Chemical Constituents of the Methanolic Extract of Leaves of Leiothrix spiralis Ruhland and Their Antimicrobial Activity
Abstract
:1. Introduction
2. Results and Discussion
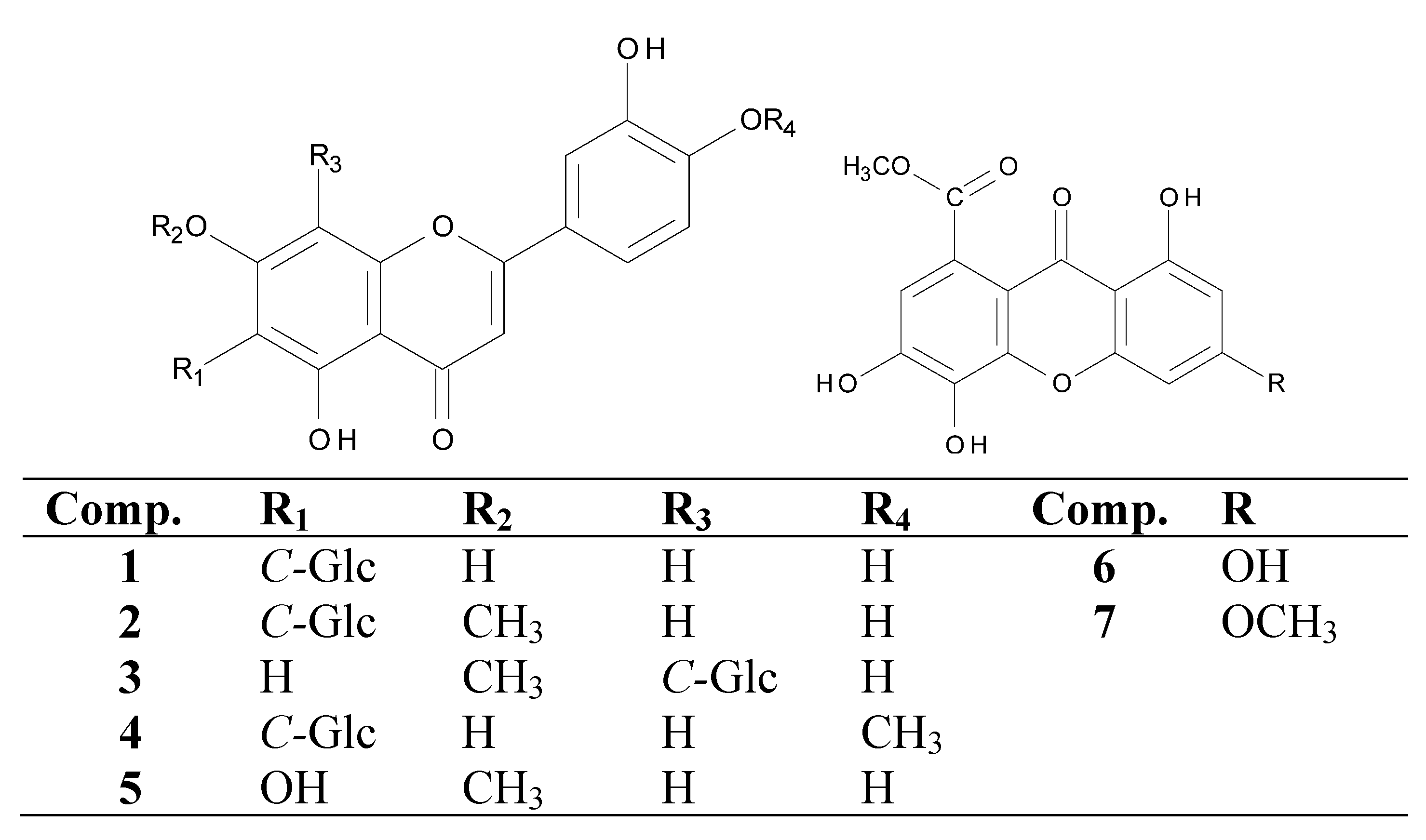
| Tested samples | MIC (MBC)a | MIC (MFC) a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram-positive bacteria | Gram-negative bacteria | Fungi | |||||||||
| S. aureus | B. subtilis | E. faecalis | E. coli | P. aeruginosa | S. setubal | C. albicans | C. krusei | C. parapsilosis | C. tropicalis | ||
| Extract | 1000 (1000) | 1000 (1000) | 500 (-) | - | - | - | 1000 (1000) | 500 (1000) | 250 (250) | 1000 (1000) | |
| Fraction (5 + 6) | 125 (250) | 250 (250) | -* | 62.5 (250) | 31.25 (250) | -* | -* | -* | 125 (125) | -* | |
| Fraction (2 + 3) | -* | -* | -* | -* | -* | -* | -* | 250 (250) | 125 (125) | 250 (250) | |
| (1) | -* | -* | -* | -* | -* | -* | -* | -* | 125 (125) | -* | |
| (4) | -* | -* | -* | -* | -* | -* | 125 (250) | -* | -* | -* | |
| (7) | 125 (-*) | 125 (125) | -* | -* | 125 (250) | -* | 62.5 (250) | 15.7(15.7) | 15.7 (15.7) | 31.25 (62.5) | |
| Positive controls b | 2.1 × 10−2 | 2.1 × 10−2 | 2.1 × 10−2 | 3.3 × 10−5 | 3.3 × 10−5 | 3.3 × 10−5 | 32 (>64) | 8 (>64) | 8 (>64) | 32 (>64) | |
| (2.1 × 10−2) | (2.1 × 10−2) | (2.1 × 10−2) | (3.3 × 10−5) | (3.3 × 10−5) | (3.3 × 10−5) | ||||||
3. Experimental
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Antibacterial Activity and Minimum Bactericidal Concentration (MBC)
3.5. Antifungal Activity and Minimum Fungicidal Concentration (MFC)
3.6. Anti-Helicobacter pylori Activity
4. Conclusions
Conflict of Interest
Acknowledgements
References and Notes
- Giulietti, A.M.; Amaral, M.C.E.; Bittrich, V. Phylogenetic analysis of inter-and infrageneric relationships of Leiothrix Ruhland (Eriocaulaceae). Kew Bull. 1995, 50, 55–71. [Google Scholar] [CrossRef]
- Coelho, R.G.; Batista, L.M.; Santos, L.C.; Souza-Brito, A.R.M.; Vilegas, W. Phytochemical study and antiulcerogenic activity of Syngonanthus bisulcatus (Eriocaulaceae). Rev. Bras. Cienc. Farm. 2006, 42, 413–417. [Google Scholar]
- Hoehne, F.C. Outras monocotyledoneas tóxicas ou suspeitas. In Plantas e substâncias vegetais tóxicas e medicinais; Hoehne, F.C., Ed.; Graphicards: São Paulo/Rio de Janeiro, Brazil, 1939; p. 97. [Google Scholar]
- Santos, L.C.; Piacente, S.; Cosimo, P.; Montoro, P.; Vilegas, V. Antioxidante de xantonas isoladas de espécies de Leiothrix (Eriocaulaceae). Rev. Braz. Farmacog. 2003, 13, 67–74. [Google Scholar]
