Use of Novel Cardanol-Porphyrin Hybrids and Their TiO2-Based Composites for the Photodegradation of 4-Nitrophenol in Water
Abstract
:1. Introduction

2. Results and Discussion
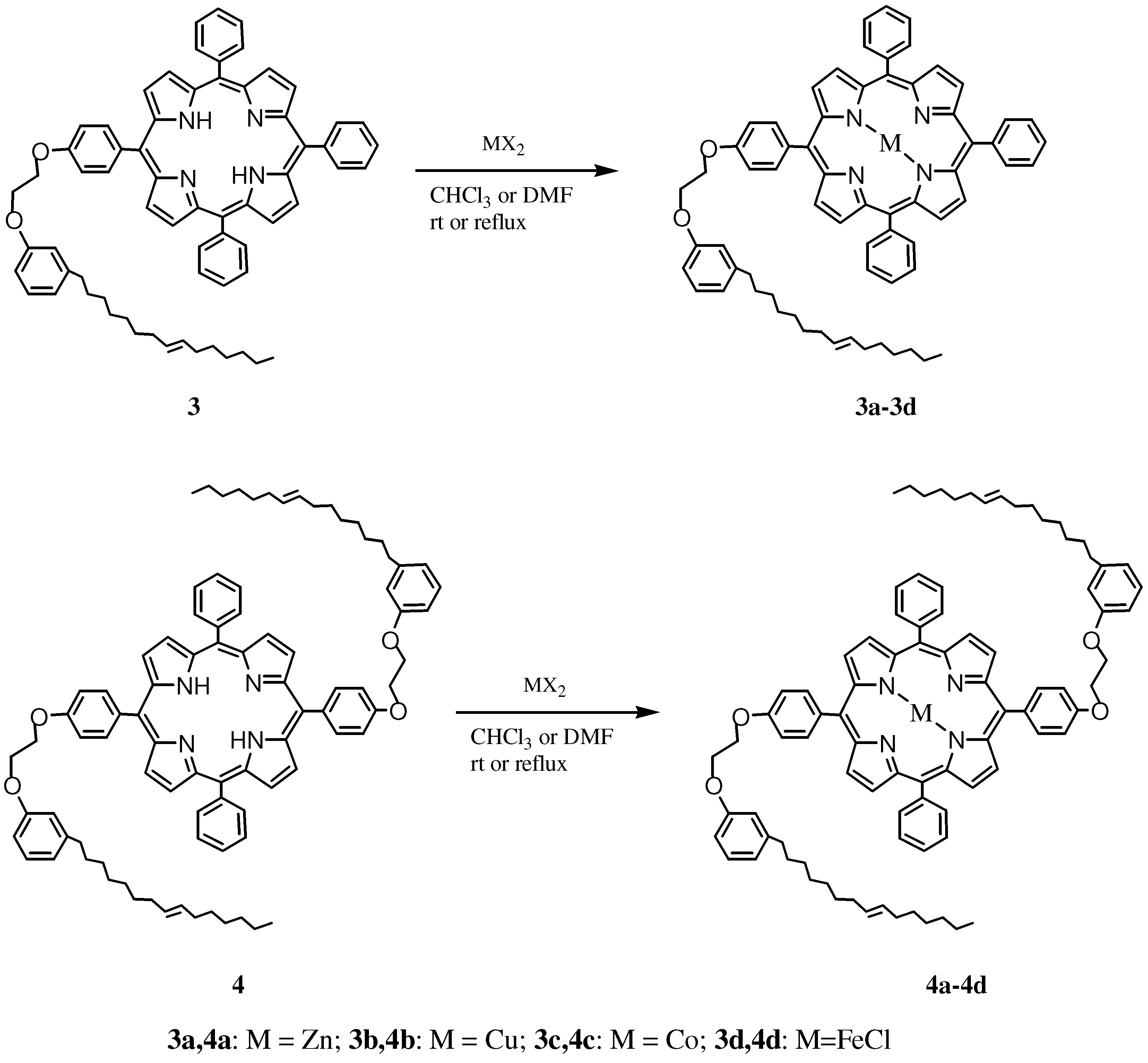
2.1. Synthesis and Characterization of Cardanol Based Porphyrins


| Compounds | M | Yields % | λmax, nm (CHCl3) | ||||
|---|---|---|---|---|---|---|---|
| Soret band | Q bands | ||||||
| 3 | 2H | 10 | 419 | 516 | 552 | 590 | 646 |
| 3a | Zn | 90 | 424 | 554 | 594 | ||
| 3b | Cu | 90 | 416 | 539 | |||
| 3c | Co | 90 | 411 | 530 | |||
| 3d | Fe | 80 | 417 | ||||
| 4 | 2H | 15 | 420 | 517 | 553 | 591 | 647 |
| 4a | Zn | 90 | 425 | 554 | 596 | ||
| 4b | Cu | 90 | 417 | 540 | |||
| 4c | Co | 90 | 412 | 530 | |||
| 4d | Fe | 80 | 419 | ||||
| cardanol-based A4-porphyrin | 2H | 14 [16] | 420 | 518 | 556 | 593 | 649 |
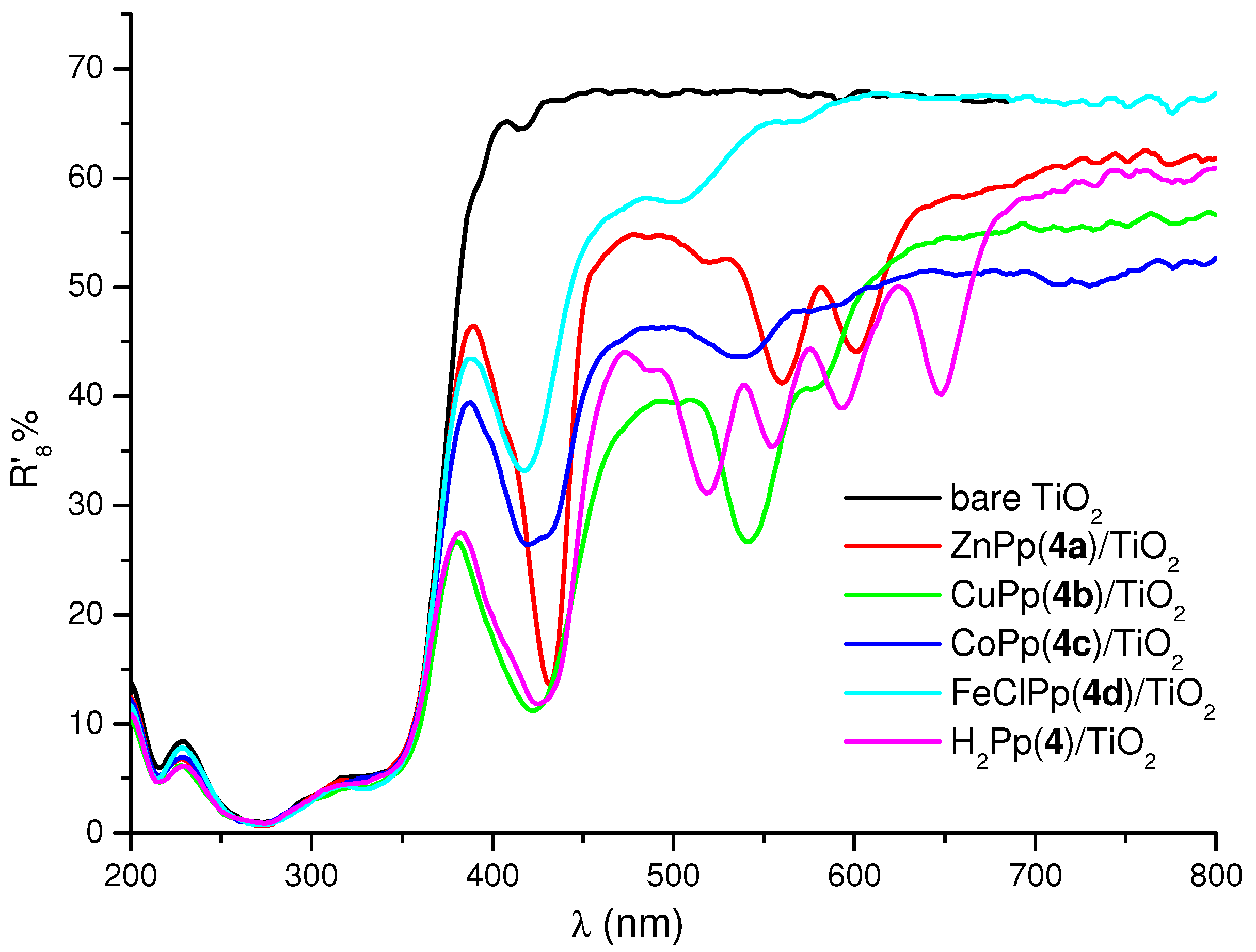
2.2. Preparation of the Cardanol Based Porphyrin/TiO2 Composites and Diffuse Reflectance (DR) Spectroscopy Characterization
