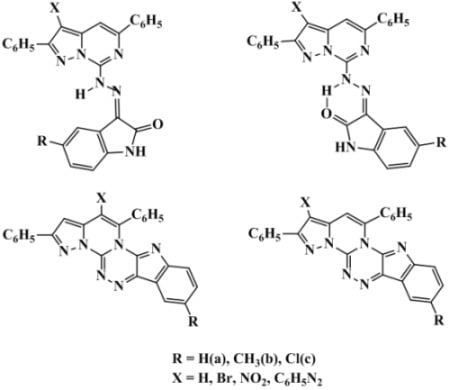
3.2. Synthesis of Compounds
3.2.1. (E)-3-{2-(2,5-Diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-ones 3a–c
A solution of 2,5-diphenyl-7-hydrazinopyrazolo[1,5-
c]pyrimidine [
30,
33] (
1, 0.30 g, 0.0010 mol) in dioxane (10 mL) was stirred with isatin (or isatin derivatives) (
2, 0.0015 mol) for 24 hours at room temperature. The products that separated out as orange needles were filtered off, washed with methanol and dried.
(E)-3-{2-(2,5-Diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-one (3a). Yield 70%; m.p. 332–334 °C; Rf 0.18 (3:1 benzene-EtOAc); IR (cm−1): 3479 (NH), 1700 (indole ring C=O), 1619 (pyrazole ring C=N), 1565 (pyrimidine ring C=N) and 1452 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δH, ppm): 6.85 (s, 1H, pyrazole-H), 7.37–8.04 (m, 10H, aromatic-H), 7.54 (s, 1H, pyrimidine-H), 8.07 (d, 2H, aromatic-H), 8.16 (d, 2H, aromatic-H), 11.09, 11.23 (s, 1H, exchangeable NH, OH) and 10.65, 14.20 (s, 1H, exchangeable NH); MS, m/z (%): 430 (7, M+), 402 (1, M+-N2), 325 (17, M+-C7H7N), 248 (1, M+-C13H10O), 234 (1, M+-C13H12N2), 194 (1, M+-C14H10N3O−), 165 (2, M+-C15H13N4O), 139 (4, M+-C16H13N5O), 132 (4, M+-C19H12N3O), 88 (3, M+-C21H18N4O), 77 (100, M+-C20H13N6O) and 62 (8, M+-C22H18N5O); Anal. Calc. for C26H18N6O (430.46): C, 72.55; H, 4.21; N, 19.52%, found: C, 72.48; H, 4.17; N, 19.47%.
(E)-3-{2-(2,5-Diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}-5-methylindolin-2-one (3b). Yield 88%; m.p. 330–332 °C; Rf 0.28 (3:1 benzene-EtOAc); IR (cm−1): 3459 (NH), 1684 (indole ring C=O), 1631 (pyrazole ring C=N), 1547 (pyrimidine ring C=N) and 1460 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δH, ppm): 2.33 (s, 3H, CH3), 7.18 (d, 1H aromatic-H) 7.23 (s, 1H, pyrazole-H), 7.44–7.56 (m, 8H, aromatic-H), 7.89 (s, 1H, pyrimidine-H), 8.11 (d, 1H, aromatic-H), 8.18 (t, 2H, aromatic-H), 8.22 (d, 1H, aromatic-H), 11.03, 11.15 (s, 1H, exchangeable NH, OH) and 10.80, 14.22 (s, 1H, exchangeable NH); MS, m/z (%): 444 (41, M+ ), 416 (36, M+-N2), 339 (100, M+-C7H7N), 262 (1, M+-C13H10O), 234 (22, M+-C14H14N2), 208 (4, M+-C14H10N3O−), 165 (1, M+-C16H15N4O), 146 (2, M+-C19H12N3O), 139 (7, M+-C17H15N5O), 88 (13, M+-C22H20N4O), 77 (21, M+-C21H15N6O) and 62 (2, M+-C23H20N5O); Anal. Calc. for C27H20N6O (444.49): C, 72.96; H, 4.54; N, 18.91%, found: C, 72.91; H, 4.51; N, 18.86%.