- Varanda, E.A.; Raddi, M.S.G.; Dias, F.L.P.; Araujo, M.C.S.; Gibran, S.C.A.; Takahashi, C.S.; Vilegas, W. Mutagenic and cytotoxic activity of an isocoumarin (paepalantine) isolated from Paepalanthus vellozioides. Teratog. Carc. Mut. 1997, 17, 85–95. [Google Scholar] [CrossRef]
- Tavares, D.C.; Varanda, E.A.; Andrade, F.P.D.; Vilegas, W.; Takahashi, C.S. Evaluation of the genotoxic potential of the isocoumarin paepalantine in in vivo and in vitro mammalian systems. J. Ethnopharmacol. 1999, 68, 115–120. [Google Scholar]
- Batista, L.M.; Almeida, A.B.; Pietro, L.M.; Toma, W.; Calvo, T.R.; Vilegas, W.; Souza, A.R.B. Gastric antiulcer activity of Syngonanthus arthrotrichus Silveira. Biol. Pharm. Bull. 2004, 27, 328–332. [Google Scholar] [CrossRef]
- Sano, P.T. Actinocephalus (Koern.) Sano (Paepalanthus sect. Actinocephalus), a new genus of Eriocaulaceae, and other taxonomic and nomenclatural changes involving Paepalanthus Mart. Taxon 2004, 53, 99–107. [Google Scholar]
- Dokkedal, A.L.; Salatino, A. Flavonoids of Brazilian species of Leiothrix (Eriocaulaceae). Biochem. Syst. Ecol. 1992, 20, 31–32. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.; Giulietti, A.M. Contents of soluble phenolic compounds of capitula of Eriocaulaceae. Quim. Nova 1990, 13, 289–292. [Google Scholar]
- Santos, L.C.; Piacente, S.; Montoro, P.; Pizza, C.; Vilegas, W. Atividade antioxidante de xantonas isoladas de espécies de Leiothix (Eriocaulaceae). Rev. Bras. Farmacog. 2003; 13, 67–74. [Google Scholar]
- Vilegas, W.; Dokkedal, A.L.; Rastrelli, L.; Piacente, S.; Pizza, C. New naphtopyranone glycoside from Paepalanthus vellozioides and Paepalanthus latipes. J. Nat. Prod. 1999, 62, 746–749. [Google Scholar] [CrossRef]
- Santos, L.C.; Piacente, S.; Riccardis, F.D.; Eletto, A.M.; Pizza, C.; Vilegas, W. Xanthones and flavonoids from Leiothrix curvifolia and Leiothrix flavescens. Phytochemistry 2001, 56, 853–856. [Google Scholar]
- Ricci, C.V.; Patricio, M.C.B.; Salatino, M.L.F.; Salatino, A.; Giulietti, A.M. Flavonoids of Syngonanthus Ruhl. (Eriocaulaceae): Taxonomic implications. Biochem. System. Ecol. 1996, 24, 577–583. [Google Scholar] [CrossRef]
- Elix, J.A.; Bennett, S.A. 6-O-methylarthothelin and 1,3,6-tri-O-methylarthothelin, two new xanthones from a Dimelaena lichen. Aust. J. Chem. 1990, 43, 1587–1590. [Google Scholar] [CrossRef]
