Radical-Scavenging Activity of Dietary Phytophenols in Combination with co-Antioxidants Using the Induction Period Method
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Radical-Scavenging Activity
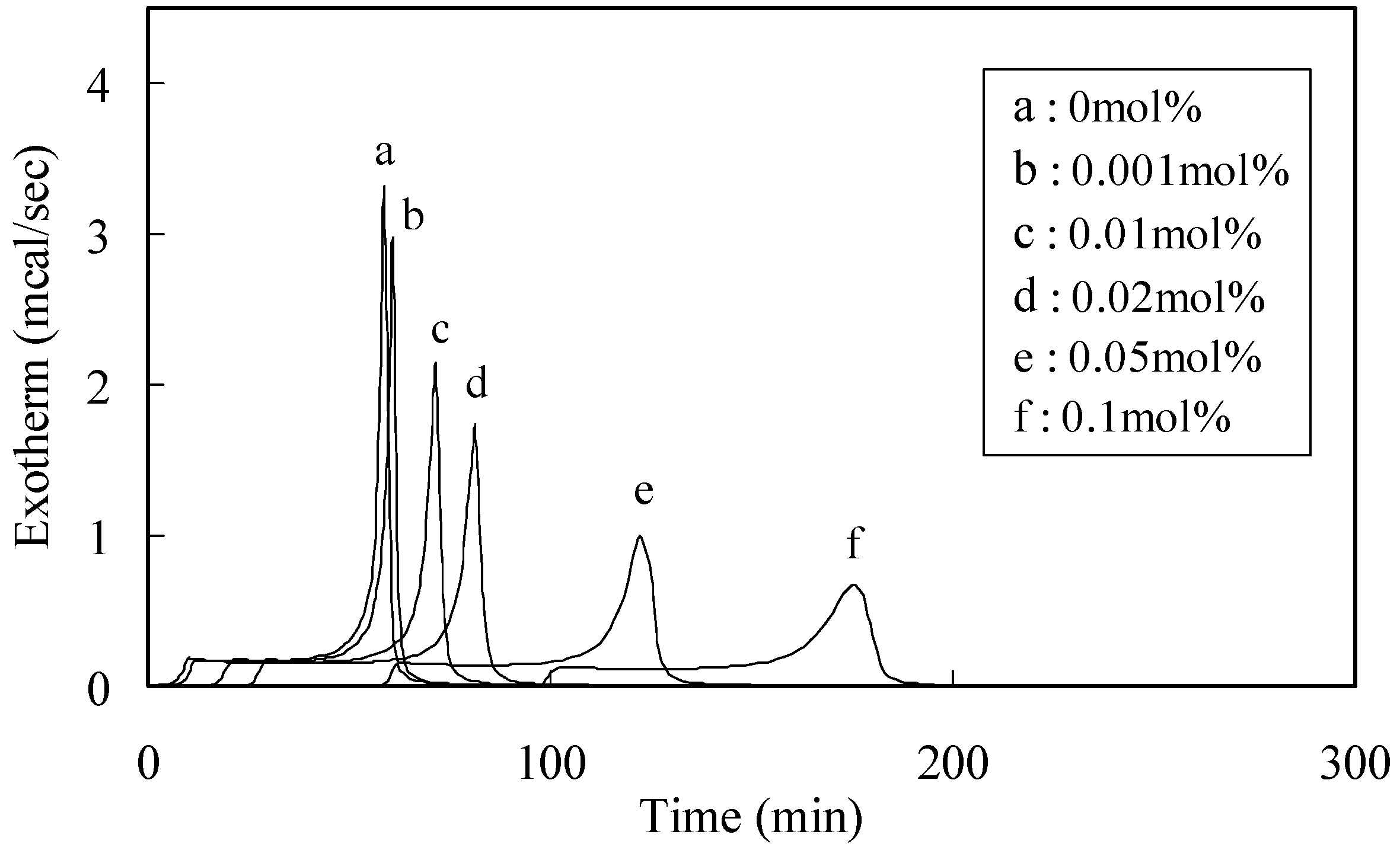
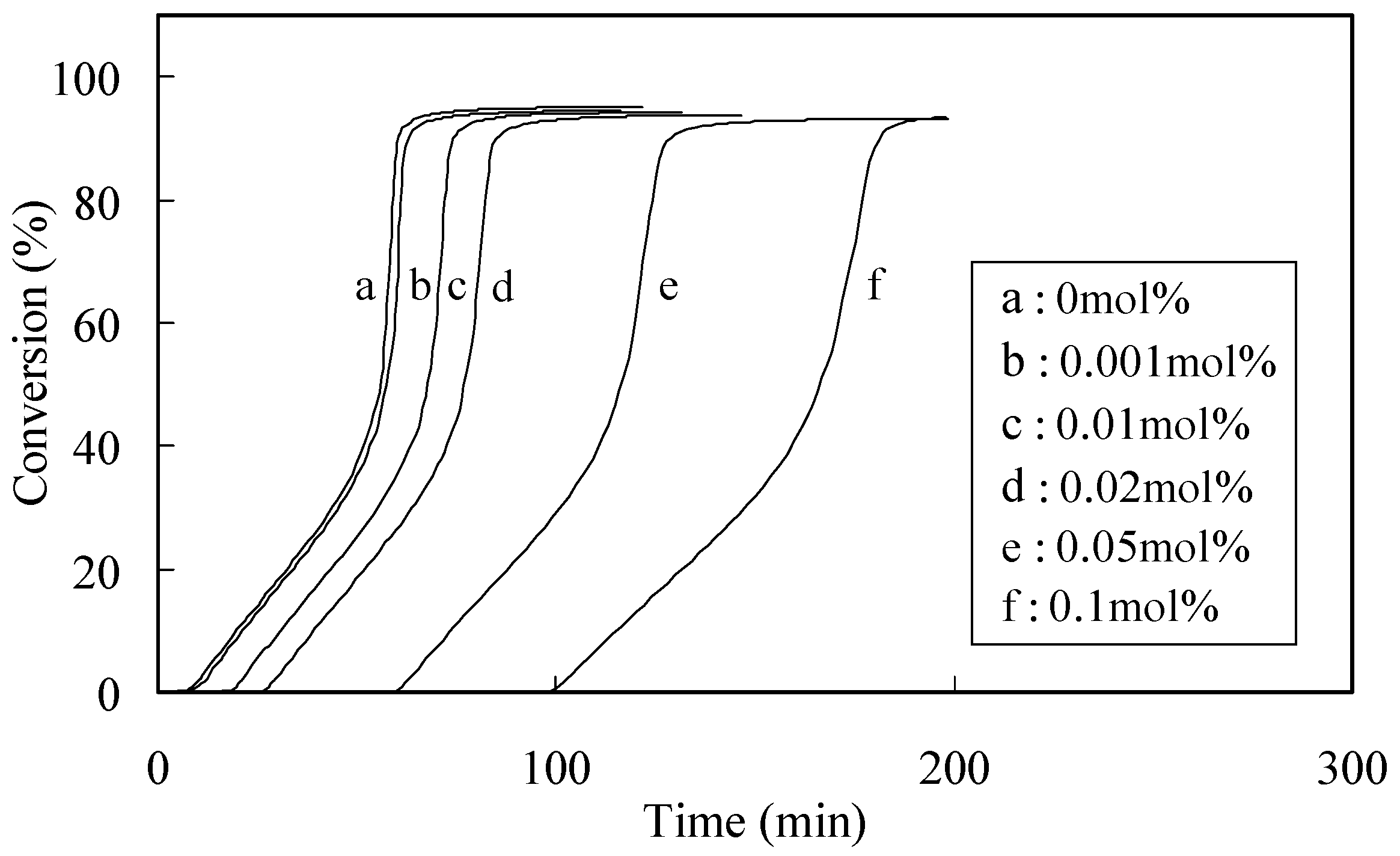
| Phenolic antioxidants | Induction period method a) | DPPH method b) | ||
|---|---|---|---|---|
| kinh × 10−3 | ||||
| n | (M−1s−1) | KCL c) | EC50 (mM) | |
| A) Phytophenols | ||||
| EGC | 2.6 | 0.85 | 478 | 0.01 |
| EGCG | 5.0 | 0.50 | 420 | 0.003 |
| Eugenol | 1.4 | 1.01 | 587 | 0.082 |
| Caffeic acid | 1.8 | 1.07 | 569 | 0.027 |
| Capsaicin | 0.6 | 1.49 | 570 | _ |
| Catechin | 3.2 | 0.66 | 501 | _ |
| Chlorogenic acid | 1.7 | 0.93 | 587 | _ |
| p-Coumaric acid | 1.1 | 1.71 | 541 | _ |
| Curcumin | 2.5 | 0.68 | 550 | 0.043 |
| trans-Cinnamic acid | nar | nar | nar | _ |
| Hesperetin | 1.0 | 2.21 | 548 | _ |
| Isoeugenol | 1.8 | 0.87 | 610 | 0.056 |
| Ferulic acid | 1.6 | 1.20 | 560 | 0.145 |
| n-Propyl gallate | 1.2 | 1.31 | 585 | 0.007 |
| Quercetin | 1.8 | 0.98 | 600 | 0.017 |
| Resveratrol | 2.3 | 0.81 | 567 | 0.11 |
| Tetrahydrocurcumin | 3.3 | 0.79 | 584 | 0.035 |
| B) Synthetic phenols | ||||
| BHT | 1.9 | 0.79 | 583 | 0.1 |
| Bisphenol A | 2.5 | 0.81 | 563 | _ |
| p-Cresol | 1.7 | 1.04 | 487 | _ |