(E)-3-{2-(2,5-Diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}-5-chloroindolin-2-one (3c). Yield 86%; m.p. 320–322 °C; Rf 0.34 (3:1 benzene-EtOAc); IR (cm−1): 3459 (NH), 1710 (indole ring C=O), 1631 (pyrazole ring C=N), 1539 (pyrimidine ring C=N) and 1459 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δH, ppm): 7.02 (d, 1H, aromatic-H), 7.16 (s, 1H, aromatic-H), 7.21 (s, 1H, pyrazole-H), 7.41–7.57 (m, 6H, aromatic-H), 7.68 (d, 1H, aromatic-H), 7.92 (s, 1H, pyrimidine-H), 8.11 (d, 2H, aromatic-H), 8.23 (d, 2H, aromatic-H), 11.36, 12.17 (s, 1H, exchangeable NH, OH) and 10.43, 14.19 (s, 1H, exchangeable NH); MS, m/z (%): 464 (29, M+), 436 (41, M+-N2), 359 (100, M+-C7H7N), 282 (2, M+-C13H10O), 234 (51, M+-C13H11ClN2), 228 (2, M+-C14H10N3O−), 166 (7, M+-C19H12N3O), 165 (5, M+-C15H12ClN4O), 139 (18, M+-C16H12ClN5O), 88 (19, M+-C21H17ClN4O), 77 (50, M+-C20H12ClN6O) and 62 (17, M+-C22H17ClN5O); Anal. Calc. for C26H17ClN6O (464.91): C, 67.17; H, 3.69; Cl, 7.63; N, 18.08%, found: C, 67.12; H, 3.65; Cl, 7.60; N, 17.95%.
3.2.2. (Z)-3-{2-(2,5-Diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-ones 3a–c
Method A: A suspension of (E)-3-{2-(2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-ones (E)-3a–c (0.0046 mol) in dioxane, xylene, pyridine, acetic acid or acetic anhydride (50 mL) was heated under reflux for twenty four hours. The products that separated out were filtered off, washed with ethanol, dried and crystallized from dioxane.
Method B: Stirring of (E)-3-{2-(2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-ones (E)-3a–c in conc. sulfuric acid (5.0 mL) was set below 15 °C and left for 2 hours. The reaction mixture was poured onto crushed ice and the separated product were filtered off, washed with water, dried and crystallized from dioxane.
The products from method A and method B showed completely similar TLC, mp, mixed mp, IR, 1H-NMR and MS spectra.
(Z)-3-{2-(2,5-Diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-one (3a). Yield 80%; m.p. 318–320 °C (crystallization from dioxane); Rf 0.66 (3:1 benzene-EtOAc); IR (cm−1): 3467 (NH), 1692 (indole ring C=O), 1626 (pyrazole ring C=N), 1557 (pyrimidine ring C=N) and 1458 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δH, ppm): 7.01 (d, 1H, aromatic-H), 7.12 (t, 1H, aromatic-H), 7.19 (s, 1H, pyrazole-H), 7.37–7.71 (m, 8H, aromatic-H), 7.91 (s, 1H, pyrimidine-H), 8.11 (d, 2H, aromatic-H), 8.22 (d, 2H, aromatic-H), 11.27 (s, 1H, exchangeable NH) and 14.21 (s, 1H, exchangeable NH); MS, m/z (%): 430 (7, M+ ), 402 (1, M+-N2), 325 (14, M+-C7H7N), 248 (1, M+-C13H10O), 234 (2, M+-C13H12N2), 194 (1, M+-C14H10N3O−), 165 (2, M+-C15H13N4O), 139 (10, M+-C16H13N5O), 132 (1, M+-C19H12N3O), 88 (48, M+-C21H18N4O), 77 (100, M+-C20H13N6O) and 62 (17, M+-C22H18N5O); Anal. Calc. for C26H18N6O (430.46): C, 72.55; H, 4.21; N, 19.52%, found: C, 72.56; H, 4.12; N, 19.12%.
(Z)-3-{2-(2,5-Diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}-5-methylindolin-2-one (3b). Yield 86%; m.p. 328–330 °C (crystallization from dioxane); Rf 0.69 (3:1 benzene-EtOAc); IR (cm−1): 3451 (NH), 1684 (indole ring C=O), 1629 (pyrazole ring C=N), 1558 (pyrimidine ring C=N) and 1459 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δH, ppm): 2.33 (s, 3H, CH3), 6.87 (d, 1H aromatic-H) 7.17 (s, 1H, aromatic-H), 7.18 (s, 1H, pyrazole-H), 7.43–7.53 (m, 7H, aromatic-H), 7.88 (s, 1H, pyrimidine-H), 8.11 (d, 2H, aromatic-H), 8.22 (d, 2H, aromatic-H), 11.14 (s, 1H, exchangeable NH) and 14.23 (s, 1H, exchangeable NH); MS, m/z (%): 444 (30, M+), 416 (40, M+-N2), 339 (100, M+-C7H7N), 262 (2, M+-C13H10O), 234 (31, M+-C14H14N2), 208(5, M+-C14H10N3O−), 165 (3, M+-C16H15N4O), 146 (8, M+-C19H12N3O), 139 (4, M+-C17H15N5O), 88 (22, M+-C22H20N4O), 77 (40, M+-C21H15N6O) and 62 (10, M+-C23H20N5O); Anal. Calc. for C27H20N6O (444.49): C, 72.96; H, 4.54; N, 18.91%, found: C, 72.89; H, 4.52; N, 18.82%.