- Tanahashi, T.; Takenaka, Y.; Ikuta, Y.; Tani, K.; Nagakura, N.; Hamada, N. Xanthones from the cultured lichen mycobionts of Pyrenula japonica and Pyrenula pseudobufonia. Phytochemistry 1999, 52, 401–405. [Google Scholar]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.G.; Nakamura, C.V.; Filho, B.P.D. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. Inst. Oswaldo Cruz 2002, 97, 1027–1031. [Google Scholar] [CrossRef]
- Gibbons, S. Phytochemicals for bacterial resistance-strengths, weaknesses and opportunities. Planta Med. 2008, 74, 594–602. [Google Scholar] [CrossRef]
- Lee, Y.J.; Liu, H.Y.; Lin, Y.C.; Sun, K.L.; Chun, C.L.; Hsueh, P.R. Fluoroquinolone resistance of Pseudomonas aeruginosa isolates causing nosocomial infection is correlated with levofloxacin but not ciprofloxacin use. Int. J. Antimicrob. Agents 2010, 35, 261–264. [Google Scholar] [CrossRef]
- Micek, S.T.; Lloyd, A.E.; Ritchie, D.J.; Reichley, R.M.; Fraser, V.J.; Kollef, M.H. Pseudomonas aeruginosa bloodstream infection: Importance of appropriate initial antimicrobial treatment. Antimicrob. Agents Chemother. 2005, 49, 1306–1311. [Google Scholar]
- Fotie, J.; Bohle, S. Pharmacological and biological activities of xanthones. Curr. Med. Chem. 2006, 5, 15–31. [Google Scholar]
- Marona, H.; Szkaradek, N.; Karczewska, E.; Trojanowska, D.; Budak, A.; Bober, P.; Przepirka, W.; Cegla, M.; Szneler, E. Antifungal and antibacterial activity of the newly synthesized 2-xanthone derivatives. Arch. Pharm. 2009, 342, 9–18. [Google Scholar] [CrossRef]
- Pinto, M.M.M.; Castanheiro, R. Natural Prenylated Xanthones: Chemistry and Biological Activities. In Natural Products: Chemistry, Biochemistry and Pharmacology; Brahmachari, G., Ed.; Narosa Publishing House PVT. LTD: New Dehli, India, 2009; pp. 520–675, Chapter 11. [Google Scholar]
- Pinto, E.; Afonso, C.; Duarte, S.; Vale-Silva, L.; Costa, E.; Sousa, E.; Pinto, M. Antifungal activity of xanthones: Evaluation of their effect on ergosterol biosynthesis by High-Performance Liquid Chromatography. Chem. Biol. Drug Des. 2011, 77, 212–222. [Google Scholar] [CrossRef]
- Dambolena, J.S.; Zygadlo, J.A.; Rubinstein, H.R. Antifumonisin activity of natural phenolic compounds. A structure-property-activity relationship study. Int. J. Food Microbiol. 2011, 145, 140–146. [Google Scholar] [CrossRef]