| DPPH | 0.8 | 3.11 | 606 | _ |
| Galvinoxyl | 0.3 | 8.24 | 578 | _ |
| Hydroquinone (HQ) | 1.0 | 7.02 | 454 | |
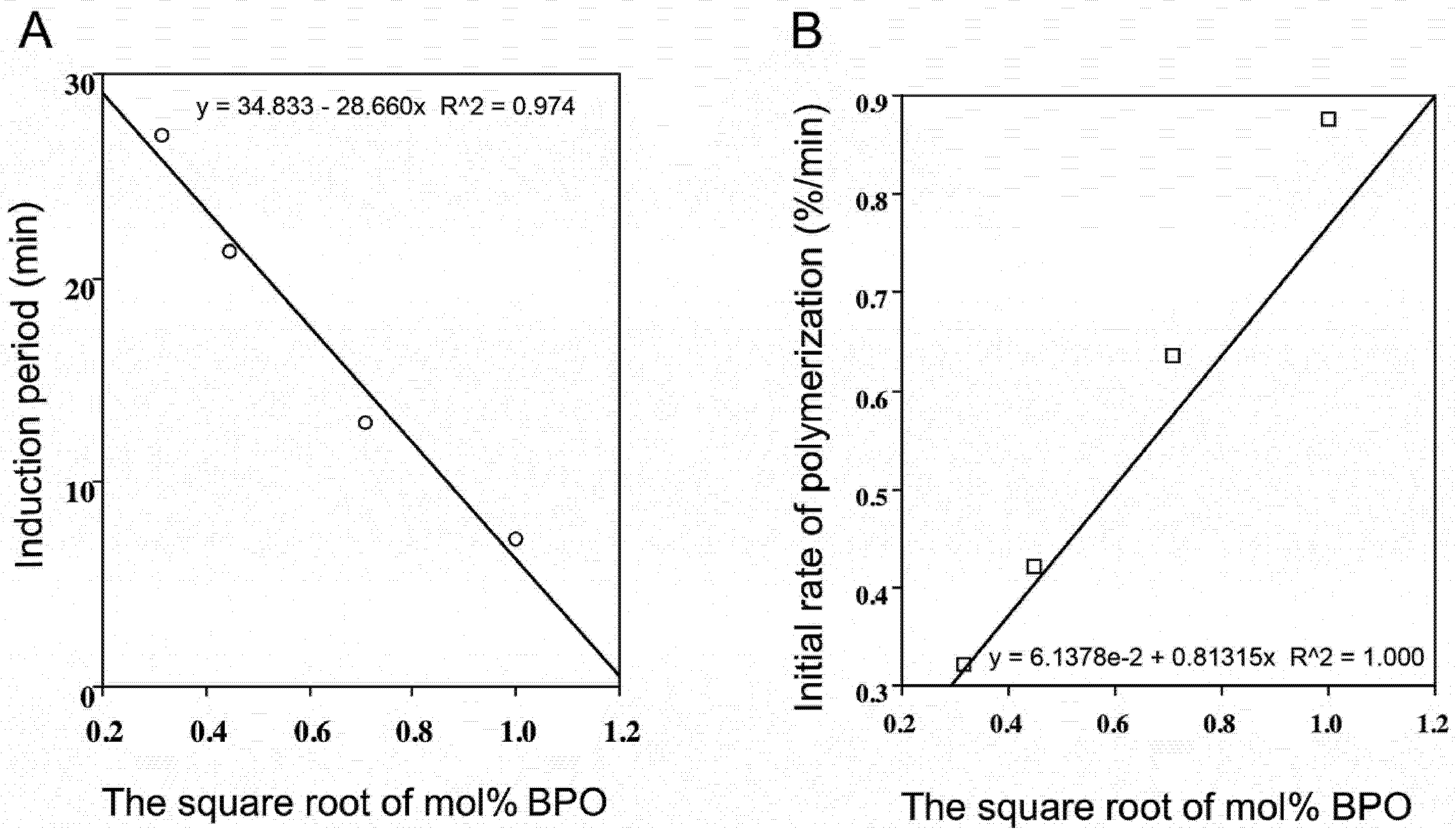
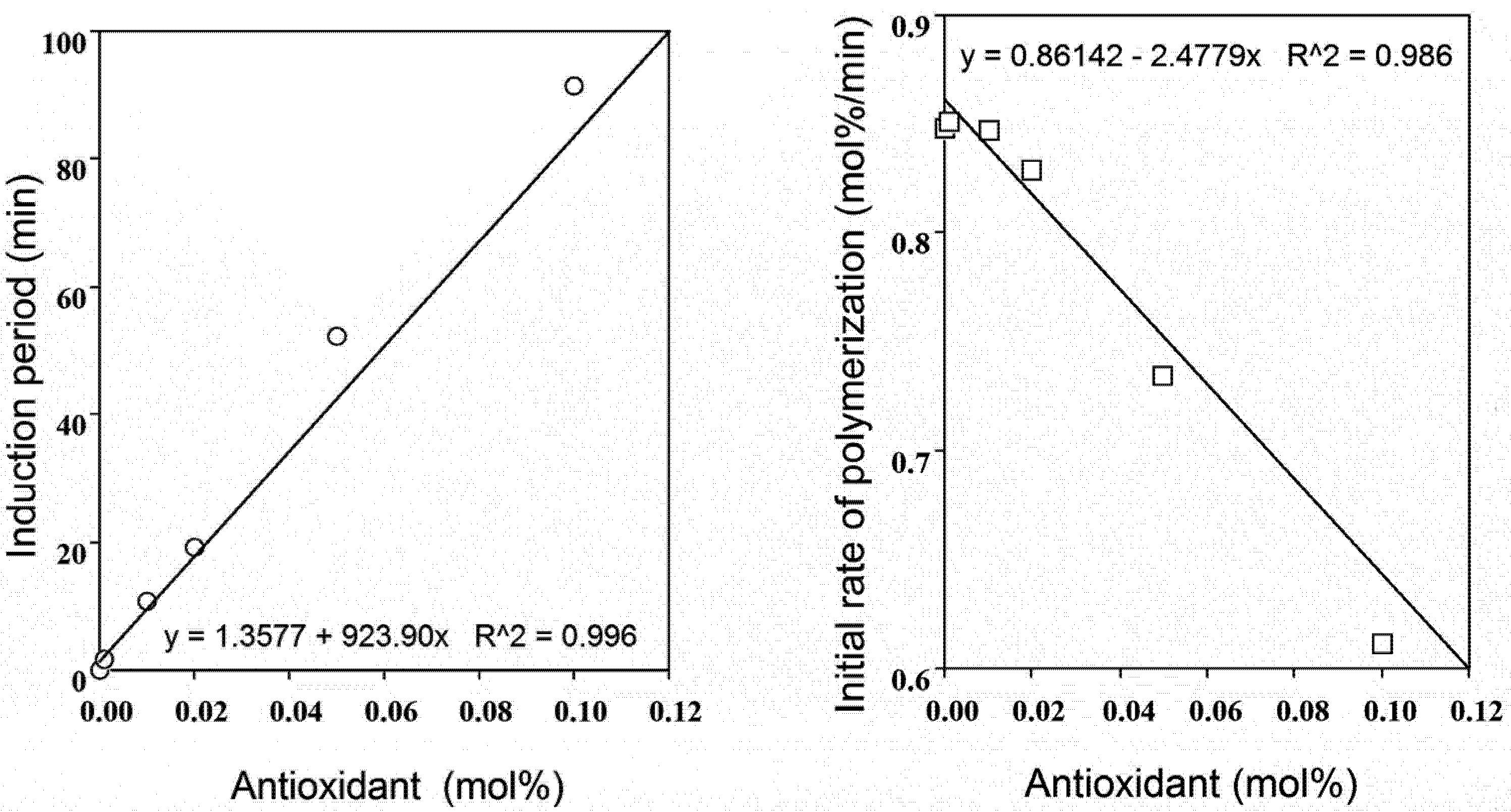
2.2. Radical-Scavenging Activity
2.3. Polyphenol Combinations
| Polyphenols a) | Induction period (IP) | Rp b) | Conversion | |||
|---|---|---|---|---|---|---|
| (min) | (min) | (%/min) | (%) | |||
| Observed (A) | Calculated (B) | B/A | ||||
| Control | 6.958 | _ | _ | 0.973 | 95.0 | |
| Epicatechin (EC) | 35.005 | _ | _ | 0.875 | 93.0 | |
| Epigallocatechin (EGC) | 30.188 | _ | _ | 0.873 | 93.9 | |
| Catechin | 34.314 | _ | _ | 0.888 | 93.2 | |
| Quercetin | 25.027 | _ | _ | 0.893 | 94.2 | |
| EC + Catechin | 69.623 | 69.319 | 1.000 | 0.797 | 92.4 | |
| EC + EGC | 60.301 | 65.193 | 0.924 | 0.866 | 92.3 | |
| EC + Quercetin | 49.968 | 65.133 | 0.767 * | 0.901 | 93.0 | |
2.4. Polyphenol: Co-Antioxidant Combination
| Polyphenols a) | Induction period | Rp b) | Conversion | ||
|---|---|---|---|---|---|
| (min) | (min) | (%/min) | (%) | ||
| Observed (A) | Calculated (B) | B/A | |||
| Control | 7.407 | _ | _ | 0.927 | 95.1 |
| Epicatechin (EC) | 34.028 | _ | _ | 0.866 | 93.4 |
| Epigallocatechin (EGC) | 26.908 | _ | _ | 0.885 | 93.6 |
| ASDB | 7.411 | _ | _ | 0.927 | 94.9 |
