Four Eremophil-9-en-8-one Derivatives from Cremanthodium stenactinium Samples Collected in China
Abstract
:1. Introduction
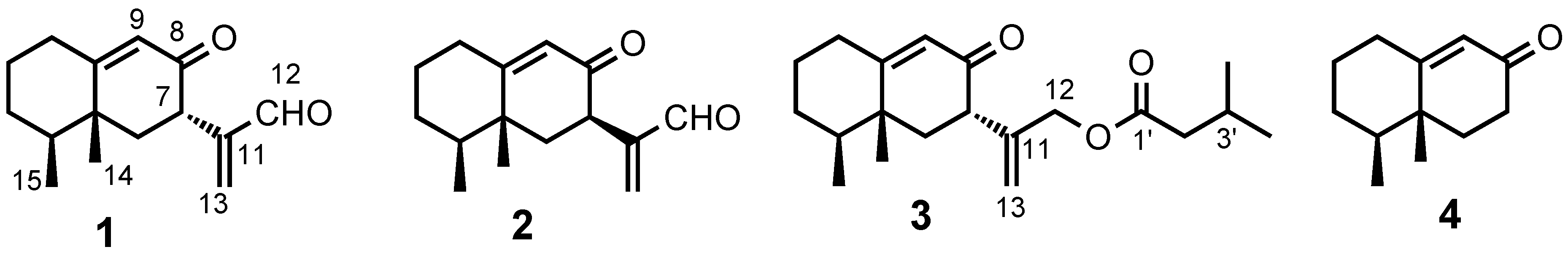
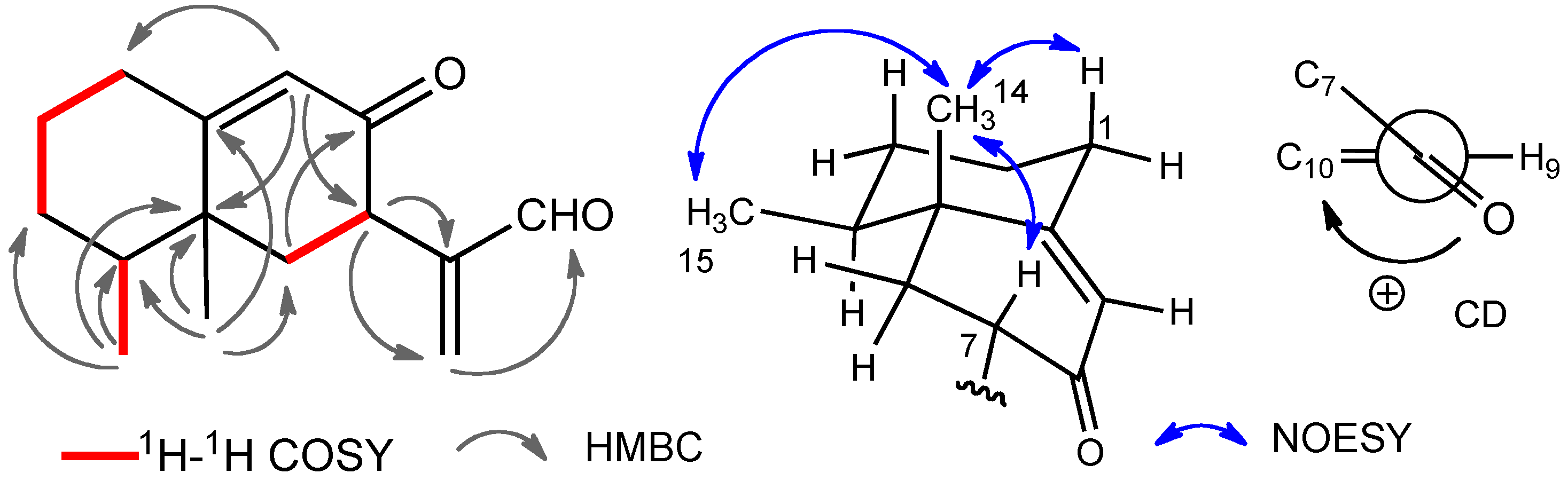
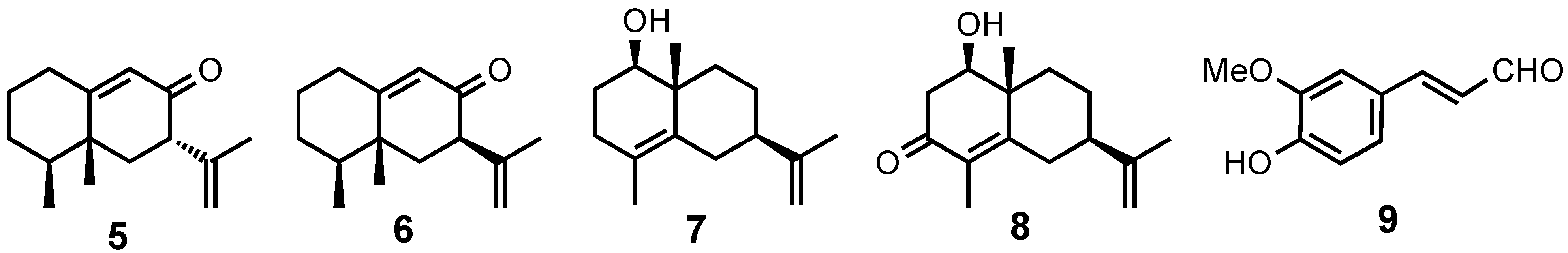
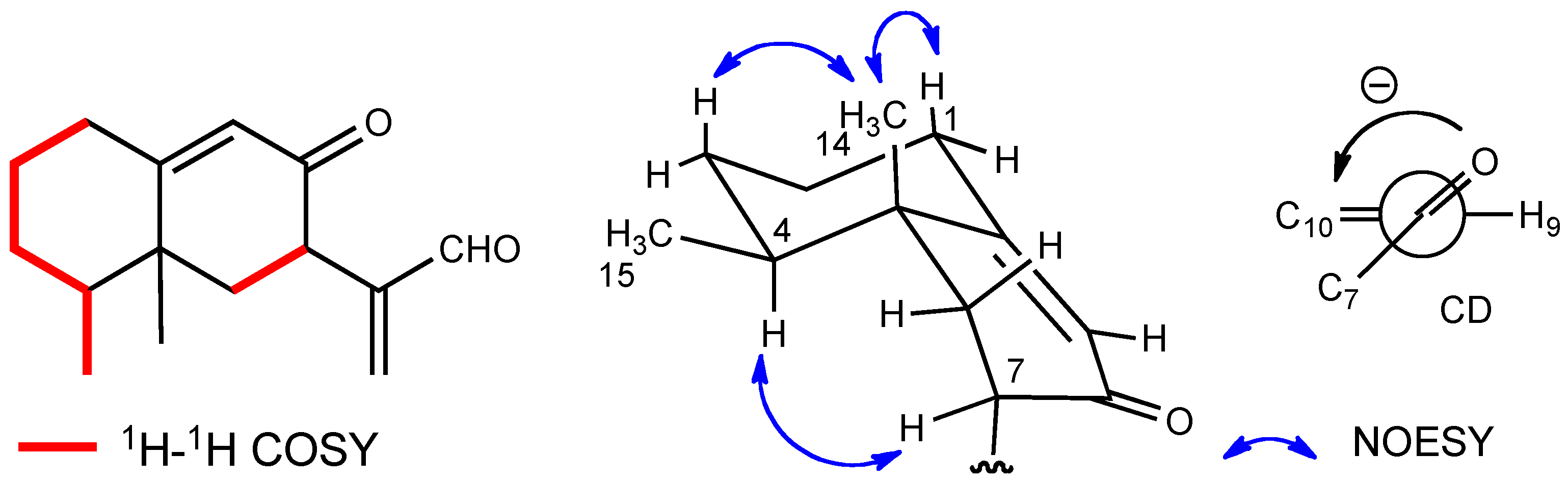
2. Results and Discussion
| position | 13C (ppm) | 1H (ppm) | |
|---|---|---|---|
| 1 | 1 | 2 | |
| 1 | 32.7 | 1.72–1.81 (2H, m) | 1.60–1.68 (m) |
| - | 1.91 (td, J = 12.2, 5.2 Hz) | ||
| 2 | 26.4 | 1.02–1.08 (m) | 0.94–1.01 (m) |
| 1.37–1.42 (m) | 1.43–1.50 (m) | ||
| 3 | 30.4 | 0.98–1.05 (m) | 0.98–1.06 (m) |
| 1.08–1.13 (m) | 1.09–1.15 (m) | ||
| 4 | 43.5 | 1.00–1.05 (m) | 1.50–1.55 (m) |
| 5 | 39.7 | - | - |
| 6 | 41.9 | 1.67 (dd, J = 13.0, 5.6 Hz) | 1.62 (t, J = 13.7 Hz) |
| 1.71 (dd, J = 13.4, 13.0 Hz) | 1.73 (dd, J = 13.7, 4.6 Hz) | ||
| 7 | 42.8 | 3.60 (dd, J = 13.4, 5.6 Hz) | 3.64 (dd, J = 13.7, 4.6 Hz) |
| 8 | 195.5 | - | - |
| 9 | 124.3 | 5.76 (d, J = 1.7 Hz) | 5.80 (d, J = 1.3 Hz) |
| 10 | 168.0 | - | - |
| 11 | 149.3 | - | - |
| 12 | 192.8 | 9.34 (s) | 9.31 (s) |
| 13 | 134.4 | 5.52 (s) | 5.52 (s) |
| 5.80 (s) | 5.88 (s) | ||
| 14 | 15.5 | 0.71 (s) | 0.63 (s) |
| 15 | 15.1 | 0.54 (d, J = 6.4 Hz) | 0.60 (d, J = 6.9 Hz) |
| position | 13C (ppm) | 1H (ppm) | ||
|---|---|---|---|---|
| 3 | 4 | 3 | 4 | |
| 1 | 32.6 | 33.1 | 1.74–1.83 (m) | 1.76 (ddt, J = 14.6, 4.2, 2.0 Hz) |
| - | 1.83 (ddd, J = 14.6, 12.8, 5.2 Hz) | |||
| 2 | 26.5 | 26.7 | 1.03–1.09 (m) | 1.03–1.09 (m) |
| 1.38–1.43 (m) | 1.39–1.44 (m) | |||
| 3 | 30.4 | 30.5 | 1.00–1.05 (m) | 0.99–1.06 (m) |
| 1.10–1.15 (m) | 1.12–1.15 (m) | |||
| 4 | 43.6 | 43.1 | 1.00–1.05 (m) | 0.96–1.02 (m) |
| 5 | 39.6 | 38.8 | - | - |
| 6 | 41.7 | 35.7 | 1.72 (dd, J = 12.9, 4.1 Hz) | 1.27 (ddd, J = 14.4, 13.4, 4.4 Hz) |
| 1.85 (dd, J = 12.9, 4.1 Hz) | 1.45 (ddd, J = 13.4, 4.9, 3.5 Hz) | |||
