.L.; Duerk, J.L. High-field magnetic resonance imaging of the response of human prostate cancer to Pc 4-based photodynamic therapy in an animal model. Lasers Surg. Med. 2007, 39, 723–730. [Google Scholar]
- Lee, R.G.; Vecchiotti, M.A.; Heaphy, J.; Panneerselvam, A.; Schluchter, M.D.; Oleinick, N.L.; Lavertu, P.; Alagramam, K.N.; Arnold, J.E.; Sprecher, R.C. Photodynamic therapy of cottontail rabbit papillomavirus-induced papillomas in a severe combined immunodeficient mouse xenograft system. Laryngoscope 2010, 120, 618–624. [Google Scholar]
- Agarwal, R.; Korman, N.J.; Mohan, R.R.; Feyes, D.K.; Jawed, S.; Zaim, M.T.; Mukhtar, H. Apoptosis is an early event during phthalocyanine photodynamic therapy-induced ablation of chemically induced squamous papillomas in mouse skin. Photochem. Photobiol. 1996, 63, 547–552. [Google Scholar]
- George, J.E., III; Ahmad, Y.; Varghai, D.; Li, X.; Berlin, J.; Jackowe, D.; Jungermann, M.; Wolfe, M.S.; Lilge, L.; Totonchi, A.; et al. Pc 4 photodynamic therapy of U87-derived human glioma in the nude rat. Lasers Surg. Med. 2005, 36, 383–389. [Google Scholar] [CrossRef]
- Oleinick, N.L.; Morris, R.L.; Belichenko, T. The role of apoptosis in response to photodynamic therapy: What, where, why, and how. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar] [CrossRef]
- Anderson, C.; Hrabovsky, S.; McKinley, Y.; Tubesing, K.; Tang, H.P.; Dunbar, R.; Mukhtar, H.; Elmets, C.A. Phthalocyanine photodynamic therapy: Disparate effects of pharmacologic inhibitorson cutaneous photosensitivity and on tumor regression. Photochem. Photobiol. 1997, 65, 895–901. [Google Scholar]
- Lam, M.; Hsia, A.H.; Liu, Y.; Guo, M.; Swick, A.R.; Berlin, J.C.; McCormick, T.S.; Kenney, M.E.; Oleinick, N.L.; Cooper, K.D.; et al. Successful cutaneous delivery of the photosensitizer silicon phthalocyanine 4 for photodynamic therapy. Clin. Exp. Dermatol. 2011, 36, 645–651. [Google Scholar] [CrossRef]
- Baron, E.D.; Malbasa, C.L.; Santo-Domingo, D.; Fu, P.; Miller, J.D.; Hanneman, K.K.; Hsia, A.H.; Oleinick, N.L.; Colussi, V.C.; Cooper, K.D. Silicon phthalocyanine (Pc 4) photodynamic therapy is a safe modality for cutaneous neoplasms: Results of a phase 1 clinical trial. Lasers Surg. Med. 2010, 42, 728–735. [Google Scholar]
- Choi, C.F.; Tsang, P.T.; Huang, J.D.; Chan, E.Y.; Ko, W.H.; Fong, W.P.; Ng, D.K. Synthesis and in vitro photodynamic activity of new hexadeca-carboxy phthalocyanines. Chem. Commun. (Camb.) 2004, 19, 2236–2237. [Google Scholar]
- Huang, J.D.; Fong, W.P.; Chan, E.Y.M.; Choi, M.T.M.; Chan, W.K.; Chan, M.C.; Ng, K.P. Photodynamic activities of a dicationic silicon(IV) phthalocyanine and its bovine serum albumin conjugates. Tetrahedron Lett. 2003, 44, 8029–8032. [Google Scholar]
- Huang, J.D.; Wang, S.Q.; Lo, P.C.; Fong, W.P.; Ko, W.H.; Ng, D.K.P. Halogenated silicon(IV) phthalocyanines with axial poly(ethylene glycol) chains. Synthesis, spectroscopic properties, complexation with bovine serum albumin and in vitro photodynamic activities. New J. Chem. 2004, 28, 348–354. [Google Scholar] [CrossRef]