| 2-ME | 9.218 | _ | _ | 9.218 | 95.0 |
| EGC + EC | 59.02 | 60.936 | 0.970 | 0.840 | 92.6 |
| EGC + ASDB | 29.612 | 34.319 | 0.860* | 0.893 | 93.3 |
| EGC +2-ME | 29.373 * | 36.126 | 0.810* | 0.891 | 93.2 |
3. Discussion
4. Experimental
4.1. Materials
4.2. Methods
4.3. Measurement of Rate of Initiation

4.4. Measurement of Stoichiometric Factor (n)
4.5. Measurement of the Inhibition Rate
5. Conclusions
Acknowledgements
References and Notes
- Chen, D.; Dou, Q.P. Tea polyphenols and their roles in cancer prevention and chemotherapy. Int. J. Mol. Sci. 2008, 9, 1196–1206. [Google Scholar] [CrossRef]
- Atsumi, T.; Ishihara, M.; Kadoma, Y.; Tonosaki, K.; Fujisawa, S. Comparative radical production and cytotoxicity induced by camphorquinone and 9-fluorenone against human pulp fibroblasts. J. Oral Rehabil. 2004, 31, 1155–1164. [Google Scholar] [CrossRef]
- Terakado, M.; Yamazaki, M.; Tsujinoto, Y.; Kawashima, T.; Nagashima, K.; Ogawa, J.; Fujita, Y.; Sugiya, T.; Sakai, T.; Furuyama, S. Lipid peroxidation as a possible cause of benzoyl peroxide toxicity in rabbit dental pulp-A microsomal lipid peroxidation in vitro. J. Dent. Res. 1984, 63, 901–905. [Google Scholar] [CrossRef]
