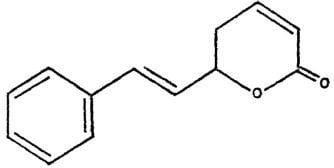
Selective Cytotoxicity of Goniothalamin against Hepatoblastoma HepG2 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.2. Discussion
3. Materials and Methods
3.1. Reagents
3.2. Cell lines
3.3. MTT cytotoxicity assay
3.4. Lactate dehydrogenase (LDH) assay
3.5. Cell cycle PI analysis
3.6. Bromodeoxyuridine (BrdU) cell proliferation assay
3.7. Viable cell counts using trypan blue dye exclusion assay
4. Conclusions
Declaration of Interest
References
- El-Serag, H.B.; Mason, A.C. Rising incidence of hepatocellular carcinoma in the United States. New. Engl. J. Med. 1999, 340, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Kubicka, S.; Rudolph, K.L.; Hanke, M.; Tietze, M.K.; Tillmann, H.L.; Trautwein, C. Hepatocellular carcinoma in Germany: A retrospective epidemiological study from a low-endemic area. Liver 2000, 20, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Wu, M.C.; Xu, G.W.; Li, D.Z.; Cheng, H.; Tu, Z.X.; Jiang, H.Q.; Gu, J.R. Overexpression of the MDR1 gene and P-glycoprotein in human hepatocellular carcinoma. J. Natl. Cancer Inst. 1992, 84, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Sbisa, E.; Tullo, A.; Papeo, P.A.; Saccone, C.; Poole, S.; Pignatelli, M.; Mitry, R.R.; Ding, S.; Isla, A.; Davies, A.; Habib, N.A. p53 mutation is a poor prognostic indicator for survival in patients with hepatocellular carcinoma undergoing surgical tumour ablation. Br. J. Cancer 1998, 77, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.; Jonas, S.; Karsten, A.; Neuhaus, P. Determination of the oncogenes p53 and C-Erb B2 in the tumour cytosols of advanced hepatocellular carcinoma (HCC) and correlation to survival time. Anticancer Res. 1999, 19, 2501–2503. [Google Scholar] [PubMed]
- Shah, S.R.; Riordan, S.M.; Karani, J.; Williams, R. Tumor ablation and hepatic decompensation rates in multi–agent chemoembolization of hepatocellular carcinoma. QJM 1998, 91, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.K. British National Formulary, 52nd ed.; Pharmaceutical Press: London, UK, 2006. [Google Scholar]
- Sam, T.W.; Sew-Yeu, C.; Matsjeh, S.; Gan, E.K.; Razak, D.; Mohamed, A.L. Goniothalamin oxide: An embryotoxic compound from Goniothalamus macrophyllus (Annonaceae). Tetrahedron Lett. 1987, 28, 2541–2544. [Google Scholar] [CrossRef]
- Chatchai, W.; Boonsong, W.; Puttachat, S.; Arunporn, I.; Niwat, K. Goniothalamin, a cytotoxic compound, isolated from Goniothalamus macrophyllus (Blume) Hook. f. & Thomson var. macrophyllus. Songklanakarin. J. Sci. Technol. 2005, 27, 479–487. [Google Scholar]
- Chen, W.Y.; Wu, C.C.; Lan, Y.H.; Chang, F.R.; Teng, C.M.; Wu, Y.C. Goniothalamin induces cell cycle-specific apoptosis by modulating the redox status in MDA-MB-231 cells. Eur. J. Pharmacol. 2005, 522, 20–29. [Google Scholar] [CrossRef] [PubMed]
