The in Vitro Structure-Related Anti-Cancer Activity of Ginsenosides and Their Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrolysate of Ginseng Extracts Preparation and Its Cytotoxicity
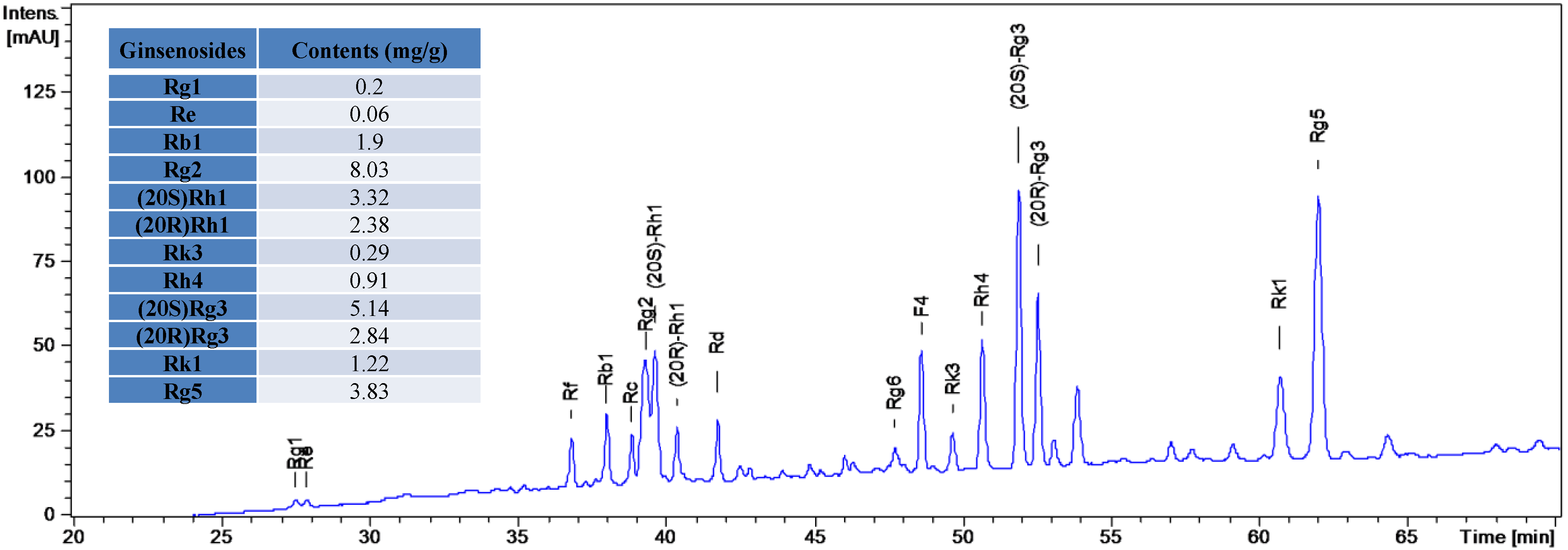
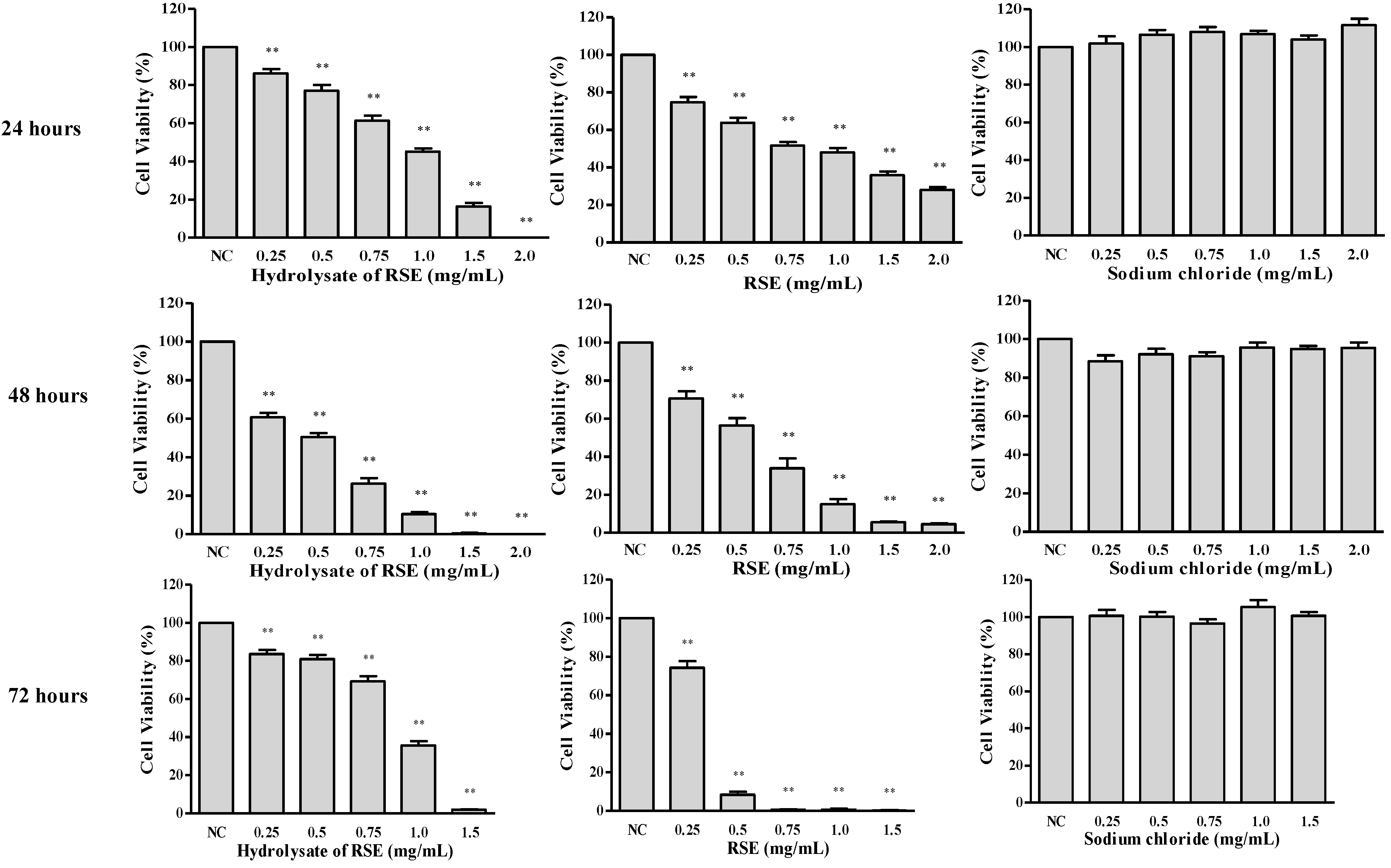
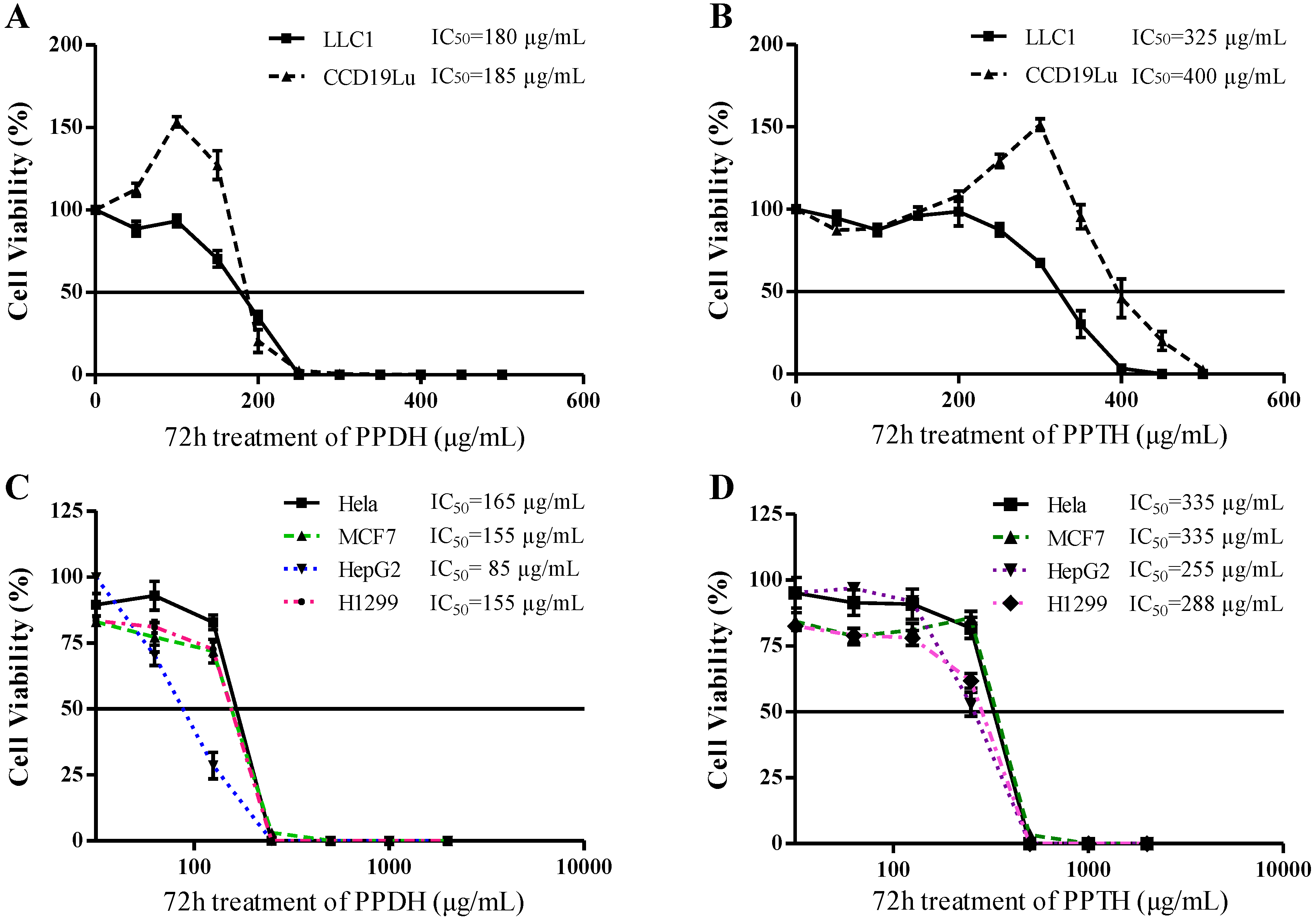
2.2. Hydrolysate of PPD- or PPT-Type Ginsenosides Fraction Preparation and Its Cytotoxicity

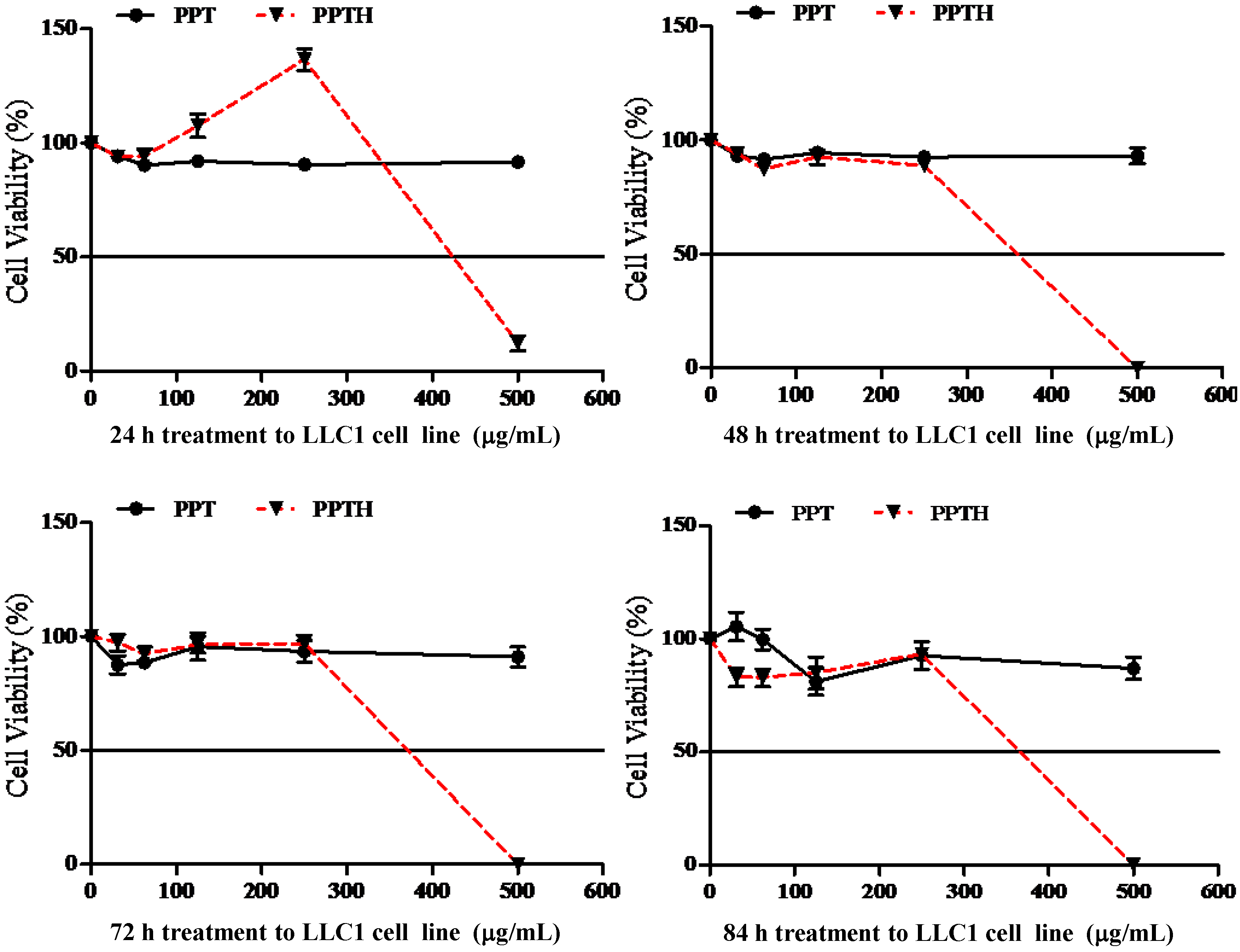
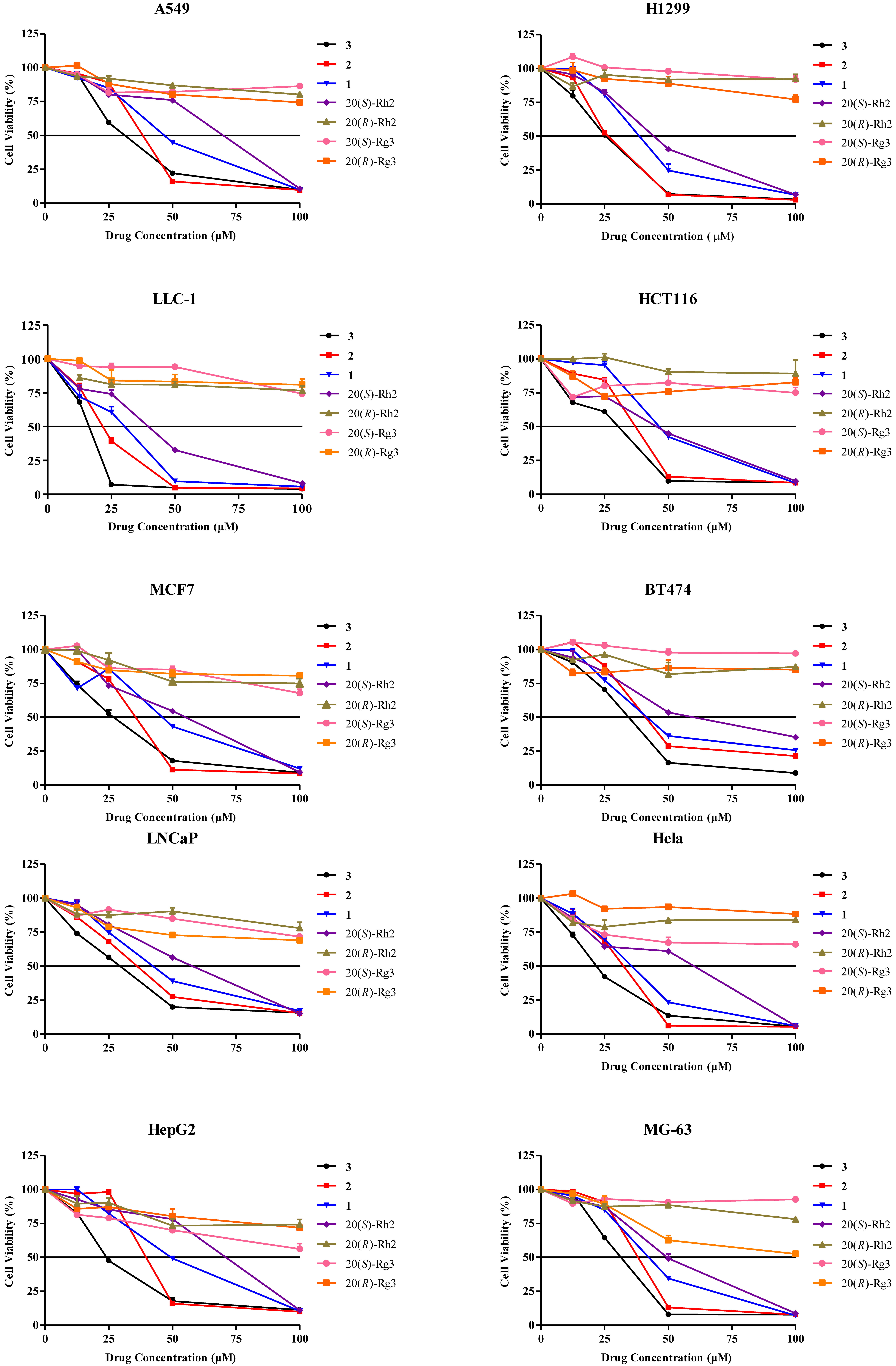
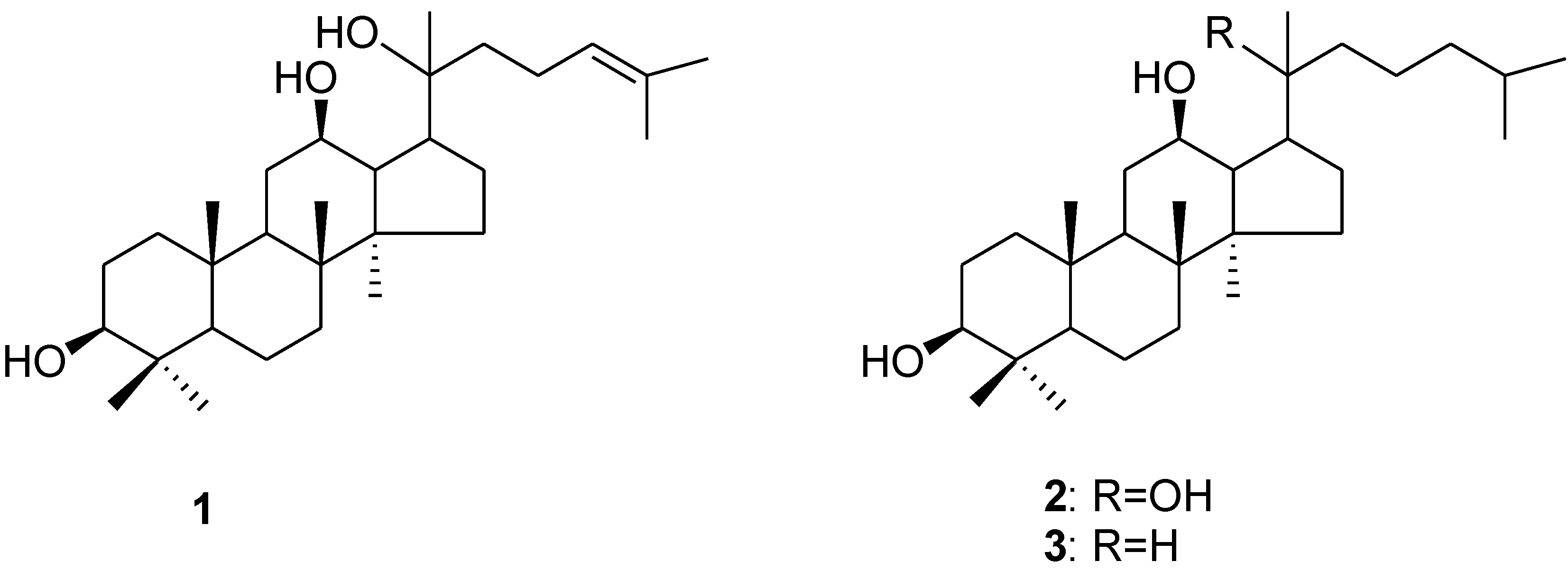
2.3. Examination of the Cytotoxic Potency of PPD-type Ginsenosides and their Structurally-Modified PPD-Derivatives
3. Experimental
3.1. Instruments and Materials