- Masuki, K.; Nomura, Y.; Bhawal, U.K.; Sawajiri, M.; Hirata, I.; Nahara, Y.; Okazaki, M. Apoptotic and necrotic influence of dental resin polymerization initiators in human gingival fibroblast cultures. Dent. Mater. J. 2007, 26, 861–869. [Google Scholar] [CrossRef]
- Weaver, R.E.; Goebel, W.M. Reactions to acrylic resin dental prostheses. J. Prosthet. Dent. 1980, 43, 138–142. [Google Scholar] [CrossRef]
- Nomura, Y.; Teshima, W.; Kawahara, T.; Tanaka, N.; Ishibashi, H.; Okazaki, M.; Arizono, K. Genotoxicity of dental resin polymerization initiators in vitro. J. Mater. Sci. Mater. Med. 2006, 17, 29–32. [Google Scholar] [CrossRef]
- Koren, E.; Ron Kohen, R.; Ginsburg, I. Polyphenols enhance total oxidant-scavenging capacities of human blood by binding to red blood cells. Exp. Biol. Med. 2010, 235, 689–699. [Google Scholar] [CrossRef]
- Fujisawa, S.; ishihara, M.; Kadoma, Y. Kinetic evaluation of the reactivity of flavonoids as radical scavengers. SAR QSAR Environ. Res. 2002, 13, 617–627. [Google Scholar] [CrossRef]
- Fujisawa, S.; Ishihara, M.; Atsumi, T.; Kadoma, Y. A quantitative approach to the free radical interaction between alpha-tocopherol or ascorbate and flavonoids. In Vivo 2006, 20, 445–452. [Google Scholar]
- Kadoma, Y.; Ishihara, M.; Okada, N.; Fujisawa, S. Free radical interaction between vitamin E (alpha-, beta-, gamma-, delta-tocopherol), ascorbate and flavonoids. In Vivo 2006, 20, 823–827. [Google Scholar]