- Lee, P.P.S.; Ngai, T.; Huang, J.D.; Wu, C.; Fong, W.P.; Ng, D.K.P. Synthesis, characterization, biodegradation, and in vitro photodynamic activities of silicon(IV) phthalocyanines conjugated axially with poly(epsilon-caprolactone). Macromolecules 2003, 36, 7527–7533. [Google Scholar] [CrossRef]
- Lee, P.P.S.; Lo, P.C.; Chan, E.Y.M.; Fong, W.P.; Ko, W.H.; Ng, D.K.P. Synthesis and in vitro photodynamic activity of novel galactose-containing phthalocyanines. Tetrahedron Lett. 2005, 46, 1551–1554. [Google Scholar] [CrossRef]
- Lo, P.C.; Leung, S.C.H.; Chan, E.Y.M.; Fong, W.P.; Ko, W.H.; Ng, D.K.P. Photodynamic effects of a novel series of silicon(IV) phthalocyanines against human colon adenocarcinoma cells. Photodiagn. Photodyn. Ther. 2007, 4, 117–123. [Google Scholar] [CrossRef]
- Master, A.M.; Rodriguez, M.E.; Kenney, M.E.; Oleinick, N.L.; Sen Gupta, A. Delivery of the photosensitizer Pc4 in PEG-PCL micelles for in vitro PDT studies. J. Pharm. Sci. 2010, 99, 2386–2398. [Google Scholar]
- Roy, I.; Ohulchanskyy, T.Y.; Pudavar, H.E.; Bergey, E.J.; Oseroff, A.R.; Morgan, J.; Dougherty, T.J.; Prasad, P.N. Ceramic-based nanoparticles entrapping water-insoluble photosensitizing anticancer drugs: A novel drug-carrier system for photodynamic therapy. J. Am. Chem. Soc. 2003, 125, 7860–7865. [Google Scholar]
- Zhao, B.Z.; Yin, J.J.; Bilski, P.J.; Chignell, C.F.; Roberts, J.E.; He, Y.Y. Enhanced photodynamic efficacy towards melanoma cells by encapsulation of Pc4 in silica nanoparticles. Toxicol. Appl. Pharm. 2009, 241, 163–172. [Google Scholar]
- Cheng, Y.; Samia, A.C.; Meyers, J.D.; Panagopoulos, I.; Fei, B.W.; Burda, C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J. Am. Chem. Soc. 2008, 130, 10643–10647. [Google Scholar]
- Li, H.; Marotta, D.E.; Kim, S.; Busch, T.M.; Wileyto, E.P.; Zheng, G. High payload delivery of optical imaging and photodynamic therapy agents to tumors using phthalocyanine-reconstituted low-density lipoprotein nanoparticles. J. Biomed. Opt. 2005, 10, 41203. [Google Scholar] [CrossRef]
- Zheng, G.; Chen, J.; Li, H.; Glickson, J.D. Rerouting lipoprotein nanoparticles to selected alternate receptors for the targeted delivery of cancer diagnostic and therapeutic agents. Proc. Natl. Acad. Sci. USA 2005, 102, 17757–17762. [Google Scholar]
- Juan, C.; Ian, R.C.; Gang, Z. Novel targeting and activation strategies for photodynamic therapy. In Proceedings of Light-activated Tissue Regeneration and Therapy Conference; Ronald, W., Darrell, B.T., Eds.; Springer: New York, NY, USA, 2008; pp. 127–147. [Google Scholar]
- Dutta, S.; Ongarora, B.G.; Li, H.; Vicente, M.G.; Kolli, B.K.; Chang, K.P. Intracellular targeting specificity of novel phthalocyanines assessed in a host-parasite model for developing potential photodynamic medicine. PLoS One 2011, 6, e20786. [Google Scholar]
- Giuliani, F.; Martinelli, M.; Cocchi, A.; Arbia, D.; Fantetti, L.; Roncucci, G. In vitro resistance selection studies of RLP068/Cl, a new Zn(II) phthalocyanine suitable for antimicrobial photodynamic therapy. Antimicrob. Agents Chemother. 2010, 54, 637–642. [Google Scholar] [CrossRef]