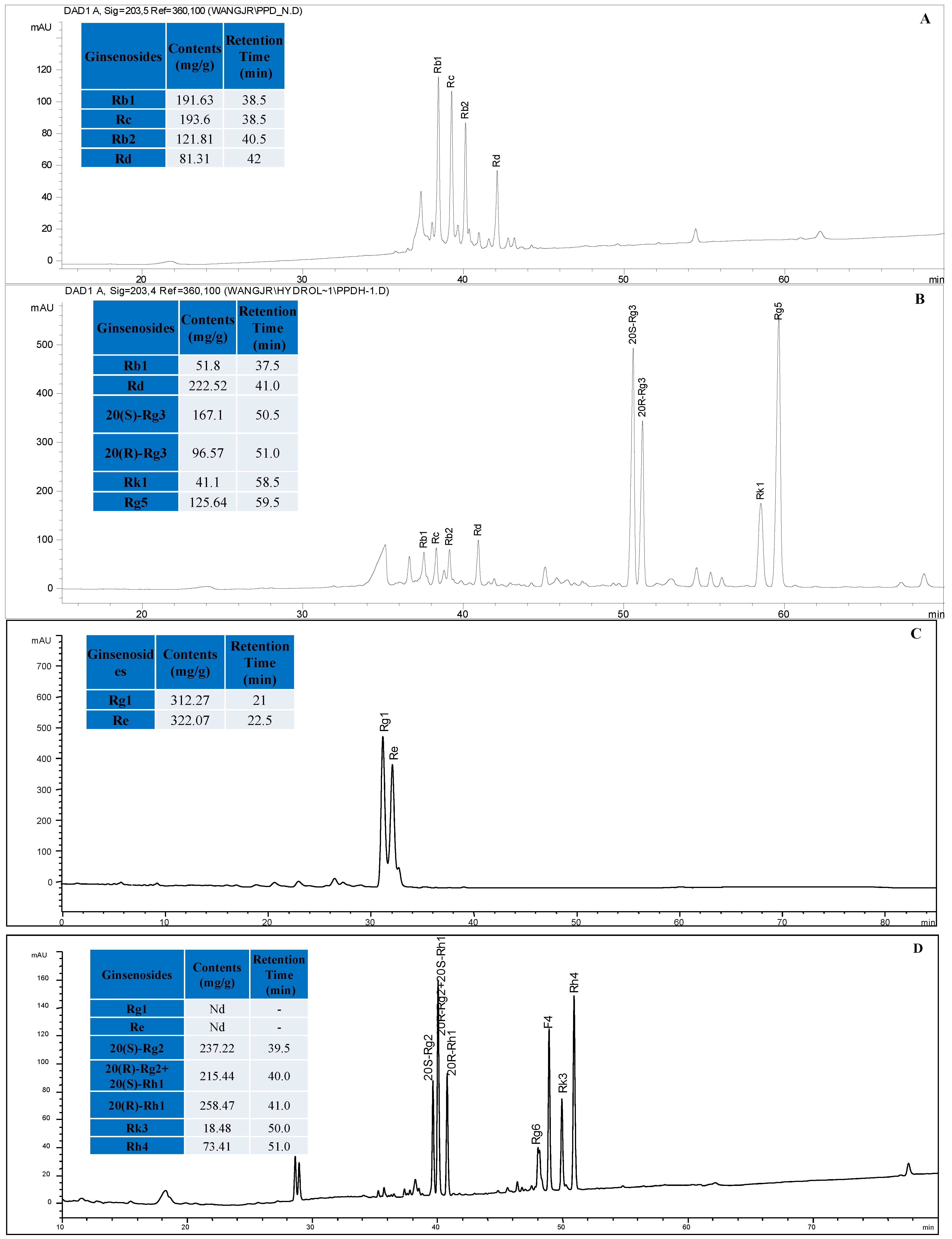
3.2. HPLC Analysis of Ginsenosides in RSEH, PPTH and PPDH
3.3. Synthesis of the PPD (1) Derivatives 2 and 3
3.4. Cancer Cell Lines and Cell Culture Conditions
3.5. Cytotoxic Activity Assay
4. Conclusions
Acknowledgements
Conflict of Interest
References and Notes
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN. Int. J. Cancer 2008, 127, 2893–2917. [Google Scholar]
- Hernandez, B.Y.; Green, M.D.; Cassel, K.D.; Pobutsky, A.M.; Vu, V.; Wilkens, L.R. Preview of Hawaii Cancer Facts and Figures. Hawaii Med. J. 2010, 69, 223–224. [Google Scholar]