| 7 | 47.5 | 34.3 | 3.12 (dd, J = 14.7, 4.1 Hz) | 2.14 (ddd, J = 16.6, 14.4, 4.9 Hz) |
| - | 2.25 (ddd, J = 16.6, 4.4, 3.5 Hz) | |||
| 8 | 196.7 | 197.3 | - | - |
| 9 | 124.4 | 124.7 | 5.73 (s) | 5.80 (s) |
| 10 | 168.0 | 168.5 | - | - |
| 11 | 143.9 | - | - | - |
| 12 | 66.3 | - | 4.98 (s) | - |
| 13 | 114.0 | - | 4.98 (s) | - |
| 5.36 (s) | - | |||
| 14 | 15.4 | 15.6 | 0.69 (s) | 0.61 (s) |
| 15 | 15.1 | 15.2 | 0.57 (d, J = 6.1 Hz) | 0.55 (d, J = 6.4 Hz) |
| 1' | 172.0 | - | - | - |
| 2' | 43.4 | - | 2.07 (d, J = 6.1 Hz) | - |
| 3' | 25.8 | - | 2.05–2.15 (m) | - |
| 4' 5' | 22.5 (2C) | - | 0.84 (d, J = 6.4 Hz) | - |


3. Experimental
General
4. Conclusions
Acknowledgments
References and Notes
- Liu, J.; Liu, S.; Ho, T.; Lu, A. Karyological studies on the Sino-Himalayan genus, Cremanthodium (Asteraceae: Senecionae). Bot. J. Linn. Soc. 2001, 135, 107–112. [Google Scholar]
- Liu, J.Q.; Wang, Y.J.; Wang, A.L.; Ohba, H.; Abbott, R.J. Radiation and diversification within the Ligularia-Cremanthodium-Parasenecio complex (Asteraceae) triggered by uplift of the Qinghai-Tibetan plateau. Mol. Phylogenet. Evol. 2006, 38, 31–49. [Google Scholar] [CrossRef]
- Yang, L.; Chen, H.; Jia, Z.J. Lignan and a coumarin from Cremanthodium ellisii Kitam. Ind. J. Chem. 1995, 34B, 975–977. [Google Scholar]
- Chen, H.; Zhu, T.; Shen, X.M.; Jia, Z.J. Four new sesquiterpene polyol esters from Cremanthodium ellisii. J. Nat. Prod. 1996, 59, 1117–1120. [Google Scholar] [CrossRef]
- Chen, H.; Jia, Z.J.; Tan, R.X. Two new oplopanol esters from Cremanthodium ellisii. Planta Med. 1997, 63, 245–247. [Google Scholar] [CrossRef]
- Su, B.N.; Zhu, Q.X.; Jia, Z.J. Nor-lignan and sesquiterpenes from Cremanthodium ellisii. Phytochemistry 2000, 53, 1103–1108. [Google Scholar]
- Wang, A.X.; Zhang, Q.; Jia, Z.J. Phenylpropanosides, lignans and other constituents from Cremanthodium ellisii. Pharmazie 2004, 59, 889–892. [Google Scholar]