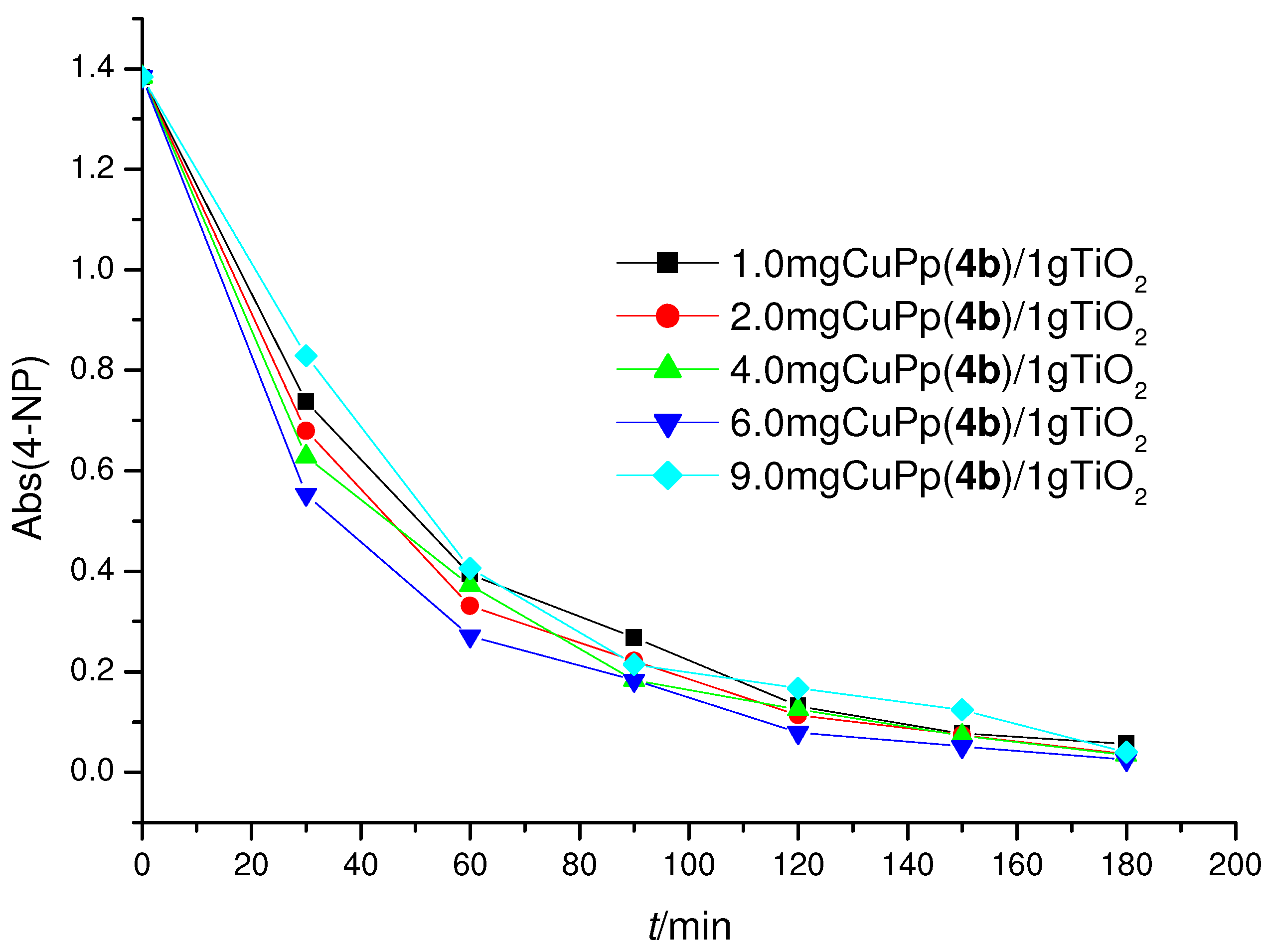
2.3. Photoreactivity Experiments
| Samples a | r0 × 109 | r0′ × 109 | 4-NP |
|---|---|---|---|
| (mol.L−1.s−1) | (mol.L−1.s−1.m−2) | (%) b converted at 180 min | |
| TiO2 | 26.59 | 33.24 | 93.5 |
| 1.0 µmol CuPp(4b)/TiO2 | 36.36 | 45.45 | 95.9 |
| 2.0 µmol CuPp(4b)/TiO2 | 39.62 | 49.52 | 97.5 |
| 4.0 µmol CuPp(4b)/TiO2 | 42.48 | 53.10 | 97.4 |
| 6.0 µmol CuPp(4b)/TiO2 | 46.70 | 58.38 | 98.2 |
| 9.0 µmol CuPp(4b)/TiO2 | 31.21 | 39.01 | 97.1 |
| 6.0 µmol ZnPp(4a)/TiO2 | 33.94 | 42.42 | 95.1 |
| 6.0 µmol CoPp(4c)/TiO2 | 34.28 | 42.85 | 95.4 |
| 6.0 µmol FeClPp(4d)/TiO2 | 18.77 | 23.46 | 92.8 |
| 6.0 µmol H2Pp(4)/TiO2 | 22.20 | 27.75 | 86.0 |
| 4.0 µmol CuPp(3b)/TiO2 | 34.34 | 42.92 | 95.8 |
| 6.0 µmol CuPp(3b)/TiO2 | 42.03 | 52.54 | 97.9 |
| 6.6 µmol CuPp(3b)/TiO2 | 41.52 | 51.90 | 96.7 |
| 6.0 µmol ZnPp(3a)/TiO2 | 33.55 | 41.94 | 94.7 |
| 6.0 µmol CoPp(3c)/TiO2 | 33.72 | 42.15 | 93.9 |
| 6.0 µmol FeClPp(3d)/TiO2 | 18.66 | 23.32 | 93.3 |
| 6.0 µmol H2Pp(3)/TiO2 | 21.36 | 26.70 | 85.5 |
