Terpenoids from Endophytic Fungi
Abstract
:1. Introduction
2. Terpenoids
2.1. Sesquiterpenes
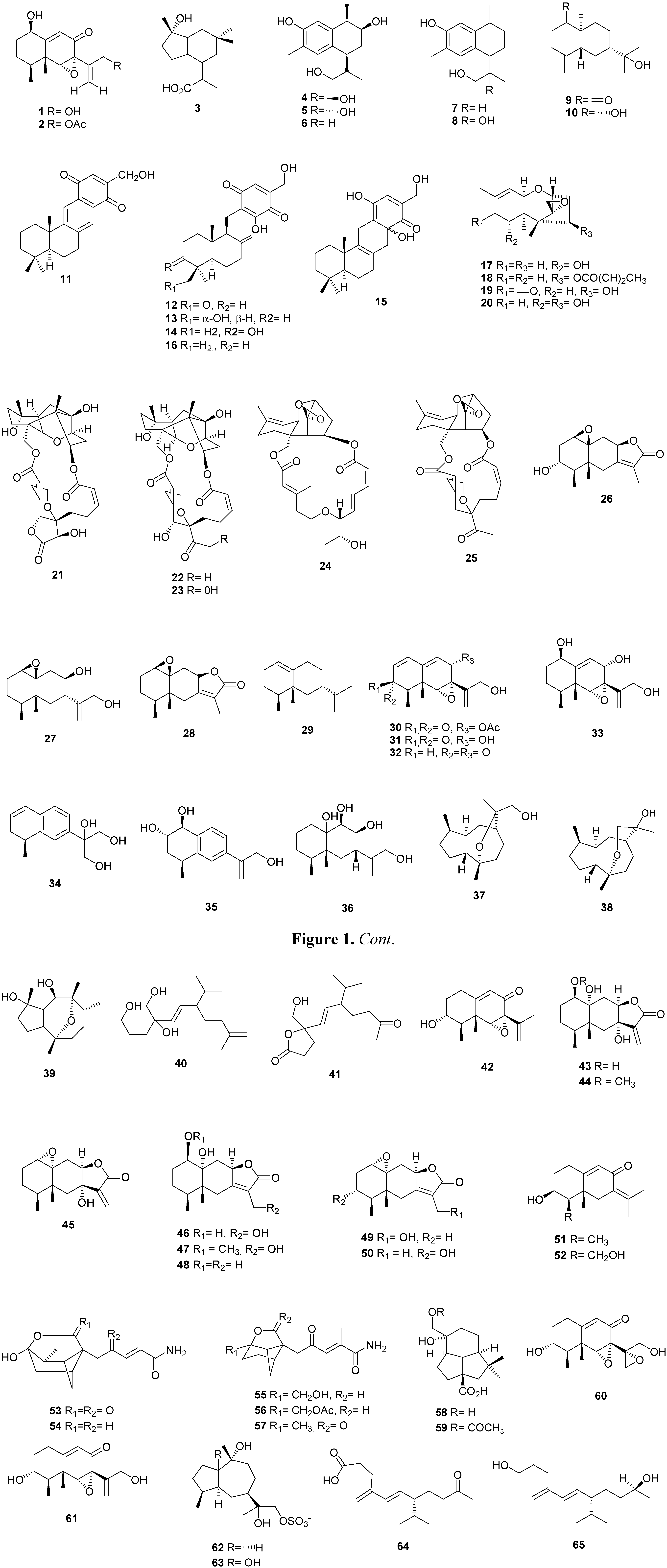
2.2. Diterpenes
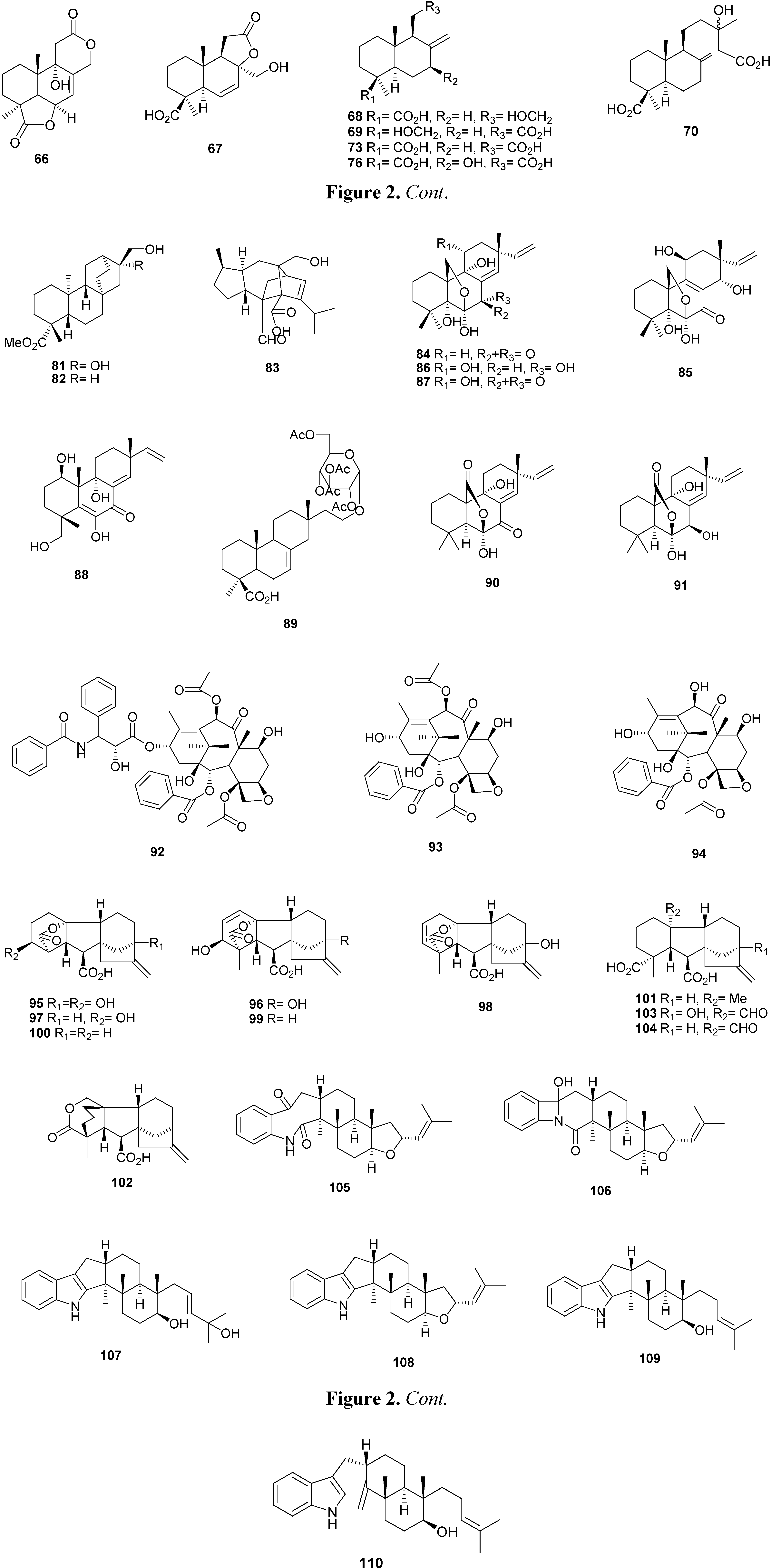
2.3. Meroterpenes

2.4. Other Terpenoids

3. Conclusions


Acknowledgements
References and Notes
- Li, L.-Y.; Ding, Y.; Groth, I.; Menzel, K.-D.; Peschel, G.; Voigt, K.; Deng, Z.-W.; Sattler, I.; Lin, W.-H. Pyrrole and indole alkaloids from an endophytic Fusarium incarnatum (HKI00504) isolated from the mangrove plant Aegiceras corniculatum. J. Asian Nat. Prod. Res. 2008, 10, 765–770. [Google Scholar] [CrossRef]
- Azevedo, J.L. Botânica: Uma ciência básica ou aplicada? Rev. Bras. Bot. 1999, 22, 225–229. [Google Scholar] [CrossRef]
- Petrini, O.; Sieber, T.N.; Toti, L.; Viret, O. Ecology, metabolite production, and substrate utilization in endophytic fungi. Nat. Toxins 1992, 1, 185–196. [Google Scholar]
- Qin, J.C.; Zhang, Y.M.; Gao, J.M.; Bai, M.S.; Yang, S.X.; Laatsch, H.; Zhang, A.L. Bioactive metabolites produced by Chaetomium globosum, an endophytic fungus isolated from Ginkgo biloba. Bioorg. Med. Chem. Lett. 2009, 19, 1572–1574. [Google Scholar]
- Macías-Rubalcava, M.L.; Hernández-Bautista, B.E.; Jiménez-Estrada, M.; González, M.C.; Glenn, A.E.; Hanlin, R.T.; Hernández-Ortega, S.; Saucedo-García, A.; Muria-González, J.M.; Anaya, A.L. Naphthoquinone spiroketal with allelochemical activity from the newly discovered endophytic fungus Edenia gomezpompae. Phytochemistry 2008, 69, 1185–1196. [Google Scholar]
- Chapela, I.H. Fungi in healthy stems and branches of American beech and aspen: A comparative study. New Phytol. 1989, 113, 65–75. [Google Scholar] [CrossRef]
- Lösgen, S.; Magull, J.; Schulz, B.; Draeger, S.; Zeeck, A. Isofusidienols: Novel chromone-3-oxepines produced by the endophytic fungus Chalara sp. Eur. J. Org. Chem. 2008, 4, 698–703. [Google Scholar]
- Guo, Z.; She, Z.; Shao, C.; Wen, L.; Liu, F.; Zheng, Z.; Lin, Y. 1H and 13C-NMR signal assignments of paecilin A and B, two new chromone derivatives from mangrove endophytic fungus Paecilomyces sp. (tree 1–7). Magn. Reson. Chem. 2007, 45, 777–780. [Google Scholar] [CrossRef]
- Lin, T.; Lin, X.; Lu, C.; Hu, Z.; Huang, W.; Huang, Y.; Shen, Y. Secondary metabolites of Phomopsis sp. XZ-26, an endophytic fungus from Camptotheca acuminate. Eur. J. Org. Chem. 2009, 2009, 2975–2982. [Google Scholar]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006, 23, 753. [Google Scholar] [CrossRef]