- Kadoma, Y.; Fujisawa, S. A comparative study of the radical-scavenging activity of the phenolcarboxylic acids, p-coumaric acid, chlorogenic acid and ferulic acid, with 2-mercaptoethanol, a thiol, using the induction period method. Molecules 2008, 13, 2488–2499. [Google Scholar] [CrossRef]
- Fujisawa, S.; Atsumi, T.; Ishihara, M.; Kadoma, Y. Cytotoxicity, ROS-generation activity and radical-scavenging Activity of curcumin and related compounds. Anticancer Res. 2004, 24, 563–569. [Google Scholar]
- Burton, G.W.; Ingold, K.U. Autoxidation of biological molecules.1. Antioxidant activity vitamin E and related chain-breaking phenolic antioxidants in vitro. J. Am. Chem. Soc. 1981, 103, 6472–6477. [Google Scholar] [CrossRef]
- Kessler, M.; Höper, J.; Harrison, D.K.; Skolasinska, K.; Klövekorn, W.P.; Sebening, F.; Volkholz, H.J.; Beier, I.; Kernbach, C.; Rettig, V. Tissue O2 supply under normal and pathological conditions. Adv. Exp. Med. Biol. 1984, 169, 69–80. [Google Scholar]
- Burton, G.W.; Ingold, K.U. beta-Carotene: An unusual type of lipid antioxidant. Science 1984, 224, 569–573. [Google Scholar]
- Kondo, K.; Kurihara, M.; Miyata, N.; Suzuki, T.; Toyoda, M. Mechanistic studies of catechins as antioxidants. Arch. Biochem. Biophys. 1999, 362, 79–86. [Google Scholar] [CrossRef]
- Javanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovic, B.; Simic, M.G. Flavonoids as antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, L.M.; Yang, L.; Liu, Z.L. Evidence for alpha-tocopherol regeneration of green tea polyphenols in SDS micells. Free Radic. Biol. Med. 2005, 38, 78–84. [Google Scholar] [CrossRef]
- Zhao, B. Antioxidant effects of green tea polyphenols. Chain. Sci. Bull. 2003, 48, 315–319. [Google Scholar]
- Guo, Q.; Zhao, B.; Shen, S.; Hou, J.; Hu, J.; Xin, W. ESR study on the structure—Antioxidant activity relationship of tea catechin and their epimers. Biochim. Biophys. Acta 1999, 1427, 13–23. [Google Scholar] [CrossRef]
- Nanjo, F.; Nori, M.; Goto, K.; Hara, Y. Radical scavenging activity of tea catechins and their related compounds. Biosci. Biotechnol. Biochem. 1999, 63, 1621–1623. [Google Scholar] [CrossRef]
- Mukai, K.; Mitani, S.; Ohara, K.; Nagaoka, S. Structure-activity relationship of the tocopherol-regeneration reaction by catechins. Free Radic. Biol. Med. 2005, 38, 1243–1256. [Google Scholar] [CrossRef]
- Frank, J.; Budek, A.; Lundh, T.; Parker, R.S.; Swanson, J.E.; Lourenço, C.F.; Gago, B.; Laranjinha, J.; Vessby, B.; Kamal-Eldin, A. Dietary flavonoids with a catechol structure increase alpha-tocopherol in rats and protect the vitamin from oxidation in vitro. J. Lipid Res. 2006, 47, 2718–2725. [Google Scholar] [CrossRef]
