Thermal and Rheological Properties of a Family of Botryosphaerans Produced by Botryosphaeria rhodina MAMB-05
Abstract
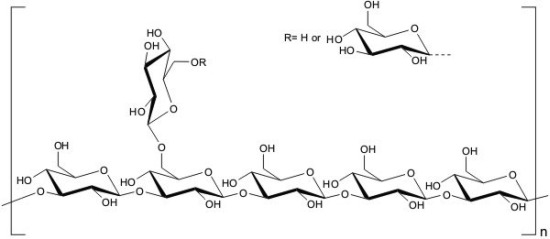
:1. Introduction
2. Results and Discussion
2.1. DSC Studies

2.2. Thermogravimetric Studies

2.3. FT-IR Spectroscopy Studies

2.4. Rheological Analysis

| Concentration (g/L) | EPSGLC | EPSSUC | EPSFRU | |||
|---|---|---|---|---|---|---|
| k * | a | k * | a | k * | a | |
| 1 | 0.005 | 0.863 | 0.002 | 0.891 | 0.002 | 0.966 |
| 2 | 0.006 | 0.860 | 0.003 | 0.900 | 0.002 | 0.960 |
| 3 | 0.008 | 0.833 | 0.003 | 0.921 | 0.003 | 0.891 |
| 4 | 0.180 | 0.560 | 0.015 | 0.736 | 0.006 | 0.834 |
| 5 | 0.265 | 0.533 | 0.084 | 0.621 | 0.018 | 0.730 |
| Samples | Extent of branching (%) * | Proportion of branches (%) * | Elution volume (mL) ** | η0 of EPS solution (mPa.s) at substrate concentration of (g/L) | ||
|---|---|---|---|---|---|---|
| Glucose | Gentiobiose | 1 | 5 | |||
| EPSGLC | 22 | 6 | 16 | 20.8 | 3.27 | 120.52 |
| EPSSUC | 21 | 12 | 9 | 21.3 | 2.08 | 33.24 |
| EPSFRU | 31 | 16 | 15 | 22.4 | 1.34 | 6.96 |
2.5. Capillary Viscometry
3. Experimental
3.1. Production of Botryosphaeran
3.2. Differential Scanning Calorimetry (DSC)
3.3. Thermogravimetric (TG) Analysis
3.4. Fourier-Transform Infra-red Spectroscopy (FT-IR)
3.5. Gel permeation Chromatography
3.6. Rheology
4. Conclusions
Acknowledgements
Conflict of Interest
- Sample Availability: Samples of the different botryosphaerans are available from the authors (Contact: Dr. Aneli M. Barbosa, E-Mail: [email protected]).
References
- Villain-Simonnet, A.; Milas, M.; Rinaudo, M. A new polysaccharide (YAS34). I. Characterization of the conformations and conformational transition. Int. J. Biol. Macromol. 2000, 27, 65–75. [Google Scholar] [CrossRef]
- Zohuriaan, M.J.; Shokrolahi, F. Thermal studies on natural and modified gums. Polym, Test. 2004, 23, 575–579. [Google Scholar] [CrossRef]
- Selbmann, L.; Stingele, F.; Petruccioli, M. Exopolysaccharide production by filamentous fungi: The example of Botryosphaeria rhodina. Antonie Leeuw. 2003, 84, 135–145. [Google Scholar] [CrossRef]
- Ikeda, S.; Nitta, Y.; Kim, S.B.; Temsiripong, T.; Pongsawatmanit, R.; Nishinari, K. Single-phase mixed gels of xyloglucan and gellan. Food Hydrocoll. 2004, 18, 669–675. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Oliver, A.E.; Tablin, F.; Crowe, J.H. A Fourier-transform infrared spectroscopy study of sugar glasses. Carbohydr. Res. 2004, 339, 1077–1085. [Google Scholar] [CrossRef]
- Ding, Q.; Labelle, M.; Yang, B.Y.; Montgomery, R. Physicochemical studies of extracellular polysaccharides of Erwinia chrysanthemi spp. Carbohydr. Polym. 2003, 51, 333–346. [Google Scholar] [CrossRef]
- Gorret, N.; Renard, C.M.G.C.; Famelart, M.H.; Maubois, J.L.; Doublier, J.L. Rheological characterization of the EPS produced by P. acidi-propionici on milk microfiltrate. Carbohydr. Polym. 2003, 51, 149–158. [Google Scholar] [CrossRef]