- Lin, X.; Lu, C.; Huang, Y.; Zheng, Z.; Su, W.; Shen, Y. Endophytic fungi from a pharmaceutical plant, Camptotheca acuminata: Isolation, identification and bioactivity. World J. Microbiol. Biotechnol. 2007, 23, 1037–1040. [Google Scholar] [CrossRef]
- Momesso, L.S.; Kawano, C.Y.; Ribeiro, P.H.; Nomizo, A.; Goldman, G.H.; Pupo, M.T. Chaetoglobosinas produzidas por Chaetomium globosum, fungo endofítico associado a Viguiera robusta GARDN. (Asteraceae). Quim. Nova 2008, 31, 1680–1685. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.L. Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef]
- Hu, Z.-Y.; Li, Y.-Y.; Huang, Y.-J.; Su, W.-J.; Shen, Y.-M. Three new sesquiterpenoids from Xylaria sp. NCY2. Helv. Chim. Acta 2008, 91, 46–52. [Google Scholar] [CrossRef]
- Silva, G.H.; Teles, H.L.; Zanardi, L.M.; Young, M.C.M.; Eberlin, M.N.; Hadad, R.; Pfenning, L.H.; Costa-Neto, C.M.; Castro-Gamboa, I.; Bolzani, V.S.; et al. Cadinane sesquiterpenoids of Phomopsis cassia, an endophytic fungus associated with Cassia spectabilis (Leguminoseae). Phytochemistry 2006, 67, 1964–1969. [Google Scholar]
- Isaka, M.; Palasarn, S.; Lapanun, S.; Chanthaket, R.; Boonyuen, N.; Lumyong, S. γ-Lactones and ent-eudesmane sesquiterpenes from the endophytic fungus Eutypella sp. BCC 13199. J. Nat. Prod. 2009, 72, 1720–1722. [Google Scholar] [CrossRef]
- Wijeratne, E.M.K.; Paranagama, P.A.; Marron, M.T.; Gunatilaka, M.K.; Arnold, A.E.; Gunatilaka, A.A.L. Sesquiterpene quinines and related metabolites from Phyllosticta spinarum, a fungal strain endophytic in Platycladus orientalis of the sonorant desert. J. Nat. Prod. 2008, 71, 218–222. [Google Scholar] [CrossRef]
- Chinworrungsee, M.; Wiyakrutta, S.; Sriubolmas, N.; Chuailua, P.; Suksamrarn, A. Cytotoxic activities of trichothecenes isolated from an endophytic fungus belonging to order hypocreales. Arch. Pharm. Res. 2008, 31, 611–616. [Google Scholar] [CrossRef]
- Shen, L.; Jiao, R.H.; Ye, Y.H.; Wang, X.T.; Xu, C.; Song, Y.C.; Zhu, H.L.; Tan, R.X. Absolute configuration of new cytotoxic and other bioactive trichothecene macrolides. Chem. Eur. J. 2006, 12, 5596–5602. [Google Scholar] [CrossRef]