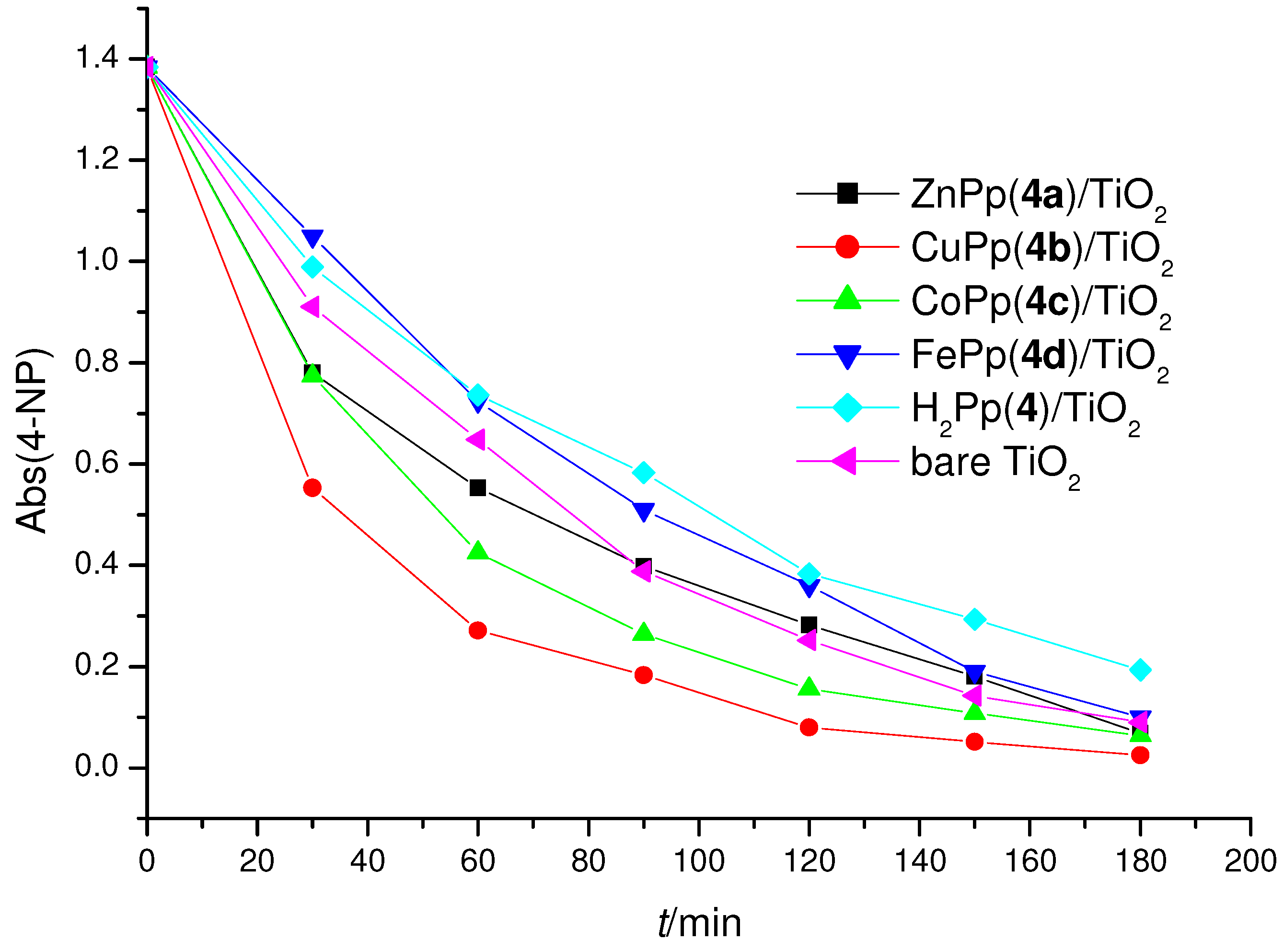
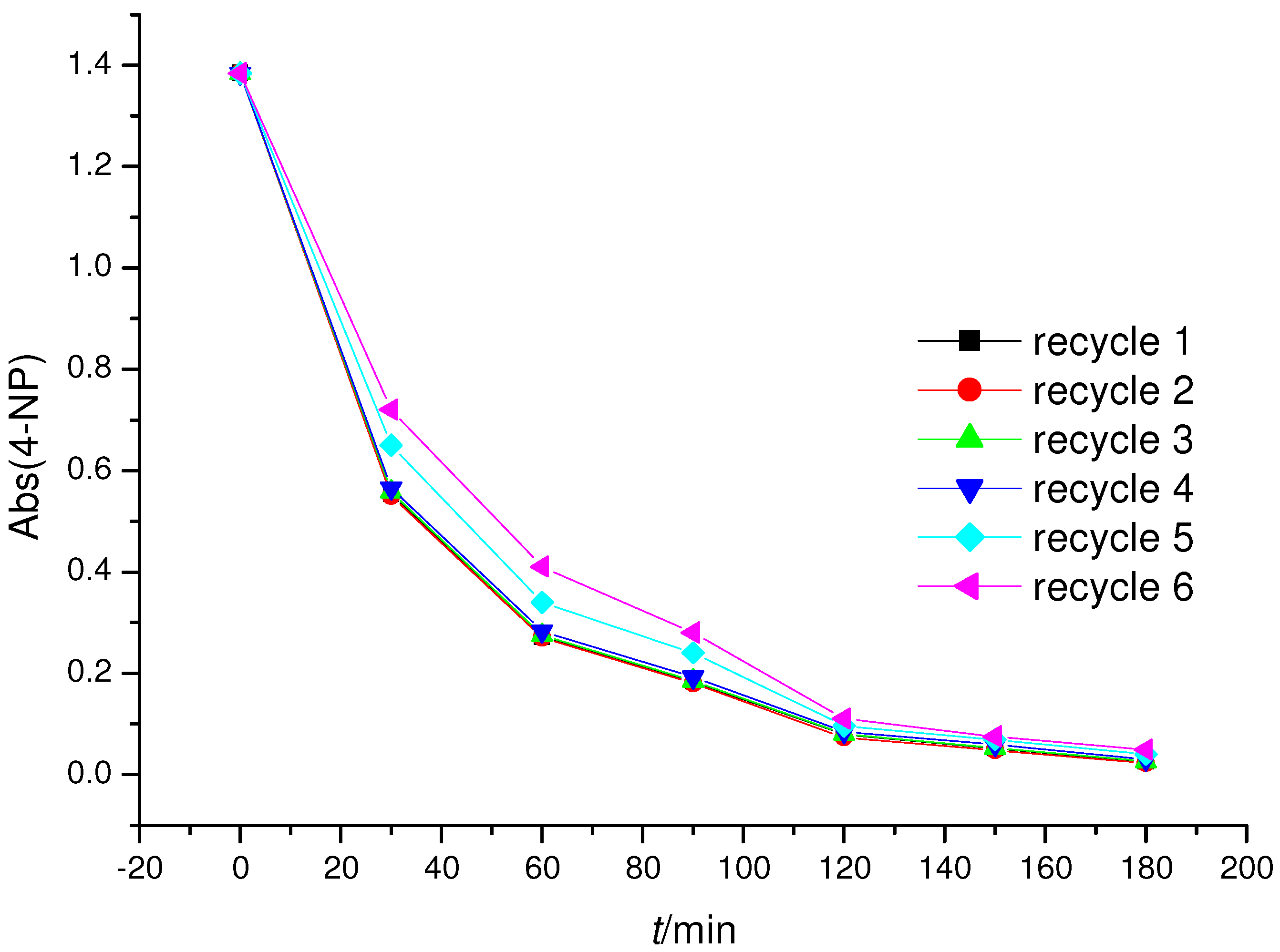

3. Experimental
3.1. Reagents
3.2. Analyses
3.3. Synthesis
3.3.1. Synthesis of the cardanol based precursors of the porphyrins
3.3.2. Synthesis and characterization of 5,10,15-triphenyl-20-mono-[4-(2-(3-pentadec-8-enyl) phenoxy)ethoxy] phenylporphyrin (3)
3.3.3. Synthesis and characterization of 5,15-diphenyl-10, 20-di-4-(2-(3-pentadec-8-enyl)phenoxy)ethoxy]phenyl porphyrin (4)
3.3.4. General procedure for the synthesis of 3a–3c, 4a–4c
3.3.5. General procedure for synthesis of 3d and 4d
Representative data for compounds 3a–3d, 4a–4d
3.4. Preparation of the Cardanol-Based Porphyrin/TiO2 Composites
3.5. Photo-Reactivity Experiments
4. Conclusions
Acknowledgments
References and Notes
- Andrighetti, L.; Bassi, G.F.; Capella, P.; De Logu, A.M.; Deolalikar, A.B.; Haeusler, G.; Malorgio, G.A.; Mavignier Cavalcante, F.; Rivoria, G.; Vannini, L.; Deserti, R. The World Cashew Economy; Nomisma: Bologna, Italy, 1994. [Google Scholar]
