Synthesis and Anticancer Activity of Some New S-Glycosyl and S-Alkyl 1,2,4-Triazinone Derivatives
Abstract
:1. Introduction
2. Results and Discussion
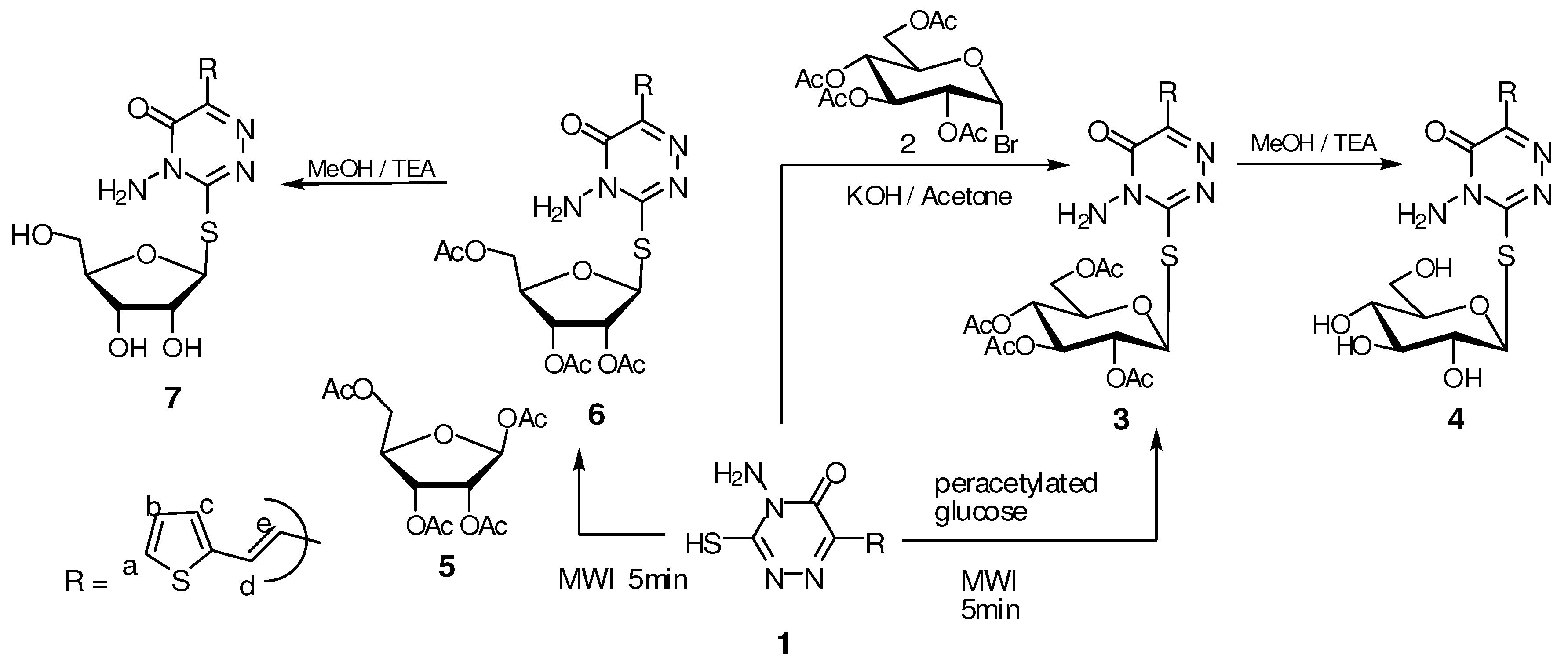

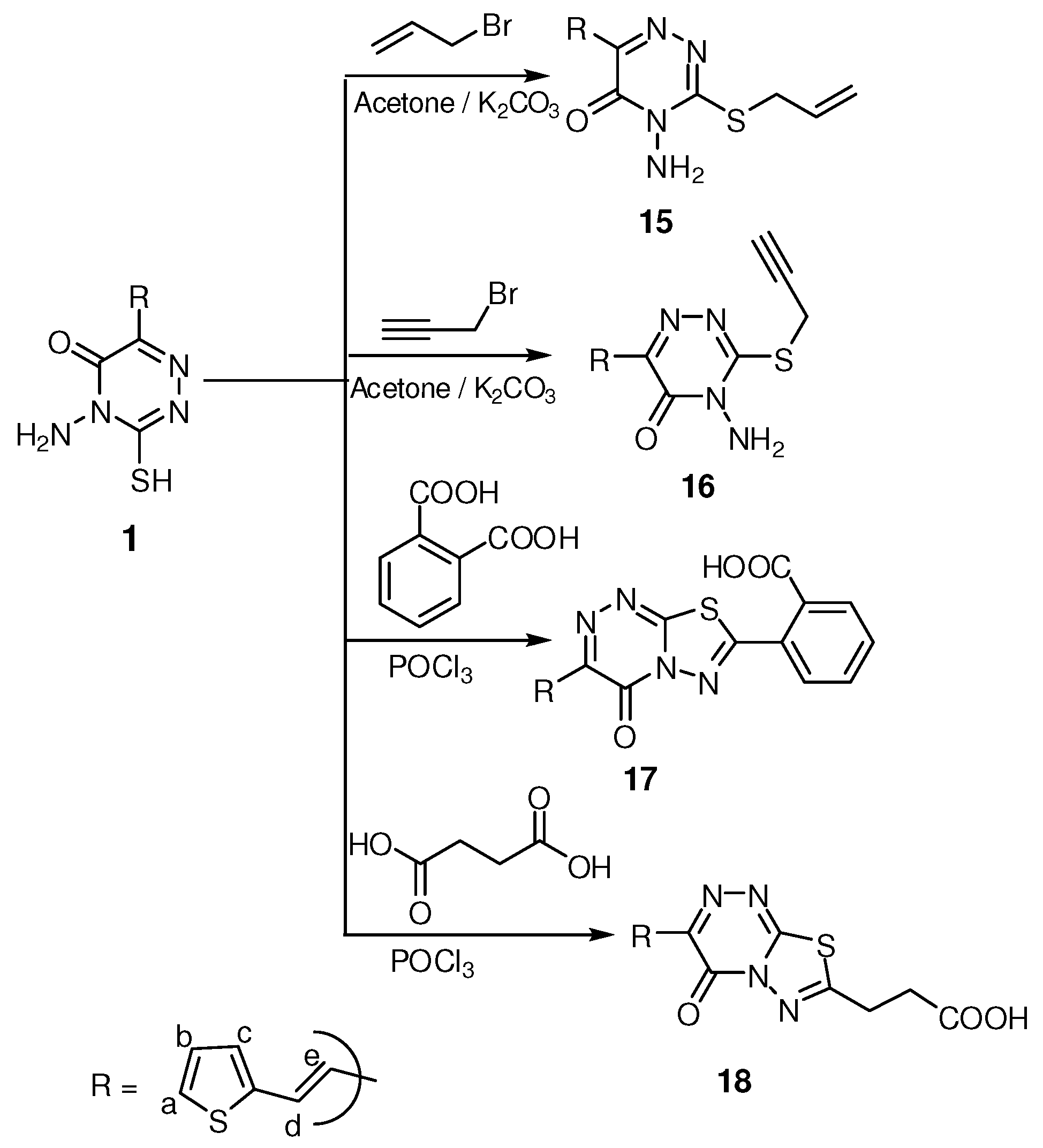
2.1. Pharmacological Studies
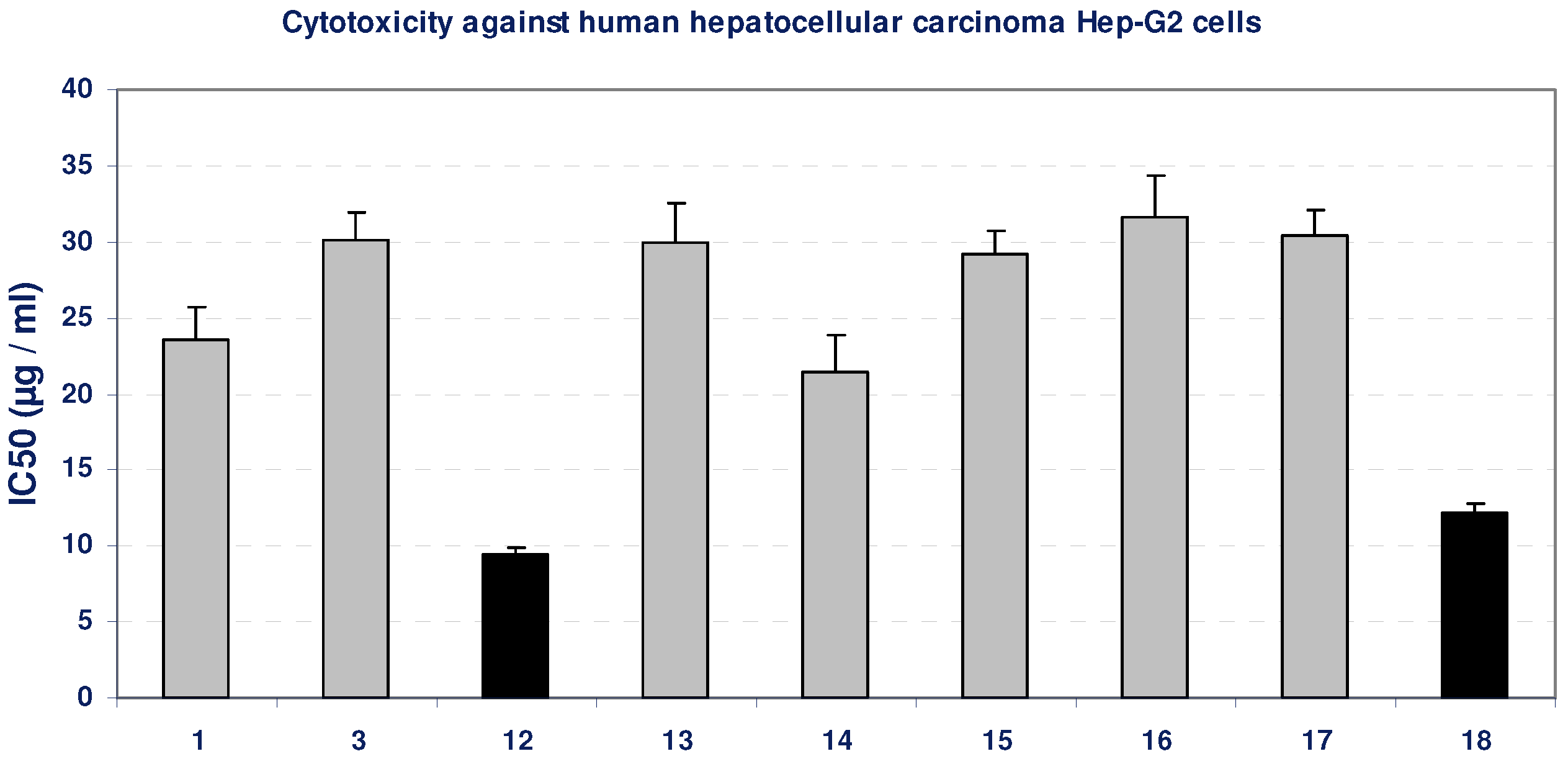
2.1.1. Cytotoxicity of the compounds against Hep-G2 Cells
| Compd. No. | Mean IC50 (µg/mL) | SE | ||||
|---|---|---|---|---|---|---|
| Hep-G2 cells | MCF-7 cells | HCT-116 cells | Hep-G2 cells | MCF-7 cells | HCT-116 cells | |
| 1 | 23.51 | 21.04 | 32.6 | 2.25 | 1.62 | 1.45 |
| 3 | 30.12 | 39.2 | 27.23 | 1.88 | 2.08 | 2.70 |
| 12 | 9.44 | 10.06 | 6.51 | 0.45 | 0.65 | 0.69 |
| 13 | 30.02 | 26.78 | 36.12 | 2.49 | 2.07 | 1.85 |
| 14 | 21.51 | 25.69 | 33.67 | 2.32 | 1.48 | 1.77 |
| 15 | 29.14 | 21.67 | 18.38 | 1.54 | 2.01 | 1.50 |
| 16 | 31.64 | 28.88 | 40.08 | 2.77 | 2.18 | 1.99 |
| 17 | 30.47 | 21.09 | 23.86 | 1.65 | 2.10 | 1.46 |
| 18 | 12.13 | 11.65 | 8.34 | 0.58 | 0.84 | 0.80 |
2.1.2. Cytotoxicity of the compounds against MCF-7 Cells

2.1.3. Cytotoxicity of the compounds against HCT-116 Cells
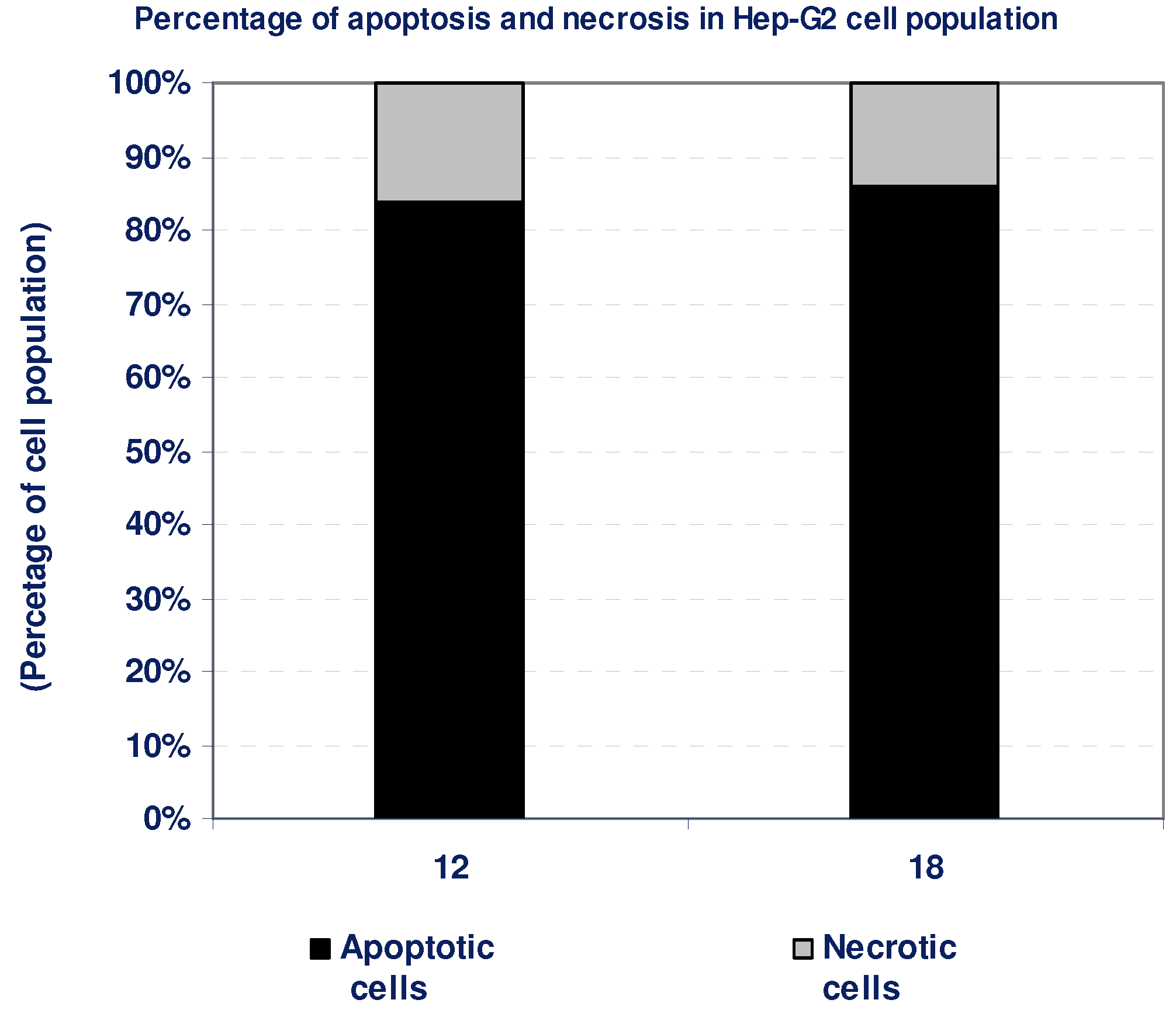
2.1.4. Percentage of induced apoptotic and necrotic cells in Hep-G2 Cells

| Compd. No. | Hep-G2 | |
|---|---|---|
| Apoptotic cells | Necrotic cells | |
| 12 | 84 | 16 |
| 18 | 86 | 14 |
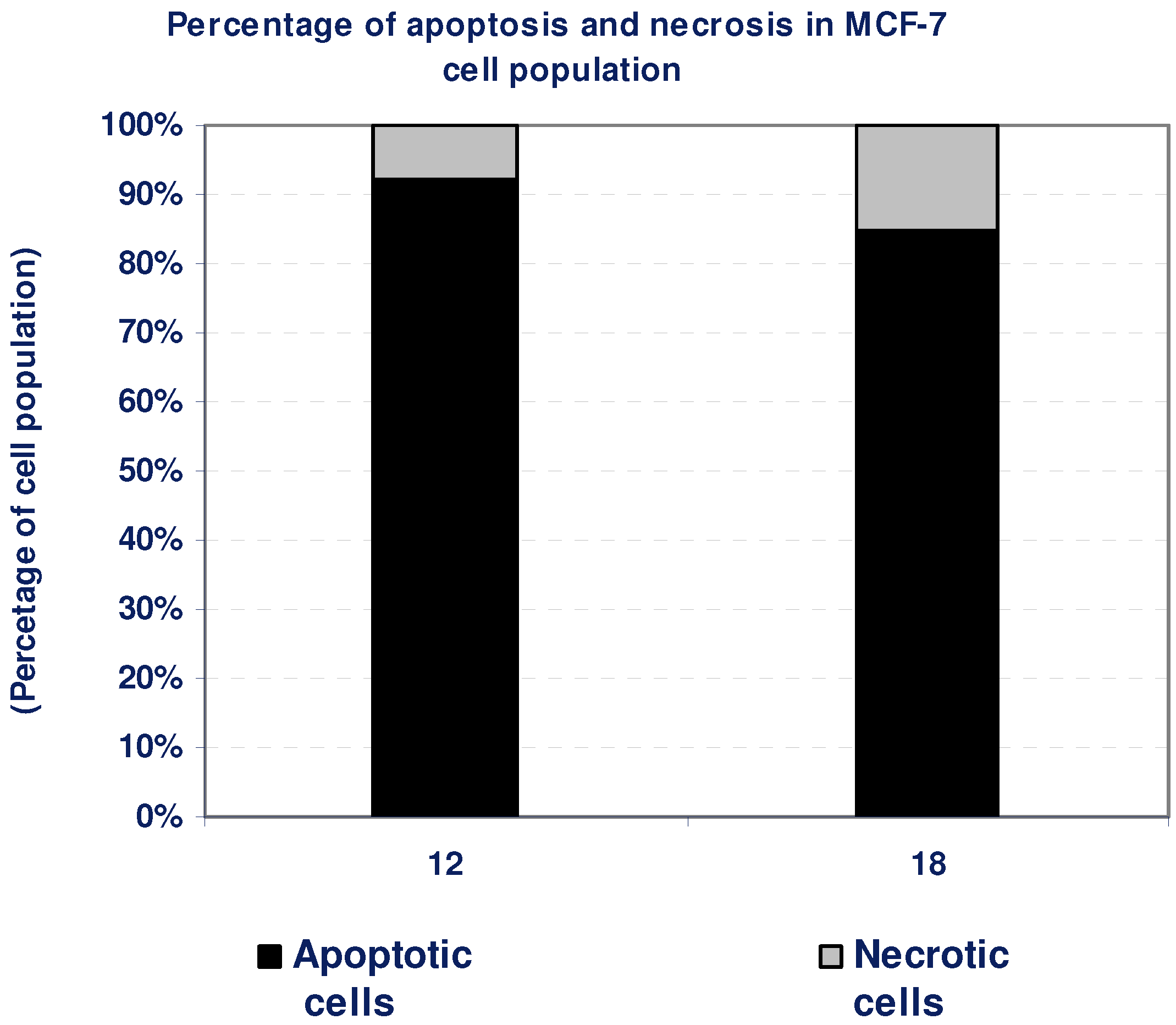
2.1.5. Percentage of induced apoptotic and necrotic cells in MCF-7 Cells
| Compd. No. | MCF-7 | |
|---|---|---|