- Amaral, L.S.; Rodrigues-Filho, E. Two novel eremophilane sesquiterpenes from an endophytic xylariaceous fungus isolated from leaves of Cupressus lusitanica. J. Braz. Chem. Soc. 2010, 21, 1446–1450. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Koseki, T.; Murayama, T.; Shiono, Y. Eremophilane sesquiterpenes from the endophyte Microdiplodia sp. KS 75-1and revision of the stereochemistries of phomadecalins C and D. Phytochem. Lett. 2010, 3, 148–151. [Google Scholar] [CrossRef]
- Xu, R.; Wang, M.-Z.; Lu, C.-H.; Zheng, Z.-H.; Shen, Y.-M. Tuberculariols A-C, new sesquiterpenes from the mutant strain M-741 of Tubercularia sp. TF 5. Helv. Chim. Acta 2009, 92, 1514–1519. [Google Scholar] [CrossRef]
- Wang, M.; Liu, S.; Li, Y.; Xu, R.; Lu, C.; Shen, Y. Protoplast mutation and genome shuffling induce the endophytic fungus Tubercularia sp. TF5 to produce new compounds. Curr. Microbiol. 2010, 61, 254–260. [Google Scholar]
- Isaka, M.; Chinthanom, P.; Boonruangprapa, T.; Rungjindamai, N.; Pinruan, U. Eremophilane-type sesquiterpenes from the fungus Xylaria sp. BCC 21097. J. Nat. Prod. 2010, 73, 683–687. [Google Scholar] [CrossRef]
- Sumarah, M.W.; Punuani, E.; Sorensen, D.; Blackwell, B.A.; Miller, J.D. Secondary metabolites from anti-insect extracts of endophytic fungi isolated from Picea rubens. Phytochemistry 2010, 71, 760–765. [Google Scholar]
- Liu, L.; Gao, H.; Chen, X.; Cai, X.; Yang, L.; Guo, L.; Yao, X.; Che, Y. Brasilamides A-D: Sesquiterpenoids from the plant endophytic fungus Paraconiothyrium brasiliense. Eur. J. Org. Chem. 2010, 17, 3302–3306. [Google Scholar]
- Silva, G.H.; Oliveira, C.M.; Teles, H.L.; Pauletti, P.M.; Gamboa, I.C.; Silva, D.H.S.; Bolzani, V.S.; Young, M.C.M.; Costa-Neto, C.M.; Pfenning, L.H.; et al. Sesquiterpenes from Xylaria sp., an endophytic fungus associated with Piper aduncum (Piperaceae). Phytochem. Lett. 2010, 3, 164–167. [Google Scholar] [CrossRef]
- Mei, W.-L.; Chen, P.; Wang, H.; Huang, J.-L.; Dai, H.-F. Two new sesquiterpenes from endophytic fungus S49 of Cephalotaxushainanensis. J. Asian Nat. Prod. Res. 2010, 12, 582–585. [Google Scholar] [CrossRef]
- Bashyal, B.P.; Gunatilaka, A.A.L. Tricinonoic acid and tricindiol, two new irregular sesquiterpenes from an endophytic strain of Fusarium tricinctum. Nat. Prod. Res. 2010, 24, 349–356. [Google Scholar] [CrossRef]
- Yuan, L.; Zhao, P.J.; Ma, J.; Lu, C.-H.; Shen, Y.-M. Labdane and tetranorlabdane diterpenoids from Botryosphaeria sp. MHF, an endophytic fungus of Maytenus hookeri. Helv. Chim. Acta 2009, 92, 1118–1125. [Google Scholar] [CrossRef]