- Zhu, Y.; Yang, L.; Jia, Z.J. Novel highly oxygenated bisabolane sesquiterpenes from Cremanthodium discoideum. J. Nat. Prod. 1999, 62, 1479–1483. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, Q.X.; Jia, Z.J. Epoxide sesquiterpenes and steroids from Cremanthodium discoideum. Aust. J. Chem. 2000, 53, 831–834. [Google Scholar] [CrossRef]
- Cremanthodium potaninii (triterpene): Yang, A.; Zeng, Y.; Yang, Z Isolation and structural elucidation of triterpenes from Cremanthodiium potaninii C. Winkl. Zhongchengyao 2010, 32, 636–639.
- Tu, Y.; Yang, R.; Shou, Q.; Wang, B.; Peng, T. Studies on chemical constituents of essential oils from Cremanthodium pleurocaule. Zhongguo Zhongyao Zazhi 2006, 31, 522–524. [Google Scholar]
- Hanai, R.; Gong, X.; Tori, M.; Kondo, S.; Otose, K.; Okamoto, Y.; Nishihama, T.; Murota, A.; Shen, Y.; Wu, S.; Kuroda, C. Chemical and Genetic Study of Ligularia tongolensis, Ligularia cymbulifera, and Ligularia atroviolacea in the Hengduan Mountains of China. Bull. Chem. Soc. Jpn. 2005, 78, 1302–1308. [Google Scholar] [CrossRef]
- Tori, M.; Honda, K.; Nakamizo, H.; Okamoto, Y.; Sakaoku, M.; Takaoka, S.; Gong, X.; Shen, Y.; Kuroda, C.; Hanai, R. Chemical constituents of Ligularia virgaurea and its diversity in southwestern Sichuan of China. Tetrahedron 2006, 62, 4988–4995. [Google Scholar]
- Tori, M.; Fujiwara, M.; Okamoto, Y.; Tanaka, M.; Gong, X.; Shen, Y.; Hanai, R.; Kuroda, C. New Oplopane-type Sesquiterpenoids from Ligularia duciformis. Nat. Prod. Commun. 2007, 2, 357–360. [Google Scholar]
- Tori, M.; Watanabe, A.; Matsuo, S.; Okamoto, Y.; Tachikawa, K.; Takaoka, S.; Gong, X.; Kuroda, C.; Hanai, R. Diversity of Ligularia kanaitzensis in sesquiterpenoid composition and neutral DNA sequences. Tetrahedron 2008, 64, 4486–4495. [Google Scholar] [CrossRef]