| Apoptotic cells | Necrotic cells | |
| 12 | 92 | 8 |
| 18 | 85 | 15 |
2.1.6. Percentage of induced apoptotic and necrotic cells in HCT-116 Cells
| Compd. No. | HCT-116 | |
|---|---|---|
| Apoptotic cells | Necrotic cells | |
| 12 | 94 | 6 |
| 15 | 61 | 39 |
| 18 | 82 | 18 |

3. Experimental
3.1. General
3.2. Material and Methods for Pharmacological Activity
3.2.1. Cell culture
3.2.2. Cytotoxicity assay
3.2.2.1. Reagents preparation
3.2.2.2. Procedure
3.2.2.3. Calculations
3.2.3. Apoptosis and necrosis staining
3.2.4. General method for preparation of compounds 3 and 6
3.2.5. General procedure for preparation of compounds 4,7,9 and 10
3.2.6. General procedure for synthesis of S-alkyl compounds 8, 10, 12, 13 and 14
3.2.7. General procedure for synthesis of compounds 15 and 16
3.2.8. General procedure for compounds 17 and 18
4. Conclusions
References
- Robins, M.J.; Walker, R.T.; De Clercq, E.; Eckstein, F. Nucleoside Analogues. In Chemistry, Biology, and Medical Applications; Plenum Press: New York, NY, USA, 1979; pp. 165–174. [Google Scholar]
- Fallon, H.J.; Frei, E.; Freireich, E.J. Correlations of the biochemical and clinical effects of 6-azauridine in patients with leukemia. Am. J. Med. 1962, 33, 526–537. [Google Scholar] [CrossRef]
- Ladislav, N.; Anna, V.; Alois, P. Polarographic properties and potential carcinogenicity of some natural nucleosides and their synthetic analogues. Bioelectrochem. Bioenerg. 1999, 48, 129–134. [Google Scholar] [CrossRef]