- Du, G.J.; Dai, Q.; Williams, S.; Wang, C.Z.; Yuan, C.S. Synthesis of protopanaxadiol derivatives and evaluation of their anticancer activities. Anticancer Drugs 2011, 22, 35–45. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Rayburn, E.R.; Zhao, Y.; Hill, D.L.; Zhang, R. 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol, a novel natural product for prostate cancer therapy: Activity in vitro and in vivo and mechanisms of action. Br. J. Cancer 2008, 98, 792–802. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Tawab, M.A.; Bahr, U.; Karas, M.; Wurglics, M.; Schubert-Zsilavecz, M. Degradation of ginsenosides in humans after oral administration. Drug Metab. Dispos. 2003, 31, 1065–1071. [Google Scholar] [CrossRef]
- Pietta, P.; Mauri, P.; Rava, A. Hydrolysis of ginsenosides in artificial gastric fluid monitored by high-performance liquid chromatography. J. Chromatogr. 1986, 362, 291–297. [Google Scholar] [CrossRef]
- Zhang, X.; Song, F.; Cui, M.; Liu, Z.; Liu, S. Investigation of the hydrolysis of ginsenosides by high performance liquid chromatography-electrospray ionization mass spectrometry. Planta Med. 2007, 73, 1225–1229. [Google Scholar] [CrossRef]
- Han, B.H.; Park, M.H.; Han, Y.N.; Woo, L.K.; Sankawa, U.; Yahara, S.; Tanaka, O. Degradation of ginseng saponins under mild acidic conditions. Planta Med. 1982, 44, 146–149. [Google Scholar] [CrossRef]
