Phenolic Enriched Extract of Baccharis trimera Presents Anti-inflammatory and Antioxidant Activities
Abstract
:1. Introduction
2. Results and Discussion
| Samples | Phenolic content a (GAE/g) |
|---|---|
| Crude ethanol | 261.59 ± 8.75 |
| Dichloromethane | 126.93 ± 5.52 |
| Ethyl acetate | 1387.95 ± 42.45 |
| Butanol | 107.71 ± 2.07 |
| Aqueous | 455.29 ± 24.9 |
| Saponin | 223.02 ± 28.86 |
| Phenolic | 1482.02 ± 50.41 |
| Samples | IC50 (µg/mL) | AEAC (g) |
|---|---|---|
| Ascorbic acid (AA) | 3.38 | 1 |
| Crude ethanol | 15.49 | 4.58 |
| Dichloromethane | 29.95 | 8.85 |
| Ethyl acetate | 6.25 | 1.85 |
| Butanol | 8.24 | 2.44 |
| Aqueous | 50.87 | 15.03 |
| Saponin | 10.25 | 3.03 |
| Phenolic | 1.57 | 0.46 |
| Quercetin | 2.41 | 0.71 |
| Luteolin | 2.84 | 0.84 |
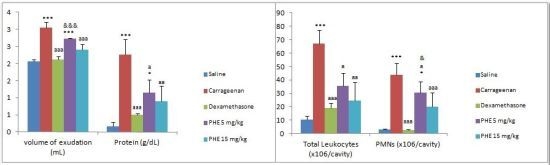
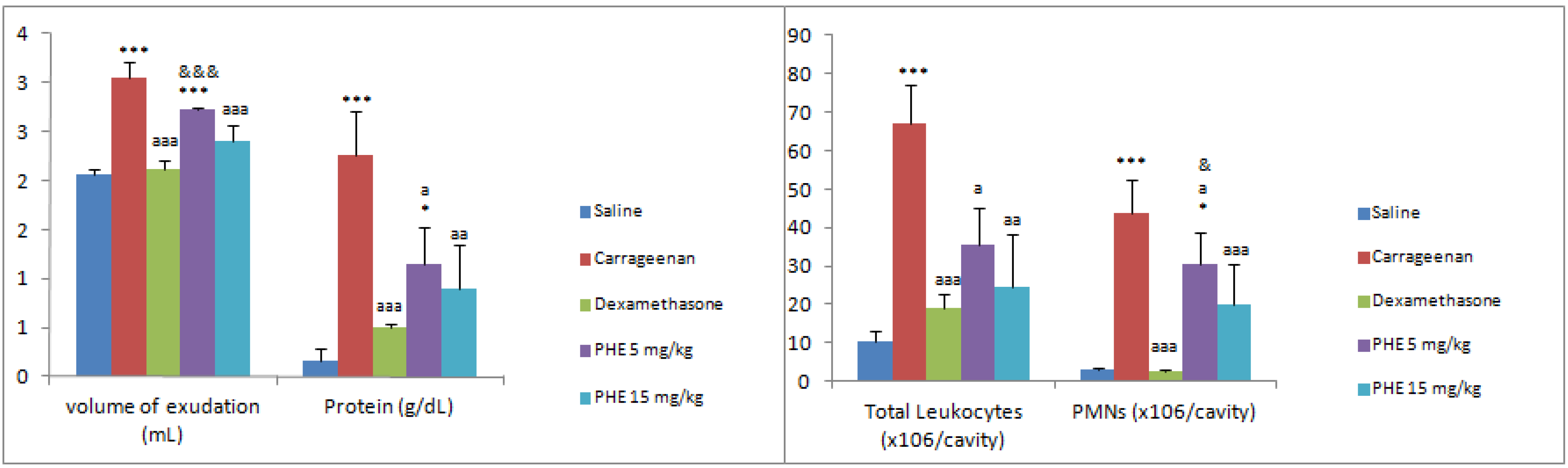
| Groups/dose | volume of exudation (mL) | Total Leukocytes (×106/cavity) | PMNs (×106/cavity) | Protein (g/dL) | NO (nmol/cavity) |
|---|---|---|---|---|---|
| Saline | 2.06 ± 0.05 | 10.57 ± 2.55 | 3.12 ± 0.38 | 0.16 ± 0.12 | 4.44 ± 3.37 |
| Carrageenan | 2.96 ± 0.15 *** | 37.47 ± 21.88 ** | 43.70 ± 18.71 *** | 2.25 ± 0.46 *** | 70.53 ± 18.06 |
| Dexamethasone | 2.12 ± 0.09 aaa | 19.05 ± 3.57 | 2.79 ± 0.46 aaa | 0.50 ± 0.04 aaa | 39.97 ± 11.73 |
| DCM 25 mg/kg | 2.46 ± 0.13 **aaa&& | 8.79 ± 2.53 aa | 5.90 ± 0.42 aa | 0.54 ± 0.20 aaa | 12.67 ± 4.48 aaa& |
| DCM 50 mg/kg | 2.42 ± 0.19 **aaa& | 7.52 ± 0.98 aa | 4.30 ± 0.49 aaa | 0.62 ± 0.34 aaa | 17.71 ± 4.17 aaa |
| DCM 75 mg/kg | 2.34 ± 0.18 aaa | 8.03 ± 1.99 aa | 4.00 ± 0.69 aaa | 0.69 ± 0.12 aaa | 19.93 ± 4.49 aaa |
| EA 25 mg/kg | 2.45 ± 0.23 **aaa&& | 17.71 ± 7.50 aa | 10.45 ± 5.16 a | 0.48 ± 0.26 aaa | 17.51 ± 6.94 aaa |
| EA 50 mg/kg | 2.35 ± 0.12 *aaa | 11.97 ± 4.04 aaa | 2.66 ± 1.96 aa | 0.59 ± 0.43 aaa | 17.69 ± 15.89 aaa |
| EA 75 mg/kg | 2.32 ± 0.08 aaa | 6.42 ± 1.34 aaa& | 2.43 ± 0.15 aa | 0.48 ±0.21 aaa | 20.62 ± 26.93 aaa |
| BU 25 mg/kg | 2.51 ± 0.23 ***aaa&&&# | 20.20 ± 14.54 *a | 15.25 ± 12.79 a | 1.31 ± 0.55 ***a& | 15.91 ± 5.54 aaa&& |
| BU 50 mg/kg | 2.33 ± 0.13 aaa | 11.45 ± 3.00 aa | 2.86 ± 0.58 aaa | 1.01 ± 0.37 **aa | 12.26 ± 4.44 aaa&& |