- Fengel, D.A.; Wegener, G. Wood Chemistry, Ultrastructure and Reactions, 1st ed; Walter de Gruyter: Berlin, Germany, 1984. [Google Scholar]
- Gray, J.S.S.; Yang, B.Y.; Montgomery, R. Montgomery, R. Extracellular polysaccharide of Erwinia chrysanthemi A350 and ribotyping of Erwinia chrysanthemi spp. Carbohydr. Res. 2000, 324, 255–267. [Google Scholar] [CrossRef]
- Zhbankov, R.G.; Korolik, E.V.; Petrov, P.T.; Lapkovski, M.P.; Tsarenkov, V.M.; Marchewka, M.K.; Ratajczak, H. Vibrational spectra and the structure of medical biopolymers. J. Mol. Struct. 2000, 555, 85–96. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G.; Izydorczyk, M.S. Molecular size effects on rheological properties of oat β-glucans in solution and gels. Food Hydrocoll. 2003, 17, 693–712. [Google Scholar] [CrossRef]
- Rodd, A.B.; Dunstan, D.E.; Boger, D.V. Characterisation of xanthan gum solutions using dynamic light scattering and rheology. Carbohydr. Polym. 2000, 42, 159–174. [Google Scholar] [CrossRef]
- Renaud, M.; Belgacem, M.N.; Rinaudo, M. Rheological behaviour of polysaccharide aqueous solutions. Polymers 2005, 46, 12348–12358. [Google Scholar] [CrossRef]
- Bueno, S.M.; Garcia-Cruz, C.H. The influence of fermentation time and the presence of salts on the rheology of the fermentation broth of polysaccharide-producing bacteria free of soil. J. Food Eng. 2001, 50, 41–42. [Google Scholar] [CrossRef]
- Dekker, R.F.H.; Barbosa, A.M. The effects of aeration and veratryl alcohol on the production of two laccases by the ascomycete Botryosphaeria sp. Enzyme Microb. Technol. 2001, 28, 81–88. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Steluti, R.M.; Dekker, R.F.H.; Cardoso, M.S.; Corradi da Silva, M.L. Structural characterization of Botryosphaeran: A (1→3,1→6)-β-D-glucan produced by the ascomyceteous fungus, Botryosphaeria sp. Carbohydr. Res. 2003, 338, 1691–1698. [Google Scholar] [CrossRef]
- Corradi da Silva, M.L.; Izeli, N.L.; Martinez, P.F.; Silva, I.R.; Constantino, C.J.L.; Cardoso, M.S.; Barbosa, A.M.; Dekker, R.F.H.; Silva, G.V.J. Purification and structural characterization of (1→3;1→6)-β-glucan (botryosphaerans) from Botryosphaeria rhodina grown on sucrose and fructose as carbon sources: a comparative study. Carbohydr. Polym. 2005, 61, 10–17. [Google Scholar] [CrossRef]
- Gomaa, K.; Kraus, J.; Rokopf, F.; Roper, M.; Franz, G. Antitumor and immunological activity of a β-1→3; 1→6 glucan from Glomerella cingulata. J. Cancer Res. Clin. Oncol. 1992, 118, 136–140. [Google Scholar] [CrossRef]
- Seviour, R.J.; Stasinopoulos, S.J.; Auer, D.P.F.; Gibbs, P.A. Production of pullulan and other exopolysaccharides by filamentous fungi. Crit. Ver. Biotechnol. 1992, 12, 279–298. [Google Scholar] [CrossRef]
- Morris, G.A.; Li, P.; Puaud, M.; Liu, Z.; Mitchell, J.R.; Harding, S.E. Hydrodynamic characterisation of the exopolysaccharide from the halophilic cyanobacterium Aphanothece halophytica GR02: A comparison with xanthan. Carbohydr. Polym. 2001, 44, 261–268. [Google Scholar] [CrossRef]