- Wu, X.; Lu, C.-H.; Shen, Y.-M. Three new ent-trachylobane diterpenoids from co-cultures of the calli of Trewia nudiflora and Fusarium sp. WXE. Helv. Chim. Acta 2009, 92, 2783–2789. [Google Scholar] [CrossRef]
- Pongcharoen, W.; Rukachaisirikul, V.; Phongpaichit, S.; Kühn, T.; Pelzing, M.; Sakayaroj, J.; Taylor, W.C. Metabolites from the endophytic fungus Xylaria sp. PSU-D14. Phytochemistry 2008, 69, 1900–1902. [Google Scholar]
- Pongcharoen, W.; Rukachaisirikul, V.; Phongpaichit, S.; Rungjindamai, N.; Sakayaroj, J. Pimarane diterpene and cytochalasin derivatives from the endophytic fungus Eutypella scoparia PSU-D44. J. Nat. Prod. 2006, 69, 856–858. [Google Scholar] [CrossRef]
- Li, Y.; Lu, C.; Hu, Z.; Huang, Y.; Shen, Y. Secondary metabolites of Tubercularia sp. TF5, an endophytic fungal strain of Taxus mairei. Nat. Prod. Res. Part A 2009, 23, 70–76. [Google Scholar] [CrossRef]
- Weber, R.W.; Kappe, R.; Paululat, T.; Mösker, E.; Anke, H. Anti-Candida metabolites from endophytic fungi. Phytochemistry 2007, 68, 886–892. [Google Scholar]
- Zhao, K.; Ping, W.; Li, Q.; Hao, S.; Zhao, L.; Gao, T.; Zhou, D. Aspergillus niger var. taxi, a new species variant of taxol-producing fungus isolated from Taxus cuspidate in China. J. Appl. Microbiol. 2009, 107, 1202–1207. [Google Scholar] [CrossRef]
- Kumaran, R.S.; Muthumary, J.; Hur, B.-K. Isolation and identification of an anticancer drug, taxol from Phyllosticta tabernaemontanae, a leaf spot fungus of an angiosperm, Wrightia tinctoria. J. Microbiol. 2009, 47, 40–49. [Google Scholar] [CrossRef]
- Guo, B.H.; Wang, Y.C.; Zhou, X.W.; Hu, K.; Tan, F.; Miao, Z.Q.; Tang, K.X. An endophytic taxol-producing fungus BT2 isolated from Taxus chinensis var. mairei. Afr. J. Biotechnol. 2006, 5, 875–877. [Google Scholar]
- Kumaran, R.S.; Muthumary, J.; Hur, B.K. Production of taxol from Phyllosticta spinarum, an endophytic fungus of Cupressus sp. Eng. Life Sci. 2008, 8, 438–446. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, W.; Zhu, H.; Tang, K. Identification of a taxol-producing endophytic fungus EFY-36. Afr. J. Biotechnol. 2009, 8, 2623–2625. [Google Scholar]
- Miao, Z.; Wang, Y.; Yu, X.; Guo, B.; Tang, K. A new endophytic taxane production fungus from Taxus chinensis. Appl. Biochem. Microbiol. 2009, 45, 81–86. [Google Scholar] [CrossRef]