- Wiegand, H.; Boesch-Saadatmandi, C.; Wein, S.; Wolffram, S.; Frank, J.; Rimbach, G. Dietary flavonoids do not affect vitamin E status in growing rats. J. Anim. Physiol. Anim. Nutr. (Berl) 2010, 94, 307–318. [Google Scholar]
- Szent-Gyorgy, A. The living state and cancer. Proc. Natl. Acad. Sci. USA 1977, 74, 2844–2847. [Google Scholar] [CrossRef]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Sample Availability: Not available.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kadoma, Y.; Fujisawa, S. Radical-Scavenging Activity of Dietary Phytophenols in Combination with co-Antioxidants Using the Induction Period Method. Molecules 2011, 16, 10457-10470. https://doi.org/10.3390/molecules161210457
Kadoma Y, Fujisawa S. Radical-Scavenging Activity of Dietary Phytophenols in Combination with co-Antioxidants Using the Induction Period Method. Molecules. 2011; 16(12):10457-10470. https://doi.org/10.3390/molecules161210457
Chicago/Turabian StyleKadoma, Yoshinori, and Seiichiro Fujisawa. 2011. "Radical-Scavenging Activity of Dietary Phytophenols in Combination with co-Antioxidants Using the Induction Period Method" Molecules 16, no. 12: 10457-10470. https://doi.org/10.3390/molecules161210457