- Fang, J.J.; Ye, G.; Chen, W.L.; Zhao, W.M. Antibacterial phenolic components from Eriocaulon buergerianum. Phytochemistry 2008, 69, 1279–1286. [Google Scholar]
- Hernández, N.E.; Tereschuk, M.L.; Abdala, L.R. Antimicrobial activity of flavonoids in medicinal plants from Tafí del Valle (Tucumán, Argentina). J. Ethnopharmacol. 2000, 73, 317–322. [Google Scholar] [CrossRef]
- Kuete, V.; Ngameni, B.; Simo, C.C.F.; Tankeu, R.K.; Ngadjui, B.T.; Meyer, J.J.M.; Lall, N.; Kuiate, R.R. Antimicrobial activity of the crude extracts and compounds from Ficus chlamydocarpa and Ficus cordata (Moraceae). J. Ethnopharmacol. 2008, 120, 17–24. [Google Scholar]
- Mbaveng, A.T.; Ngameni, B.; Kuete, V.; Simo, I.K.; Ambassa, T.; Roy, R.; Bezabih, M.; Etoa, F.X.; Ngadjui, B.T.; Abegaz, B.M.; et al. Antimicrobial activity of the crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moraceae). J. Ethnopharmacol. 2008, 116, 483–489. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- da Silva, M.A.; Cardoso, C.A.; Vilegas, W.; dos Santos, L.C. High-performance liquid chromatographic quantification of flavonoids in Eriocaulaceae species and their antimicrobial activity. Molecules 2009, 14, 4644–4654. [Google Scholar]
- Moraes, T.M.; Silva, M.A.; Rodrigues, C.M.; Santos, L.C.; Sannomiy, M.; Rocha, L.R.M.; Brito, A.R.M.S.; Bauab, T.M.; Vilegas, W.; Hiruma-Lima, C.A. Antioxidant, Antisecretory and Gastroprotective Activities from Leiothrix Flavescens. In Comprehensive Bioactive Natural Products. Efficacy, Safety & Clinical Evaluation I.; Gupta, V.K., Ed.; Studium Press LLC: Houston, TX, USA, 2010; Volume 2, p. 265, Chapter 13. [Google Scholar]
- Rinaldo, D.; Rodrigues, C.M.; Rodrigues, J.; Sannomiya, M.; dos Santos, L.C.; Vilegas, W. New flavone from the leaves of Neea theifera (Nyctaginaceae). J. Braz. Chem. Soc. 2007, 18, 1132–1135. [Google Scholar] [CrossRef]
- Miyake, Y.; Yamamoto, K.; Morimitsu, Y.; Osawa, T. Isolation of C-glucosylflavone from lemon peel and antioxidative activity of flavonoid compounds in lemon fruit. J. Agric. Food Chem. 1997, 45, 4619–4623. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Zhou, Z.; Lai, C.S.; Zhang, L.; Quan, Z.; Shao, X.; Pan, M.H.; Ho, C.T. Flavonoids from Rabdosia rubescens exert anti-inflammatory and growth inhibitory effect against human leukemia HL-60 cells. Food Chem. 2010, 122, 831–835. [Google Scholar] [CrossRef]