- Deng, B.W.; Liu, K.H.; Chen, W.Q.; Ding, X.W.; Xie, X.C. Fusarium solani, tax-3, a new endophytic taxol-producing fungus from Taxus chinensis. World J. Microbiol. Biotechnol. 2009, 25, 139–143. [Google Scholar] [CrossRef]
- Liu, K.; Ding, X.; Deng, B.; Chen, W. Isolation and characterization of endophytic taxol-producing fungi from Taxus chinensis. J. Ind. Microbiol. Biotechnol. 2009, 36, 1171–1177. [Google Scholar] [CrossRef]
- Li, Y.-C.; Tao, W.-Y. Cheng, L. Paclitaxel production using co-culture of Taxus suspension cells and paclitaxel-producing endophytic fungi in a co-bioreactor. Appl. Microbiol. Biotechnol. 2009, 83, 233–239. [Google Scholar] [CrossRef]
- Li, Y.-C.; Tao, W.-Y. Interactions of taxol-producing endophytic fungus with its host (Taxus spp.) during taxol accumulation. Cell Biol. Int. 2009, 54, 106–112. [Google Scholar]
- Kumaran, R.S.; Hur, B.-K. Screening of species of the endophytic fungus Phomopsis for the production of the anticancer drug taxol. Biotechnol. Appl. Biochem. 2009, 54, 21–30. [Google Scholar] [CrossRef]
- Chi, Y.; Zhao, D.-L.; Zhou, D.-P. Identification of taxol biosynthesis stage-enriched transcripts in Nodulisporium sylviforme, using suppression subtractive hybridization. World J. Microbiol. Biotechnol. 2008, 24, 2601–2605. [Google Scholar] [CrossRef]
- Li, Y.-C.; Tao, W.-Y. Paclitaxel-producing fungal endophyte stimulates the accumulation of taxoids in suspension cultures of Taxus cuspidate. Sci. Hortic. 2009, 121, 97–102. [Google Scholar]
- Zhang, P.; Zhou, P.-P.; Yu, L.-J. An endophytic taxol-producing fungus from Taxus media, Cladosporium cladosporioides MD2. Curr. Microbiol. 2009, 59, 227–232. [Google Scholar]
- Sreekanth, D.; Syed, A.; Sarkar, S.; Sarkar, D.; Santhakumari, B.; Ahmad, A.; Khan, M.I. Production, purification, and characterization of taxol and 10-DABIII from a new endophytic fungus Gliocladium sp. isolated from the Indian yew tree Taxus baccata. J. Microbiol. Biotechnol. 2009, 19, 1342–1347. [Google Scholar]
- Gangadevi, V.; Muthumary, J. Taxol production by Pestalotiopsis terminaliae, an endophytic fungus of Terminalia arjuna (arjun tree). Biotechnol. Appl. Biochem. 2009, 52, 9–15. [Google Scholar] [CrossRef]
- Gangadevi, V.; Muthumary, J. Taxol, an anticancer drug produced by an endophytic fungus Bartalinia robillardoids Tassi, isolated from a medicinal plant, Aegle marmelos Correa ex Roxb. World J. Microbiol. Biotechnol. 2008, 24, 717–724. [Google Scholar] [CrossRef]