- Blazdell, P. The mighty cashew. Int. Sci. Rev. 2000, 28, 220–226. [Google Scholar]
- Attanasi, O.A.; Filippone, P. Cardanolo: Una preziosa materia prima rinnovabile. Chim. Ind. 2003, 85, 11–12. [Google Scholar]
- Tyman, J.H.P. Synthetic and Natural Phenols; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Mazzetto, S.E.; Lomonaco, D.; Mele, G. Cashew nut oil: Opportunities and challenges in the context of sustainable industrial development. Quím. Nova. 2009, 32, 732–741. [Google Scholar] [CrossRef]
- Mele, G.; Vasapollo, G. Fine chemicals and new hybrid materials from cardanol. Mini Rev. Org. Chem. 2008, 5, 243–253. [Google Scholar] [CrossRef]
- Mele, G.; Li, J.; Margapoti, E.; Martina, F.; Vasapollo, G. Synthesis of novel porphyrins cardanol based via cross methathesis. Catal. Today 2009, 140, 37–43. [Google Scholar]
- Photocatalysis and Environment: Trends and Applications; Schiavello, M. (Ed.) Kluwer: Dordrecht, The Netherlands, 1988.
- Ollis, D.; Pelizzetti, E.; Serpone, N. Photocatalyzed destruction of water contaminants. Environ. Sci. Technol. 1991, 25, 1522–1525. [Google Scholar] [CrossRef]
- Marcì, G.; Mele, G.; Palmisano, L.; Pulito, P.; Sannino, A. Environmentally sustainable production of cellulose-based superabsorbent hydrogels. Green Chem. 2006, 8, 439–444. [Google Scholar] [CrossRef]
- Takahashi, N.; Nakai, T.; Satoh, Y.; Katoh, Y. Variation of biodegradability of nitrogenous organic compounds by ozonation. Water Res. 1994, 28, 1563–1570. [Google Scholar] [CrossRef]
- Daneshvar, N.; Behnajady, M.A.; Asghar, Y.Z. Photooxidative degradation of 4-nitrophenol (4-NP) in UV/H2O2 process: Influence of operational parameters and reaction mechanism. J. Hazard. Mater. 2007, B139, 275–279. [Google Scholar]
- Wei, L; Zhu, H.; Mao, X.H.; Gan, F.X. Electrochemical oxidation process combined with UV photolysis for the mineralization of nitrophenol in saline wastewater. Sep. Purif. Technol. 2011, 77, 18–25. [Google Scholar] [CrossRef]