- Ibrahim, Y.A. Facile approach for the selective glycosidation of cyclic asymmetric amides and thioamides. Carbohyd. Lett. 1996, 1, 425–432. [Google Scholar]
- Ibrahim, Y.A. Synthesis and structure of 2-deoxyribosyl-6-azauracil derivatives. Carbohyd. Lett. 1996, 2, 189–195. [Google Scholar] [CrossRef]
- Mansour, A.K.; Ibrahim, Y.A.; Khalil, N.A.S.M. Selective Synthesis and Structure of 6-Arylvinyl-2- and 4-Glucosyl-1,2,4-triazines of Expected Interesting Biological Activity. Nucleos. Nucleot. 1999, 18, 2265–2283. [Google Scholar] [CrossRef]
- Roy, D.P. Analogues of Nucleic Acid Compounds; Springer Verlag: Berlin, Germany, 1970; pp. 42–45. [Google Scholar]
- Robins, R.K. Synthetic Antiviral Agents. Chem. Eng. News 1986, 64, 28–40. [Google Scholar] [CrossRef]
- Bonini, C.; Chiummiento, L.; Bonis, M.D.; Funicello, M.; Lupattelli, P.; Suanno, G.; Berti, F.; Campaner, P. Synthesis, biological activity and modelling studies of two novel anti HIV PR inhibitors with a thiophene containing hydroxyethylamino core. Tetrahedron 2005, 61, 6580–6589. [Google Scholar] [CrossRef]
- Brault, L.; Migianu, E.; Néguesque, A.; Battaglia, E.; Bagrel, D.; Kirsch, G. New thiophene analogues of kenpaullone: synthesis and biological evaluation in breast cancer cells. Eur. J. Med. Chem. 2005, 40, 757–763. [Google Scholar] [CrossRef]
- Kumar, P.R.; Raju, S.; Goud, P.S.; Sailaja, M.; Sarma, M.R.; Reddy, G.O.; Kumar, M.P.; Reddy, V.V.R.M.K.; Suresha, T.; Hegdeb, P. Synthesis and biological evaluation of thiophene [3,2-b] pyrrole derivatives as potential anti-inflammatory agents. Bioorg. Med. Chem. 2004, 12, 1221–1230. [Google Scholar] [CrossRef]