- Bjoroy, O.; Rayyan, S.; Fossen, T.; Kalberg, K.; Andersen, O.M. C-glycosylanthocyanidins synthesized from C-glycosylflavones. Phytochemistry 2009, 70, 278–287. [Google Scholar]
- Wang, R.F.; Yang, X.W.; Ma, C.M.; Liu, H.Y.; Shang, M.Y.; Zhang, Q.Y.; Cai, S.Q.; Park, J.H. Trollioside, a new compound from the flowers of Trollius chinensis. J. Asian Nat. Prod. Res. 2004, 6, 139–144. [Google Scholar] [CrossRef]
- Ramesh, P.; Nair, A.G.R.; Subramanian, S.S. Flavone glycosides of Vitex trifolia. Fitoterapia 1986, 57, 282–283. [Google Scholar]
- Clinical and Laboratory Standards Institute document M7-A8. In Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard, 7th ed; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009.
- Kuete, V.; Nana, F.; Ngameni, B.; Mbaveng, A.T.; Keumedjio, F.; Ngadjui, B.T. Antimicrobial activity of the crude extract, fractions and compounds from stem bark of Ficus ovata (Moraceae). J. Ethnopharmacol. 2009, 124, 556–561. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute document M27-A3. In Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard, 3th ed; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 22.
- Mégraud, F.; Lehours, P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin. Microbiol. Rev. 2007, 20, 280–322. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute document M02-A10. In Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard, 10th ed; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009.
- Sample Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Araújo, M.G.d.F.; Hilário, F.; Nogueira, L.G.; Vilegas, W.; Santos, L.C.d.; Bauab, T.M. Chemical Constituents of the Methanolic Extract of Leaves of Leiothrix spiralis Ruhland and Their Antimicrobial Activity. Molecules 2011, 16, 10479-10490. https://doi.org/10.3390/molecules161210479
Araújo MGdF, Hilário F, Nogueira LG, Vilegas W, Santos LCd, Bauab TM. Chemical Constituents of the Methanolic Extract of Leaves of Leiothrix spiralis Ruhland and Their Antimicrobial Activity. Molecules. 2011; 16(12):10479-10490. https://doi.org/10.3390/molecules161210479
Chicago/Turabian StyleAraújo, Marcelo Gonzaga de Freitas, Felipe Hilário, Leonardo Gorla Nogueira, Wagner Vilegas, Lourdes Campaner dos Santos, and Taís Maria Bauab. 2011. "Chemical Constituents of the Methanolic Extract of Leaves of Leiothrix spiralis Ruhland and Their Antimicrobial Activity" Molecules 16, no. 12: 10479-10490. https://doi.org/10.3390/molecules161210479