- Lai, T.-L.; Yong, K.-F.; Yu, J.-W.; Chena, J.-H.; Shu, Y.-Y.; Wang, C.-B. High efficiency degradation of 4-nitrophenol by microwave-enhanced catalytic method. J. Hazard. Mater. 2011, 185, 366–372. [Google Scholar] [CrossRef]
- Attanasi, O.A.; Del Sole, R.; Filippone, P.; Mazzetto, S.E.; Mele, G.; Vasapollo, G. Synthesis of novel cardanol based porphyrins. J. Porphyr. Phthalocya. 2004, 8, 1276–1284. [Google Scholar] [CrossRef]
- Guo, Y.C.; Xiao, W.J.; Mele, G.; Martina, F.; Margapoti, E.; Mazzetto, S.E.; Vasapollo, G. Synthesis of new meso-tetraaryl porphyrins bearing cardanol and further transformation of the unsaturated chains. J. Porphyr. Phthalocya. 2006, 10, 1071–1079. [Google Scholar] [CrossRef]
- Mele, G.; Del Sole, R.; Vasapollo, G.; Garcìa Lòpez, E.; Palmisano, L.; Mazzetto, S.E.; Attanasi, O.A.; Filippone, P. Polycrystalline TiO2 impregnated with cardanol-based porphyrins for the photocatalytic degradation of 4-nitrophenol. Green Chem. 2004, 6, 604–608. [Google Scholar] [CrossRef]