- Mantareva, V.; Angelov, I.; Kussovski, V.; Dimitrov, R.; Lapok, L.; Wöhrle, D. Photodynamic efficacy of water-soluble Si(IV) and Ge(IV) phthalocyanines towards Candida albicans planktonic and biofilm cultures. Eur. J. Med. Chem. 2011, 46, 4430–4440. [Google Scholar] [CrossRef]
- Longo, J.P.; Leal, S.C.; Simioni, A.R.; de Fatima, M.A.-S.; Tedesco, A.C.; Azevedo, R.B. Photodynamic therapy disinfection of carious tissue mediated by aluminum-chloride-phthalocyanine entrapped in cationic liposomes: An in vitro and clinical study. Lasers Med. Sci. 2011. Epub ahead of print.. [Google Scholar]
- Montanari, J.; Maidana, C.; Esteva, M.I.; Salomon, C.; Morilla, M.J.; Romero, E.L. Sunlight triggered photodynamic ultradeformable liposomes against Leishmania braziliensis are also leishmanicidal in the dark. J. Control. Release 2010, 147, 368–376. [Google Scholar] [CrossRef]
- van den, B.H. Photodynamic therapy of age-related macular degeneration: History and principles. Semin. Ophthalmol. 2001, 16, 181–200. [Google Scholar]
- Lange, N.; Jichlinski, P.; Zellweger, M.; Forrer, M.; Marti, A.; Guillou, L.; Kucera, P.; Wagnieres, G.; van den, B.H. Photodetection of early human bladder cancer based on the fluorescence of 5-aminolaevulinic acid hexylester-induced protoporphyrin IX: A pilot study. Br. J. Cancer 1999, 80, 185–193. [Google Scholar] [CrossRef]
- Stepp, H.; Beck, T.; Pongratz, T.; Meinel, T.; Kreth, F.W.; Tonn, J.C.; Stummer, W. ALA and malignant glioma: Fluorescence-guided resection and photodynamic treatment. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 157–164. [Google Scholar] [CrossRef]
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: A prospective study in 52 consecutive patients. J. Neurosurg. 2000, 93, 1003–1013. [Google Scholar]
- Marcus, S.L.; Sobel, R.S.; Golub, A.L.; Carroll, R.L.; Lundahl, S.; Shulman, D.G. Photodynamic therapy (PDT) and photodiagnosis (PD) using endogenous photosensitization induced by 5-aminolevulinic acid (ALA): Current clinical and development status. J. Clin. Laser Med. Surg. 1996, 14, 59–66. [Google Scholar]
- Zellweger, M.; Radu, A.; Monnier, P.; van den, B.H.; Wagnieres, G. Fluorescence pharmacokinetics of Lutetium Texaphyrin (PCI-0123, Lu-Tex) in the skin and in healthy and tumoral hamster cheek-pouch mucosa. J. Photochem. Photobiol. B 2000, 55, 56–62. [Google Scholar]
- Glanzmann, T.; Forrer, M.; Blant, S.A.; Woodtli, A.; Grosjean, P.; Braichotte, D.; van den, B.H.; Monnier, P.; Wagnieres, G. Pharmacokinetics and pharmacodynamics of tetra(m-hydroxyphenyl)chlorin in the hamster cheek pouch tumor model: Comparison with clinical measurements. J. Photochem. Photobiol. B 2000, 57, 22–32. [Google Scholar] [CrossRef]
- Fotinos, N.; Campo, M.A.; Popowycz, F.; Gurny, R.; Lange, N. 5-Aminolevulinic acid derivatives in photomedicine: Characteristics, application and perspectives. Photochem. Photobiol. 2006, 82, 994–1015. [Google Scholar]