- Graff, J.; Harder, S.; Wahl, O.; Scheuermann, E.H.; Gossmann, J. Anti-inflammatory effects of clopidogrel intake in renal transplant patients: Effects on platelet-leukocyte interactions, platelet CD40 ligand expression, and proinflammatory biomarkers Anti-inflammatory effects of clopidogrel intake in renal transplant patients: Effects on platelet-leukocyte interactions, platelet CD40 ligand expression, and proinflammatory biomarkers. Clin. Pharmacol. Ther. 2005, 78, 468–476. [Google Scholar] [CrossRef]
- Hymete, A.; Rohloff, J.; Kjosen, H.; Iversen, T.H. Acetylenic thiophenes from the roots of Echinops ellenbeckii from Ethiopia. Nat. Prod. Res. 2005, 19, 755–761. [Google Scholar] [CrossRef]
- Tapia, R.A.; Alegria, L.; Pessoa, C.D.; Salas, C.; Cortes, M.J.; Valderrama, J.A.; Sarciron, M.E.; Pautet, F.; Walchshofer, N.; Fillion, H. Synthesis and antiprotozoal activity of naphthofuranquinones and naphthothiophenequinones containing a fused thiazole ring. Bioorg. Med. Chem. 2003, 11, 2175–2182. [Google Scholar]
- Dallemagne, P.; Khanh, L.P.; Alsaidi, A.; Varlet, I.; Collot, V.; Paillet, M.; Bureau, R.; Rault, S. Synthesis and biological evaluation of five-Membered heterocycles fused to cyclopenta[c]thiophene as new antitumor agents. Bioorg. Chem. 2003, 11, 1161–1167. [Google Scholar] [CrossRef]
- Kumar, M.P.; Panda, G.; Manju, Y.K.; Chaturvedi, V.; Sinha, S. Thiophene containing triarylmethanes as antitubercular agents. Bioorg. Med. Chem. Lett. 2008, 18, 289–292. [Google Scholar]