- Attanasi, O.A.; Del Sole, R.; Filippone, P.; Ianne, R.; Mazzetto, S.E.; Mele, G.; Vasapollo, G. Synthesis of fullerene-cardanol derivatives. Synlett 2004, 5, 799–802. [Google Scholar]
- Mele, G.; Del Sole, R.; Vasapollo, G.; García-López, E.; Palmisano, L.; Schiavello, M. Photocatalytic degradation of 4-nitrophenol in aqueous suspension by using polycrystalline TiO2 impregnated with functionalized Cu(II)-porphyrin or Cu(II)-phthalocyanine. J. Catal. 2003, 217, 334–342. [Google Scholar]
- Mele, G.; Del Sole, R.; Vasapollo, G.; Marcì, G.; García-López, E.; Palmisano, L.; Coronado, J.M.; Hernandez-Alonso, M.D.; Malitesta, C.; Guascito, M.R. TRMC, XPS, and EPR characterizations of polycrystalline TiO2 porphyrin impregnated powders and their catalytic activity for 4-nitrophenol photodegradation in aqueous suspension. J. Phys. Chem. B 2005, 109, 12347–12352. [Google Scholar]
- Mele, G.; Del Sole, R.; Vasapollo, G.; García-López, E.; Palmisano, L.; Li, J.; Słota, R.; Dyrda, G. TiO2-based photocatalysts impregnated with metallo-porphyrins employed for degradation of 4-nitrophenol in aqueous solutions: Role of metal and macrocycle. Res. Chem. Intermed. 2007, 33, 433–448. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vasapollo, G.; Mele, G.; Sole, R.D.; Pio, I.; Li, J.; Mazzetto, S.E. Use of Novel Cardanol-Porphyrin Hybrids and Their TiO2-Based Composites for the Photodegradation of 4-Nitrophenol in Water. Molecules 2011, 16, 5769-5784. https://doi.org/10.3390/molecules16075769
Vasapollo G, Mele G, Sole RD, Pio I, Li J, Mazzetto SE. Use of Novel Cardanol-Porphyrin Hybrids and Their TiO2-Based Composites for the Photodegradation of 4-Nitrophenol in Water. Molecules. 2011; 16(7):5769-5784. https://doi.org/10.3390/molecules16075769
Chicago/Turabian StyleVasapollo, Giuseppe, Giuseppe Mele, Roberta Del Sole, Iolanda Pio, Jun Li, and Selma Elaine Mazzetto. 2011. "Use of Novel Cardanol-Porphyrin Hybrids and Their TiO2-Based Composites for the Photodegradation of 4-Nitrophenol in Water" Molecules 16, no. 7: 5769-5784. https://doi.org/10.3390/molecules16075769