- Kumaran, R.S.; Muthumary, J.; Hur, B.-K. Isolation and identification of taxol, an anticancer drug from Phyllosticta melochiae Yates, an endophytic fungus of Melochia corchorifolia L. Food Sci. Biotechnol. 2008, 17, 1246–1253. [Google Scholar]
- Hamayun, M.; Khan, S.A.; Khan, M.A.; Khan, A.L.; Kang, S.-M.; Kim, S.-K.; Joo, G.-J.; Lee, I.-J. Gibberellins production by pure cultures of a new strain of Aspergillus fumigates. World J. Microbiol. Biotechnol. 2009, 25, 1785–1792. [Google Scholar] [CrossRef]
- Khan, S.A.; Hamayun, M.; Kim, H.-Y.; Yoon, H.-J.; Seo, J.-C.; Choo, Y.-S.; Lee, I.-J.; Kim, S.-D.; Rhee, I.-K.; Kim, J.-G. A new strain of Arthrinium phaeospermum isolated from Carex kobomugi Ohwi is capable of gibberellins production. Biotechnol. Lett. 2009, 31, 283–287. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, S.A.; Ahmad, N.; Tang, D.-S.; Kang, S.-M.; Na, C.-I.; Sohn, E.-Y.; Hwang, Y.-H.; Shin, D.-H.; Lee, B.-H.; et al. Cladosporium sphaerospermum as a new plant growth-promoting endophyte from the roots of Glycine max (L.) Merr. World J. Microbiol. Biotechnol. 2009, 25, 627–632. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, S.A.; Kim, H.-Y.; Chaudhary, M.F.; Hwang, Y.-H.; Shin, D.-H.; Kim, I.-K.; Lee, B.-H.; Lee, I.-J. Gibberellin production and plant growth enhancement by newly isolated strain of Scolecobasidium tshawytschae. J. Microbiol. Biotechnol. 2009, 19, 560–565. [Google Scholar]
- Qiao, M.-F.; Ji, N.-Y.; Liu, X.-H.; Li, K.; Zhu, Q.-M.; Xue, Q.-Z. Indoloditerpenes from an algicolous isolate of Aspergillus oryzae. Bioorg. Med. Chem. Lett. 2010, 20, 5677–5680. [Google Scholar] [CrossRef]
- Fill, T.P.; Pereira, G.K.; Santos, M.G.; Rodrigues-Filho, E. Four additional meroterpenes produced by Penicillium sp. found in association with Melia azedarach. Possible biosynthetic intermediates to Austin. Z. Naturforsch. B: Chem. Sci. 2007, 62, 1035–1044. [Google Scholar]
- Fill, T.P.; Santos, R.M.G.; Barisson, A.; Rodrigues-Filho, E.; Souza, A.Q.L. Co-production of bisphenylpropanoid amides and meroterpenes by an endophytic Penicillium brasilianum found in the root bark of Melia azedarach. Z. Naturforsch. C: Biosci. 2009, 64, 355–360. [Google Scholar]