- Esgalhado, M.E.; Roseiro, J.C.; Collaço, M.T.A. Interactive effects of pH and temperature on cell growth and polymer production by Xanthomonas campestris. Process Biochem. 1995, 30, 667–671. [Google Scholar]
- Ding, Q.; Yang, B.Y.; Montgomery, R. Structure and hydrodynamic properties of the extracellular polysaccharide from a mutant strain (RA3W) of Erwinia chrysanthemi RA3. Carbohydr. Res. 2004, 339, 2049. [Google Scholar] [CrossRef]
- Yang, B.Y.; Ding, Q.; Montgomery, R. Extracellular polysaccharides of a bacterium associated with a fungal canker disease of Eucalyptus sp. Carbohydr. Res. 2002, 337, 731–742. [Google Scholar] [CrossRef]
- Lucas, E.F.; Soares, B.G.; Monteiro, E. Caracterização de Polímeros: Determinação de Peso Molecular e Análise Térmica (in Portuguese); E-papers: Rio de Janeiro, Brazil, 2001, 1st ed. pp. 125–149. Available online: http://www.e-papers.com.br/sumario.asp?codigo_produto=1339.
- Freifelder, D.M. Physical Biochemistry: Applications to Biochemistry and Molecular Biology, 2nd ed; W.H. Freeman: New York, NY, USA, 1982; pp. 470–491. [Google Scholar]
- Navarini, L.; Abatangelo, A.; Bertocchi, C.; Conti, E.; Bosco, M.; Picotti, F. Isolation and characterization of the exopolysaccharide produced by Streptococcus thermophilus SFi20. Int. J. Biol. Macromol. 2001, 28, 219–226. [Google Scholar] [CrossRef]
- Ren, Y.; Ellis, P.R.; Sutherland, I.W.; Ross-Murphy, S.B. Dilute and semi-dilute solution properties of an exopolysaccharide from Escherichia coli strain S61. Carbohydr. Polym. 2003, 52, 189–195. [Google Scholar] [CrossRef]
- Eteshola, E.; Gottlieb, M.; Arad, S.M. Dilute solution viscosity of red microalga exopolysaccharide. Chem. Eng. Sci. 1996, 51, 1487–1494. [Google Scholar] [CrossRef]
- Goh, K.K.T.; Hemar, Y.; Singh, H. Viscometric and static light scattering studies on an exopolysaccharide produced by Lactobacillus delbrueckii subspecies bulgaricus NCFB 2483. Biopolymers 2005, 77, 98–106. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fonseca, P.R.M.S.; Dekker, R.F.H.; Barbosa, A.M.; Silveira, J.L.M.; Vasconcelos, A.F.D.; Monteiro, N.K.; Aranda-Selverio, G.; Da Silva, M.d.L.C. Thermal and Rheological Properties of a Family of Botryosphaerans Produced by Botryosphaeria rhodina MAMB-05. Molecules 2011, 16, 7488-7501. https://doi.org/10.3390/molecules16097488
Fonseca PRMS, Dekker RFH, Barbosa AM, Silveira JLM, Vasconcelos AFD, Monteiro NK, Aranda-Selverio G, Da Silva MdLC. Thermal and Rheological Properties of a Family of Botryosphaerans Produced by Botryosphaeria rhodina MAMB-05. Molecules. 2011; 16(9):7488-7501. https://doi.org/10.3390/molecules16097488
Chicago/Turabian StyleFonseca, Paulo R.M.S., Robert F.H. Dekker, Aneli M. Barbosa, Joana L.M. Silveira, Ana F.D. Vasconcelos, Nilson K. Monteiro, Gabriel Aranda-Selverio, and Maria de Lourdes Corradi Da Silva. 2011. "Thermal and Rheological Properties of a Family of Botryosphaerans Produced by Botryosphaeria rhodina MAMB-05" Molecules 16, no. 9: 7488-7501. https://doi.org/10.3390/molecules16097488