- Tori, M.; Okamoto, Y.; Tachikawa, K.; Mihara, K.; Watanabe, A.; Sakaoku, M.; Takaoka, S.; Tanaka, M.; Gong, X.; Kuroda, C.; Hattori, M.; Hanai, R. Isolation of new eremophilane-type sesquiterpenoids, subspicatins A–D and subspicatolide from Ligularia subspicata, and chemical and genetic diversity of the species. Tetrahedron 2008, 64, 9136–9142. [Google Scholar]
- Nagano, H.; Torihata, A.; Matsushima, M.; Hanai, R.; Saito, Y.; Baba, M.; Tanio, Y.; Okamoto, Y.; Takashima, Y.; Ichihara, M.; Gong, X.; Kuroda, C.; Tori, M. Chemical and Genetic Study of Ligularia cyathiceps in Yunnan Province of China. Helv. Chim. Acta 2009, 92, 2071–2081. [Google Scholar] [CrossRef]
- Saito, Y.; Hattori, M.; Iwamoto, Y.; Takashima, Y.; Mihara, K.; Sasaki, Y.; Fujiwara, M.; Sakaoku, M.; Shimizu, A.; Chao, X.; Kuroda, C.; Gong, X.; Hanai, R.; Tori, M. Overlapping chemical and genetic diversity in Ligularia lamarum and Ligularia subspicata. Isolation of ten new eremophilanes and a new seco-bakkane compound. Tetrahedron 2011, 67, 2220–2231. [Google Scholar] [CrossRef]
- Djerassi, C.; Rbcords, R.; Bunnenberg, E.; Mislow, K.; Moscowitz, A. Inherently dissymmetric chromophores. Optical rotatory dispersion of α,β-unsaturated ketones and conformational analysis of cyclohexenones. J. Am. Chem. Soc. 1962, 84, 870–872. [Google Scholar] [CrossRef]
- Paquette, L.A.; Wang, T.Z.; Philippo, M.G.; Wang, S. Total synthesis of the cembranoid diterpene lactone (+)-cleomeolide. Some remarkable conformational features of nine-membered belts linked in 2,6-fashion to a methylenecyclohexanone core. J. Am. Chem. Soc. 1994, 116, 3367–3374. [Google Scholar] [CrossRef]
- Guerriero, A.; D’Ambrosio, M.; Cuomo, V.; Vanzanella, F.; Pietra, F. Dendrophiellin A, the first fungal trinor-eremophilane. Isolation from the marine deuteromycete Dendryphiella salina (SUTHERLAND) PUGH et NICOT. Helv. Chim. Acta 1988, 71, 57–61. [Google Scholar] [CrossRef]