- Bae, E.A.; Han, M.J.; Kim, E.J.; Kim, D.H. Transformation of ginseng saponins to ginsenoside Rh2 by acids and human intestinal bacteria and biological activities of their transformants. Arch. Pharm. Res. 2004, 27, 61–67. [Google Scholar] [CrossRef]
- Usami, Y.; Liu, Y.N.; Lin, A.S.; Shibano, M.; Akiyama, T.; Itokawa, H.; Morris-Natschke, S.L.; Bastow, K.; Kasai, R.; Lee, K.H. Antitumor agents. 261. 20(S)-protopanaxadiol and 20(S)-protopanaxatriol as antiangiogenic agents and total assignment of (1)H NMR spectra. J. Nat. Prod. 2008, 71, 478–481. [Google Scholar] [CrossRef]
- Tanaka, N.; Tanaka, O.; Shibata, S. Chemical studies on the oriental plant drugs. XXVIII. Saponins and sapogenins of ginseng; Stereochemistry of sapogenin of ginsenoside Rb1, Rb2 and Rc. Chem. Pharm. Bull. 1972, 20, 1212–1216. [Google Scholar] [CrossRef]
- Park, M.H.; Park, T.Y. Treatment and Prevention of Cancer with New Ginsenoside Derivatives. WIPO Patent WO/2005/116042, 42 April 2005. [Google Scholar]
- Chen, J.; Peng, H.; Ou-Yang, X.; He, X. Research on the antitumor effect of ginsenoside Rg3 in B16 melanoma cells. Melanoma Res. 2008, 18, 322–329. [Google Scholar] [CrossRef]
- Keum, Y.S.; Han, S.S.; Chun, K.S.; Park, K.K.; Park, J.H.; Lee, S.K.; Surh, Y.J. Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced cyclooxygenase-2 expression, NF-kappaB activation and tumor promotion. Mutat. Res. 2003, 523-524, 75–85. [Google Scholar] [CrossRef]