| BU 75 mg/kg | 2.25 ± 0.14 aaa | 9.93 ± 2.40 aaa | 2.80 ± 0.42 aaa | 0.92± 0.28 *aaa | 10.67 ± 3.44 aaa&& |
| AQ 25 mg/kg | 2.35 ± 0.34 aaa | 15.12 ± 11.99 aa | 38.15 ± 4.59 * | 0.99 ±0.81 *aaa | 26.75 ± 17.00 aaa |
| AQ 50 mg/kg | 2.16 ± 0.15 aaa | 7.28 ± 6.40 aaa | 2.00 ± 0.28 aa | 0.44 ± 0.22 aaa | 25.97 ± 17.00 aaa |
| AQ 75 mg/kg | 2.16 ± 0.12 aaa | 8.96 ± 5.46 aaa | 3.70 ± 1.10 aa | 0.34 ± 0.26 aaa | 28.77 ± 24.49 aaa |


3. Experimental
3.1. Plant Material
3.2. Chemical
3.3. Extraction
3.4. Chromatographic Analysis
3.5. Total Phenolic Determination
3.6. DPPH Assay

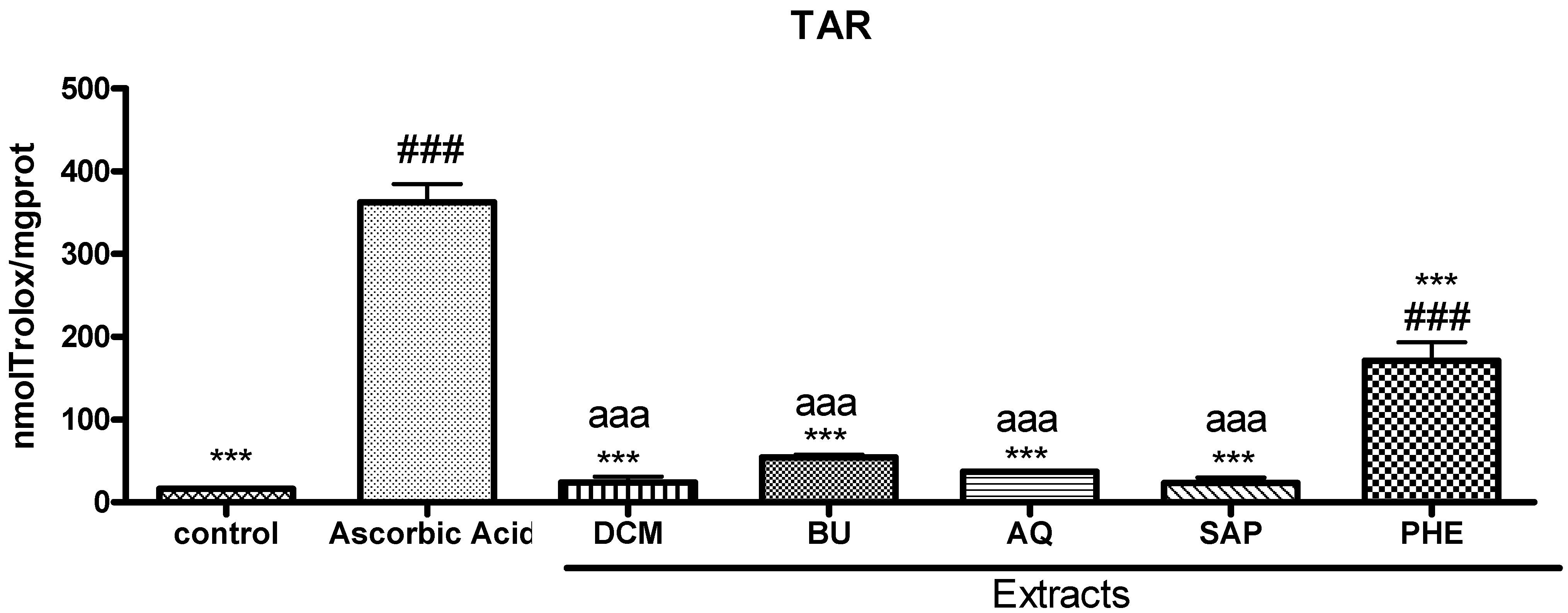
3.7. Total Antioxidant Reactivity (TAR)
3.8. Animals
3.9. Carrageenan-induced Pleurisy
3.10. Data Analysis
4. Conclusions
Acknowledgements
References and Notes
- Abad, M.J.; Bermejo, M. Baccharis (Compositae): A review update. ARKIVOC 2007, vii, 76–96. [Google Scholar]
- Farmacopéia Brasileira, 5th ed; Anvisa: Brasília, Brasil, 2010.
- Herz, W.; Pilotti, A.; Söderholm, A.C.; Shuhama, I.K.; Vichnewski, W. New ent-clerodane-type diterpenoids from Baccharis trimera. J. Org. Chem. 1977, 50, 3913–3916. [Google Scholar]
- Simões-Pires, C.A.; Debenedetti, S.; Spegazzini, E.; Mentz, L.A.; Matzenbacher, N.I.; Limberger, R.P.; Henriques, A.T. Investigation of the essential oil from eight species of Baccharis belonging to sect. Caulopterae (Asteraceae, Astereae): A taxonomic approach. Plant Syst. Evol. 2005, 253, 23–32. [Google Scholar] [CrossRef]
- de Oliveira, S.Q.; Barbon, G.; Gosmann, G. Differentiation of south Brazilian Baccharis species by TLC. J. Liq. Chromatogr. Rel. Technol. 2006, 29, 2603–2609. [Google Scholar] [CrossRef]
- Borella, J.C.; Duarte, D.P.; Novaretti, A.A.G.; Menezes, A., Jr.; França, S.C.; Rufato, C.B.; Santos, P.A.S.; Veneziani, R.C.S.; Lopes, N.P. Seasonal variability in the content of saponins from Baccharis trimera (Less.) DC (carqueja) and isolation of flavone. Braz. J. Pharmacogn. 2006, 16, 557–561. [Google Scholar]
- Gosmann, G.; Oliveira, C.B.; Comunello, L.N. Baccharis trimera (Less.) DC. Carqueja. In Recent Progress in Medicinal Plants: Drug Plants II; Awaad, A.S., Singh, V.K., Govil, J.N., Eds.; Studium Press LLC: Houston, TX, USA, 2010; Volume 28. [Google Scholar]