- Abdel-Rahman, R.M. Chemistry of uncondensed 1,2,4-triazines: Part II sulfur containing 5-oxo-1,2,4-triazin-3-yl moiety. An overview. Phosphor. Sulfur Silicon 2000, 166, 315–357. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.M. Synthesis and chemistry of fluorine containing bioactive 1,2,4-triazines: an overview: chemistry of uncondensed 1,2,4-triazines. Part III. Pharmazie 1999, 54, 791–803. [Google Scholar]
- Saad, H.A.; Allimony, H.A.; El-Mariah, F.A.A. Synthesis and antimicrobial activity of some nitrogen heterobicyclic systems. Part 2. Indian J. Chem. 1998, 37B, 1142–1148. [Google Scholar]
- El-Mariah, F.A.A.; Saad, H.A.; Allimony, H.A.; Abdel-Rahman, R.M. Synthesis and antimicrobial activity of some nitrogen heterobicyclic systems. Part 3. Indian J. Chem. 2000, 39B, 36–41. [Google Scholar]
- Salimon, J.; Salih, N. Synthesis, characterization and biological activity of some new 1,2,4-triazine derivatives. Int. J. PharmTech Res. 2010, 2, 1041–1045. [Google Scholar]
- Sangshetti, J.N.; Shinde, D.B. One pot synthesis and SAR of some novel 3-substituted 5,6-diphenyl-1,2,4-triazines as antifungal agents. Bioorg. Med. Chem. Lett. 2010, 20, 742–745. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.M. Role of uncondensed 1,2,4-triazine compounds and related heterocyclic systems as therapeutic agents: A review. Pharmazie 2001, 56, 18–22. [Google Scholar]