- Yue, P.Y.; Wong, D.Y.; Wu, P.K.; Leung, P.Y.; Mak, N.K.; Yeung, H.W.; Liu, L.; Cai, Z.; Jiang, Z.H.; Fan, T.P.; Wong, R.N. The angiosuppressive effects of 20(R)- ginsenoside Rg3. Biochem. Pharmacol. 2006, 72, 437–445. [Google Scholar]
- Wong, V.K.; Cheung, S.S.; Li, T.; Jiang, Z.H.; Wang, J.R.; Dong, H.; Yi, X.Q.; Zhou, H.; Liu, L. Asian ginseng extract inhibits in vitro and in vivo growth of mouse lewis lung carcinoma via modulation of ERK-p53 and NF-kappaB signaling. J. Cell Biochem. 2010, 111, 899–910. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dong, H.; Bai, L.-P.; Wong, V.K.W.; Zhou, H.; Wang, J.-R.; Liu, Y.; Jiang, Z.-H.; Liu, L. The in Vitro Structure-Related Anti-Cancer Activity of Ginsenosides and Their Derivatives. Molecules 2011, 16, 10619-10630. https://doi.org/10.3390/molecules161210619
Dong H, Bai L-P, Wong VKW, Zhou H, Wang J-R, Liu Y, Jiang Z-H, Liu L. The in Vitro Structure-Related Anti-Cancer Activity of Ginsenosides and Their Derivatives. Molecules. 2011; 16(12):10619-10630. https://doi.org/10.3390/molecules161210619
Chicago/Turabian StyleDong, Hang, Li-Ping Bai, Vincent Kam Wai Wong, Hua Zhou, Jing-Rong Wang, Yan Liu, Zhi-Hong Jiang, and Liang Liu. 2011. "The in Vitro Structure-Related Anti-Cancer Activity of Ginsenosides and Their Derivatives" Molecules 16, no. 12: 10619-10630. https://doi.org/10.3390/molecules161210619