- Soicke, H.; Leng-Peschlow, E. Characterization of flavonoids from Baccharis trimera and their antihepatotoxic properties. Planta Med. 1987, 53, 37–39. [Google Scholar] [CrossRef]
- Gené, R.M.; Cartañá, C.; Adzet, T.; Marin, E.; Parella, T.; Cañigueral, S. Anti-inflammatory and analgesic activity of Baccharis trimera: Identification of active constituents. Planta Med. 1996, 62, 232–235. [Google Scholar] [CrossRef]
- Simões-Pires, C.A.; Queiroz, E.F.; Henriques, A.T.; Hostettmann, K. Isolation and on-line identification of antioxidant compounds from three Baccharis species by HPLC-UV-MS/MS with post-column derivatisation. Phytochem. Anal. 2005, 16, 307–314. [Google Scholar] [CrossRef]
- Nakasugi, T.; Komai, K. Antimutagens in the Brazilian folk medicinal plant Carqueja (Baccharis trimera Less.). J. Agric. Food Chem. 1998, 46, 2560–2564. [Google Scholar] [CrossRef]
- de Oliveira, S.Q.; Dal-Pizzol, F.; Moreira, J.C.F.; Schenkel, E.P.; Gosmann, G. Antioxidant activity of Baccharis spicata, Baccharis trimera and Baccharis usterii. Acta Farm. Bonaerense 2004, 23, 365–368. [Google Scholar]
- Paul, E.L.; Lunardelli, A.; Caberlon, E.; Oliveira, C.B.; Santos, R.C.V.; Biolchi, V.; Bastos, C.M.A.; Moreira, K.B.; Nunes, F.B.; Gosmann, G.; et al. Anti-inflammatory and immunomodulatory effects of Baccharis trimera aqueous extract on induced pleurisy in rats and lymphoproliferation in vitro. Inflammation 2009, 32, 419–425. [Google Scholar] [CrossRef]
- Parejo, I.; Viladomat, F.; Bastida, J.; Rosas-Romero, A.; Saavedra, G.; Murcia, M.A.; Jiménez, A.M.; Codina, C. Investigation of Bolivian plant extracts for their radical scavenging activity and antioxidant activity. Life Sci. 2003, 73, 1667–1681. [Google Scholar] [CrossRef]
- Galotta, A.L.Q.A.; Boaventura, M.A.D.; Lima, L.A.R.S. Antioxidant and cytotoxic activities of ‘‘Açai” (Euterpe precatoria Mart). Quim. Nova 2008, 31, 1427–1430. [Google Scholar] [CrossRef]