- El-Gendy, Z.; Morsy, J.M.; Allimony, H.A.; Abdel-Monem, W.R.; Abdel-Rahman, R.M. Synthesis of heterobicyclic nitrogen systems bearing the 1,2,4-triazine moiety as anti-HIV and anticancer drugs. Part III. Pharmazie 2001, 56, 376–383. [Google Scholar]
- Gill, C.; Jadhav, G.; Shaikh, M.; Kale, R.; Ghawalkar, A.; Nagargoje, D.; Shiradkar, M. Clubbed [1,2,3]triazoles by fluorine benzimidazole: A novel approach to H 37Rv inhibitors as a potential treatment for tuberculosis. Bioorg. Med. Chem. Lett. 2008, 18, 6244–6247. [Google Scholar]
- Mullick, P.; Khan, S.A.; Begum, T.; Verma, S.; Kaushik, D.; Alam, O. Synthesis of 1,2,4-triazine derivatives as potential anti-anxiety and anti-inflammatory agents. Acta Pol. Pharm. Drug Res. 2009, 66, 379–385. [Google Scholar]
- Hynes, J.; Kanner, S.B.; Yang, X.; Tokarski, J.S.; Schieven, G.L.; Dyckman, A.J.; Lonial, H.; Zhang, R.; Sack, J.S.; Lin, S.; et al. Design, synthesis, and anti-inflammatory properties of orally active 4-(phenylamino)-pyrrolo[2,1-f][1,2,4]triazine p38α mitogen-activated protein kinase Inhibitors. J. Med. Chem. 2008, 51, 4–16. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.M. Role of uncondensed 1,2,4-triazine derivatives as biocidal plant protection agents: A review. Pharmazie 2001, 56, 195–204. [Google Scholar]
- Abdel-Rahman, R.M.; El-Gendy, Z.; Mahmoud, M.B. Synthesis of some new 3-substituted 1,2,4-triazino-indolederivatives and related compounds of potential antifungal activity. Indian J. Chem. 1990, 29B, 352–358. [Google Scholar]