- Rao, J.; Dragulescu-Andrasi, A.; Yao, H. Fluorescence imaging in vivo: Recent advances. Curr. Opin. Biotechnol. 2007, 18, 17–25. [Google Scholar] [CrossRef]
- Dumoulin, F.; Durmus, M.; Ahsen, V.; Nyokong, T. Synthetic pathways to water-soluble phthalocyanines and close analogs. Coordin. Chem. Rev. 2010, 254, 2792–2847. [Google Scholar]
- Gabriel, D.; Zuluaga, M.F.; Lange, N. On the cutting edge: protease-sensitive prodrugs for the delivery of photoactive compounds. Photochem. Photobiol. Sci. 2011, 10, 689–703. [Google Scholar] [CrossRef]
- Weissleder, R.; Tung, C.H.; Mahmood, U.; Bogdanov, A., Jr. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 1999, 17, 375–378. [Google Scholar] [CrossRef]
- Campo, M.A.; Gabriel, D.; Kucera, P.; Gurny, R.; Lange, N. Polymeric photosensitizer prodrugs for photodynamic therapy. Photochem. Photobiol. 2007, 83, 958–965. [Google Scholar] [CrossRef]
- Gabriel, D.; Campo, M.A.; Gurny, R.; Lange, N. Tailoring protease-sensitive photodynamic agents to specific disease-associated enzymes. Bioconjug. Chem. 2007, 18, 1070–1077. [Google Scholar]
- Gabriel, D.; Busso, N.; So, A.; van den, B.H.; Gurny, R.; Lange, N. Thrombin-sensitive photodynamic agents: A novel strategy for selective synovectomy in rheumatoid arthritis. J. Control. Release 2009, 138, 225–234. [Google Scholar]
- Segalla, A.; Milanesi, C.; Jori, G.; Capraro, H.G.; Isele, U.; Schieweck, K. CGP 55398, a liposomal Ge(IV) phthalocyanine bearing two axially ligated cholesterol moieties: A new potential agent for photodynamic therapy of tumours. Br. J. Cancer 1994, 69, 817–825. [Google Scholar] [CrossRef]
- de Oliveira, K.T.; de Assis, F.F.; Ribeiro, A.O.; Neri, C.R.; Fernandes, A.U.; Baptista, M.S.; Lopes, N.P.; Serra, O.A.; Iamamoto, Y. Synthesis of phthalocyanines-ALA conjugates: Water-soluble compounds with low aggregation. J. Org. Chem. 2009, 74, 7962–7965. [Google Scholar]
- Seotsanyana-Mokhosi, I.; Kresfelder, T.; Abrahamse, H.; Nyokong, T. The effect of Ge, Si and Sn phthalocyanine photo sensitizers on cell proliferation and viability of human oesophageal carcinoma cells. J. Photochem. Photobiol. B 2006, 83, 55–62. [Google Scholar]
- Durmus, M.; Ahsen, V. Water-soluble cationic gallium(III) and indium(III) phthalocyanines for photodynamic therapy. J. Inorg. Biochem. 2010, 104, 297–309. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sekkat, N.; Bergh, H.v.d.; Nyokong, T.; Lange, N. Like a Bolt from the Blue: Phthalocyanines in Biomedical Optics. Molecules 2012, 17, 98-144. https://doi.org/10.3390/molecules17010098
Sekkat N, Bergh Hvd, Nyokong T, Lange N. Like a Bolt from the Blue: Phthalocyanines in Biomedical Optics. Molecules. 2012; 17(1):98-144. https://doi.org/10.3390/molecules17010098
Chicago/Turabian StyleSekkat, Nawal, Hubert van den Bergh, Tebello Nyokong, and Norbert Lange. 2012. "Like a Bolt from the Blue: Phthalocyanines in Biomedical Optics" Molecules 17, no. 1: 98-144. https://doi.org/10.3390/molecules17010098