- Busnardo, T.C.P.M.; Padoani, C.; Mora, T.C.; Biavatti, M.W.; Fröde, T.S.; Bürger, C.; Claudino, V.D.; Dalmarco, E.M.; de Souza, M.M. Anti-inflammatory evaluation of Coronopus didymus in the pleurisy and paw oedema models in mice. J. Ethnopharmacol. 2010, 122, 519–525. [Google Scholar]
- Stahl, E. Thin-layer Chromatography: A Laboratory Handbook, 2nd ed; Springer: Berlin, Germany, 1969. [Google Scholar]
- Bonoli, M.; Verardo, V.; Marconi, E.; Caboni, M.F. Antioxidant phenols in barley (Hordeum vulgare L.) flour: Comparative spectrophotometric study among extraction methods of free and bound phenolic compounds. J. Agric. Food Chem. 2004, 52, 5195–5200. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Lissi, E.A.; Pascual, C.; del Castillo, M.D. Luminol luminescence induced by 2,2'-azo-bis(2-amidinopropane) therrnolysis. Free Radic. Res. Commun. 1992, 17, 299–311. [Google Scholar] [CrossRef]
- Habashy, R.R.; Abdel-Naim, A.B.; Khalifa, A.E.; Al-Azizi, M. Anti-inflammatory effects of jojoba liquid wax in experimental models. Pharmacol. Res. 2005, 51, 95–105. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the extracts are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Oliveira, C.B.; Comunello, L.N.; Lunardelli, A.; Amaral, R.H.; Pires, M.G.S.; Da Silva, G.L.; Manfredini, V.; Vargas, C.R.; Gnoatto, S.C.B.; De Oliveira, J.R.; et al. Phenolic Enriched Extract of Baccharis trimera Presents Anti-inflammatory and Antioxidant Activities. Molecules 2012, 17, 1113-1123. https://doi.org/10.3390/molecules17011113
De Oliveira CB, Comunello LN, Lunardelli A, Amaral RH, Pires MGS, Da Silva GL, Manfredini V, Vargas CR, Gnoatto SCB, De Oliveira JR, et al. Phenolic Enriched Extract of Baccharis trimera Presents Anti-inflammatory and Antioxidant Activities. Molecules. 2012; 17(1):1113-1123. https://doi.org/10.3390/molecules17011113
Chicago/Turabian StyleDe Oliveira, Cristiane B., Lucimara N. Comunello, Adroaldo Lunardelli, Robson H. Amaral, Melissa G. S. Pires, Gabriela Lucas Da Silva, Vanusa Manfredini, Carmen Regla Vargas, Simone C. B. Gnoatto, Jarbas R. De Oliveira, and et al. 2012. "Phenolic Enriched Extract of Baccharis trimera Presents Anti-inflammatory and Antioxidant Activities" Molecules 17, no. 1: 1113-1123. https://doi.org/10.3390/molecules17011113