- De Fátima, A.; Kohn, L.K.; Antônio, M.A.; de Carvalho, J.E.; Pilli, R.A. (R)- Goniothalamin: Total syntheses and cytotoxic activity against cancer cell lines. Bioorg. Med. Chem. 2005, 13, 2927–2933. [Google Scholar] [CrossRef] [PubMed]
- Newall, C.A.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines. A Guide for Health-care Professionals; Pharmaceutical Press: London, UK, 1996. [Google Scholar]
- Schulz, V.; Rudolf, H.; Tyler, V.E. Rational Phytotherapy. A Physician’s Guide to Herbal Medicine, 3rd ed.; Springer: Berlin, Germany, 1998. [Google Scholar]
- Adams, J. Proteasome inhibition in cancer: Development of PS-341. Sem. Oncol. 2001, 28, 613–619. [Google Scholar] [CrossRef]
- Pihie, A.H.; Stanslas, J.; Din, L.B. Non-steroid receptor mediated antiproliferative activity of styrylpyrone derivative (SPD) in human breast cancer cell lines. Anticancer Res. 1998, 18, 1739–1744. [Google Scholar] [PubMed]
- Andreas, P.S.; Kerstin, M.; Patricia, G.; Gesine, B.; Kirsten, V.; Michael, H.; Antje, K.; Bernhard, H.; Harald, S.; Rajan, S.; Detlef, S.; Martin, Z.; Hans, S. Peripheral benzodiazepine receptor ligands induce apoptosis and cell cycle arrest in human hepatocellular carcinoma cells and enhance chemosensitivity to paclitaxel, docetaxel, doxorubicin and the Bcl-2 inhibitor HA14-1. J. Hepatol. 2004, 41, 799–807. [Google Scholar]
- Wahl, A.F.; Donaldson, K.L.; Fairchild, C.; Lee, F.Y.; Foster, S.A.; Demers, G.W.; Galloway, D.A. Loss of normal p53 function confers sensitization to Taxol by increasing G2/M arrest and apoptosis. Nat. Med. 1996, 2, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Lau, T.C.; Ng, I.O. Doxorubicin-induced apoptosis and chemosensitivity in hepatoma cell lines. Cancer Chemother. Pharmacol. 2002, 49, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Gratzner, H.G. Monoclonal antibody to 5-bromo- and 5- Iododeoxyuridine: A new reagent for detection of DNA replication. Science 1982, 218, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Rajab, N.F.; Ishak, M.H.A.; Ali, A.M.; Yusoff, K.; Din, L.B.; Inayat-Hussain, S.H. Goniothalamin induces apoptosis in vascular smooth muscle cells. Chem. Biol. Interact. 2006, 159, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Inayat-Hussain, S.H.; Annuar, B.O.; Din, L.B.; Ali, A.M.; Ross, D. Loss of mitochondrial transmembrane potential and caspase-9 activation during apoptosis induced by the novel styryl-lactone goniothalamin in HL-60 leukemia cells. Toxicol. In Vitro 2003, 17, 433–439. [Google Scholar] [CrossRef]
- Tay, N.; Chan, S.H.; Ren, E.C. Detection of integrated hepatitis B virus DNA in hepatocellular carcinoma cell lines by nonradioactive in situ hybridization. J. Med. Virol. 1990, 30, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Jemmerson, R.; LaPlante, B.; Treeful, A. Release of intact, monomeric cytochrome c from apoptotic and necrotic cells. Cell Death Differ. 2002, 9, 538–548. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the goniothalamin are available from the authors |