- Abdel-Rahman, R.M.; Abdel-Malik, M.S. Synthesis of some new 3,6-dihydroaryl-1,2,4-triazin-5-ones and their effect on amylolytic activity of some fungi. Pak. J. Sci. Ind. Res. 1990, 33, 142–147. [Google Scholar]
- Abdel-Rahman, R.M.; Seada, M.; El-Gendy, Z.; Islam, I.E.; Mahmoud, M.B. Synthesis of some new 4,6-disubstituted 1,2,4-triazin-3,5(2H)-diones and related compounds of potential antifungal activity. Farmaco 1993, 48, 407–416. [Google Scholar]
- Al-Juwaiser, I.A.; Ibrahim, M.R.; Al-Awadi, N.A.; Ibrahim, Y.A. An Improved Direct Synthetic Approach to Anhydronucleosides (II). Tetrahedron 2008, 64, 8206–8212. [Google Scholar] [CrossRef]
- Saad, H.A.; Youssef, M.M.; Mosselhi, A.M. Microwave Assisted Synthesis of Some New Fused 1,2,4-Triazines Bearing Thiophene Moieties With Expected Pharmacological Activity. Molecules 2011, 16, 4937–4957. [Google Scholar]
- Krim, J.; Bisillahi, T.M.; Rakib, E.M.; Engles, J.W. Microwave-assisted click chemistry: Synthesis of mono and bis-1,2,3-triazole acyclonucleoside analogues of ACV via copper(I)-catalyzed cycloaddition. Arkivoc 2009, xiii, 142–152. [Google Scholar]
- El-Sayed, H.A.; Moustafa, A.H.; Haikal, A.Z.; El-Ashry, E.S.H. Synthesis and Evaluation of Antimicrobial Activity of Some Pyrimidine Glycosides. Nucleos. Nucleot. Nucleic Acids 2008, 27, 1061–1071. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Moustafa, A.H.; Haikal, A.Z.; El-Ashry, E.S.H. Synthesis and antibacterial activity of some glucosyl- and ribosyl-pyridazin-3-ones. Nucleos. Nucleot. Nucleic Acids 2009, 28, 184–192. [Google Scholar] [CrossRef]