- Maurer, B.; Frachenboud, M.; Grieder, A.; Ohloff, G. Zur Kenntnis der sesquiterpenoiden C12-Ketone des ätherichen Öls von Vetiveria zizanioides (L.) Nash. Helv. Chim. Acta 1972, 55, 2371–2382. [Google Scholar] [CrossRef]
- Hikino, H.; Hikino, Y.; Koakutsu, S.; Takemoto, T. Structure and absolute configuration of narchinol A. Phytochemistry 1972, 11, 2097–2099. [Google Scholar]
- Bohlmann, F.; Kramp, W.; Robinson, H.; King, R.M. A norsequiterpene from Senecio humillimus. Phytochemistry 1981, 20, 1739–1740. [Google Scholar]
- Zdero, C.; Bohlmann, F. Eremophilanolides, eudesmanolides, guaianolides and other constituents from Ondetia lineari. Phytochemistry 1989, 28, 1653–1660. [Google Scholar] [CrossRef]
- Saito, Y.; Ichihara, M.; Okamoto, Y.; Gong, X.; Kuroda, C.; Tori, M. Five new subspicatins and noreremophilane from Parasenecio petasitoides collected in China. Tetrahedron Lett. 2011, 52, 6388–6391 and references cited therein. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–9 are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saito, Y.; Ichihara, M.; Okamoto, Y.; Gong, X.; Kuroda, C.; Tori, M. Four Eremophil-9-en-8-one Derivatives from Cremanthodium stenactinium Samples Collected in China. Molecules 2011, 16, 10645-10652. https://doi.org/10.3390/molecules161210645
Saito Y, Ichihara M, Okamoto Y, Gong X, Kuroda C, Tori M. Four Eremophil-9-en-8-one Derivatives from Cremanthodium stenactinium Samples Collected in China. Molecules. 2011; 16(12):10645-10652. https://doi.org/10.3390/molecules161210645
Chicago/Turabian StyleSaito, Yoshinori, Mayu Ichihara, Yasuko Okamoto, Xun Gong, Chiaki Kuroda, and Motoo Tori. 2011. "Four Eremophil-9-en-8-one Derivatives from Cremanthodium stenactinium Samples Collected in China" Molecules 16, no. 12: 10645-10652. https://doi.org/10.3390/molecules161210645