| Cells | Goniothalamin IC50 (μM) | Doxorubicin IC50 (μM) |
|---|---|---|
| HepG2 | 4.63 | 1.06 |
| Chang | 35.01 | 0.28 |
| SI | 7.6 | 3.7 |
| IC50-GTN / IC50-Doxo | 4.4 | 125.0 |
| Cell line | Duration (hours) | Cytotoxicity assay | |
|---|---|---|---|
| IC50 from MTT (µM) | IC50 from LDH (µM) | ||
| HepG2 | 24 | 9.20 | 13.43 |
| 48 | 8.30 | 8.29 | |
| 72 | 4.63 | 5.20 | |
| Chang | 24 | 79.10 | 69.58 |
| 48 | 63.75 | 55.11 | |
| 72 | 35.01 | 32.47 | |
| (a) | SubG0/G1 (%) | G0/G1 (%) | S (%) | G2+M (%) | |
| 24h | Control | 2.49 ± 0.66 | 81.17 ± 2.07 | 6.82 ± 1.07 | 9.52 ± 0.43 |
| Goniothalamin (9.2 µM) | 17.44 ± 1.67 | 68.92 ± 1.27 | 7.68 ± 0.12 | 5.94 ± 0.22 | |
| Doxorubicin (2.2 µM) | 3.36 ± 1.02 | 76.46 ± 3.80 | 8.54 ± 0.38 | 11.64 ± 3.02 | |
| 48h | Control | 3.16 ± 0.81 | 74.53 ± 0.75 | 4.85 ± 1.23 | 17.46 ± 0.09 |
| Goniothalamin (8.3 µM) | 46.59 ± 2.79 | 39.71 ± 3.50 | 8.03 ± 0.29 | 5.67 ± 4.13 | |
| Doxorubicin (4.3 µM) | 5.14 ± 2.53 | 65.17 ± 12.80 | 15.15 ± 10.96 | 14.54 ± 1.06 | |
| 72h | Control | 3.69± 1.93 | 76.81± 2.84 | 5.37 ± 2.07 | 14.13 ± 0.58 |
| Goniothalamin (4.6 µM) | 58.83 ± 2.09 | 31.79 ± 5.82 | 7.07 ± 0.18 | 2.31 ± 4.19 | |
| Doxorubicin (1.06 µM) | 30.79 ± 0.57 | 60.64 ± 0.79 | 5.91 ± 0.15 | 2.66 ± 0.37 | |
| (b) | SubG0/G1 (%) | G0/G1 (%) | S (%) | G2+M (%) | |
| 24h | Control | 4.51 ± 0.21 | 61.27 ± 0.94 | 15.62 ± 0.12 | 18.60 ± 0.99 |
| Goniothalamin (79.10 µM) | 17.38 ± 7.29 | 55.08 ± 3.60 | 12.65 ± 2.79 | 14.89 ± 5.53 | |
| Doxorubicin (0.83 µM) | 10.71 ± 7.28 | 50.84 ± 6.03 | 22.09 ± 0.03 | 16.36 ± 1.28 | |
| 48h | Control | 3.74 ± 0.23 | 69.88 ± 1.27 | 10.32 ± 2.33 | 16.06 ± 2.09 |
| Goniothalamin (63.75 µM) | 33.00 ± 3.40 | 58.68 ± 9.45 | 5.82 ± 0.16 | 2.5 ± 5.89 | |
| Doxorubicin (0.41 µM) | 11.89 ± 6.49 | 62.43 ± 9.80 | 15.31 ± 3.10 | 10.37 ± 0.21 | |
| 72h | Control | 4.12 ± 2.39 | 66.84 ± 0.81 | 11.50 ± 1.37 | 17.54 ± 1.08 |
| Goniothalamin (35.01 µM) | 80.99 ± 0.64 | 11.60 ± 0.07 | 4.91 ± 0.13 | 2.5 ± 0.44 | |
| Doxorubicin (0.28 µM) | 22.06 ± 5.17 | 54.12 ± 1.52 | 15.19 ± 2.83 | 8.63 ± 2.17 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Qubaisi, M.; Rozita, R.; Yeap, S.-K.; Omar, A.-R.; Ali, A.-M.; Alitheen, N.B. Selective Cytotoxicity of Goniothalamin against Hepatoblastoma HepG2 Cells. Molecules 2011, 16, 2944-2959. https://doi.org/10.3390/molecules16042944
Al-Qubaisi M, Rozita R, Yeap S-K, Omar A-R, Ali A-M, Alitheen NB. Selective Cytotoxicity of Goniothalamin against Hepatoblastoma HepG2 Cells. Molecules. 2011; 16(4):2944-2959. https://doi.org/10.3390/molecules16042944
Chicago/Turabian StyleAl-Qubaisi, Mothanna, Rosli Rozita, Swee-Keong Yeap, Abdul-Rahman Omar, Abdul-Manaf Ali, and Noorjahan B. Alitheen. 2011. "Selective Cytotoxicity of Goniothalamin against Hepatoblastoma HepG2 Cells" Molecules 16, no. 4: 2944-2959. https://doi.org/10.3390/molecules16042944