- Yuan, L.; Zhao, P.-J.; Ma, J.; Li, G.-H.; Shen, Y.-M. Tricycloalternarenes A–E: Five new mixed terpenoids from the endophytic fungal strain Alternaria alternate Ly83. Helv. Chim. Acta 2008, 91, 1588–1594. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, Q.; Sun, F.-M.; An, T. Gentiopicrin-producing endophytic fungus isolated from Gentiana macrophylla. Phytomedicine 2009, 16, 793–797. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lin, C.-W.; Chen, G.-Y.; Ou, G.-C. 3β-Hydroxyfriedela-17β-carboxylic acid. Acta Crystallogr. Sect. E: Struct. Rep. Online 2008, 64, 890–894. [Google Scholar]
- Zhao, J.; Mou, Y.; Shan, T.; Li, Y.; Zhou, L.; Wang, M.; Wang, J. Antimicrobial metabolites from the endophytic fungus Pichia guilliermondii isolated from Paris polyphylla var. yunnanensis. Molecules 2010, 15, 7961–7970. [Google Scholar] [CrossRef]
- Jarvis, B.B.; Midiwo, J.O.; Bean, G.A.; Aboul-Nasr, M.B.; Barros, C.S. The mystery of trichothecene antibiotics in Baccharis species. J. Nat. Prod. 1988, 51, 736–744. [Google Scholar] [CrossRef]
- Habermehl, G.G. Secondary and tertiary metabolites as plant toxins. Toxicon 1998, 36, 1707–1719. [Google Scholar] [CrossRef]
- Santos-Filho, F.C.; Amaral, L.S.; Rodrigues-Filho, E. Composition of essential oil from Cupressus lusitanica and a Xylariaceous fungus found on its leaves. Biochem. Syst. Ecol. 2011, 39, 485–490. [Google Scholar] [CrossRef]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Cogoon, P.; Mcphail, A.T. Plant as antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Amer. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar]
- Harborne, J.B. Advances in chemical ecology. Nat. Prod. Rep. 1993, 10, 327–348. [Google Scholar] [CrossRef]
- Mahato, S.B.; Sen, S. Advances in triterpenoids research, 1990–1994. Phytochemistry 1997, 44, 1185–1236. [Google Scholar]
- Sample Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Souza, J.J.d.; Vieira, I.J.C.; Rodrigues-Filho, E.; Braz-Filho, R. Terpenoids from Endophytic Fungi. Molecules 2011, 16, 10604-10618. https://doi.org/10.3390/molecules161210604
Souza JJd, Vieira IJC, Rodrigues-Filho E, Braz-Filho R. Terpenoids from Endophytic Fungi. Molecules. 2011; 16(12):10604-10618. https://doi.org/10.3390/molecules161210604
Chicago/Turabian StyleSouza, Jucimar Jorgeane de, Ivo José Curcino Vieira, Edson Rodrigues-Filho, and Raimundo Braz-Filho. 2011. "Terpenoids from Endophytic Fungi" Molecules 16, no. 12: 10604-10618. https://doi.org/10.3390/molecules161210604