- Moustafa, A.H.; Saad, H.A.; Shehab, W.S.; El-Mobayed, M.M. Synthesis of Some New Pyrimidine Derivatives of Expected Antimicrobial Activity. Phosphor. Sulfur Silicon 2008, 183, 115–135. [Google Scholar]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
- Ribble, D.; Goldstein, N.B.; Norris, D.A.; Shellman, Y.G. A simple technique for quantifying apoptosis in 96-well plates. PMC Biotechnol. 2005, 10, 5–12. [Google Scholar]
- Martin, D.; Leonardo, M. Related isolation procedures and functional assays. In Current Protocols in Immunology; Coligan, J.E., Kruisbeek, A.M., Margulies, D.H., Shevach, E.M., Strober, W., Eds.; John Wiley & Sons: New York, NY, USA, 1998; Volume 1.3, p. 3.17.11. [Google Scholar]
- Sample Availability: Not Available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saad, H.A.; Moustafa, A.H. Synthesis and Anticancer Activity of Some New S-Glycosyl and S-Alkyl 1,2,4-Triazinone Derivatives. Molecules 2011, 16, 5682-5700. https://doi.org/10.3390/molecules16075682
Saad HA, Moustafa AH. Synthesis and Anticancer Activity of Some New S-Glycosyl and S-Alkyl 1,2,4-Triazinone Derivatives. Molecules. 2011; 16(7):5682-5700. https://doi.org/10.3390/molecules16075682
Chicago/Turabian StyleSaad, Hosam A., and Ahmed H. Moustafa. 2011. "Synthesis and Anticancer Activity of Some New S-Glycosyl and S-Alkyl 1,2,4-Triazinone Derivatives" Molecules 16, no. 7: 5682-5700. https://doi.org/10.3390/molecules16075682