(Z)-3-{2-(2,5-Diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}-5-chloroindolin-2-one (3c). Yield 91%; m.p. 312–314 °C (crystallization from dioxane); Rf 0.63 (3:1 benzene-EtOAc); IR (cm−1): 3464 (NH), 1689 (indole ring C=O), 1628 (pyrazole ring C=N), 1556 (pyrimidine ring C=N) and 1455 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δH, ppm): 7.03 (d, 1H, aromatic-H), 7.15 (s, 1H, aromatic-H), 7.22 (s, 1H, pyrazole-H), 7.43–7.59 (m, 6H, aromatic-H), 7.71 (d, 1H, aromatic-H), 7.95 (s, 1H, pyrimidine-H), 8.13 (d, 2H, aromatic-H), 8.27 (d, 2H, aromatic-H), 11.37 (s, 1H, exchangeable NH) and 14.22 (s, 1H, exchangeable NH); MS, m/z (%): 464 (26, M+), 436 (41, M+-N2), 359 (100, M+-C7H7N), 282 (3, M+-C13H10O), 234 (56, M+-C13H11ClN2), 228 (2, M+-C14H10N3O−), 166 (9, M+-C19H12N3O), 165 (6, M+-C15H12ClN4O), 139 (21, M+-C16H12ClN5O), 88 (39, M+-C21H17ClN4O), 77 (72, M+-C20H12ClN6O) and 62 (23, M+-C22H17ClN5O); Anal. Calc. for C26H17ClN6O (464.91): C, 67.17; H, 3.69; Cl, 7.63; N, 18.08%, found: C, 67.11; H, 3.63; Cl, 7.58; N, 17.91%.
3.2.3. (Z)-3-{2-(3-Bromo-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-ones 4a–c
A solution of bromine (0.06 mL, 0.0012 mol) in acetic acid (10 mL) was gradually added to a suspension of (E orZ)-3-{2-(2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-ones 3a‑c (0.0010 mol) in acetic acid (10 mL) with stirring for three hours at room temperature. The reaction mixture was then poured onto crushed ice, filtered off, washed with water, dried and crystallized from dioxane as orange needles.
(Z)-3-{2-(3-Bromo-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-one (4a) Yield 96%; m.p. 288–290 °C; Rf 0.65 (3:1 benzene-EtOAc); IR (cm−1): 3460 (NH), 1687 (indole ring C=O), 1622 (pyrazole ring C=N), 1559 (pyrimidine ring C=N) and 1456 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δH, ppm): 6.98 (d, 1H, aromatic-H), 7.13 (t, 1H, aromatic-H), 7.39–7.71 (m, 8H, aromatic-H), 7.75 (s, 1H, pyrimidine-H), 8.05 (d, 2H, aromatic-H), 8.30 (d, 2H, aromatic-H), 11.29 (s, 1H, exchangeable NH) and 14.17 (s, 1H, exchangeable NH); MS, m/z (%): 510 (64, M+), 481 (26, M+-HN2), 405 (52, M+-C7H7N), 403 (91, M+-C6H7N2), 325 (100, M+-C7H7BrN), 312 10, M+-C12H12N3), 271 (27, M+-C14H13N3O−), 243 (23, M+-C14H13N5O), 234 (27, M+-C13H12BrN2), 165 (4, M+-C15H13BrN4O), 140 (4, M+-C21H18N6O), 139 (23, M+-C16H13BrN5O), 131 (9, M+-C19H13BrN3O), 88 (38, M+-C21H18BrN4O), 76 (90, M+-C20H14BrN6O) and 62 (35, M+-C22H18BrN5O); Anal. Calc. for C26H17BrN6O (509.36): C, 61.31; H, 3.36; Br, 15.69; N, 16.50%, found: C, 61.27; H, 3.32; Br, 15.52; N, 16.33%.
(Z)-3-{2-(3-Bromo-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}-5-methylindolin-2-one (4b) Yield 97%; m.p. 320–322 °C; Rf 0.77 (3:1 benzene-EtOAc); IR (cm−1): 3465 (NH), 1689 (indole ring C=O), 1628 (pyrazole ring C=N), 1559 (pyrimidine ring C=N) and 1451 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δ, ppm): 2.36 (s, 3H, CH3), 6.88 (d, 1H aromatic-H) 7.19 (s, 1H, aromatic-H), 7.52–7.62 (m, 7H, aromatic-H), 7.73 (s, 1H, pyrimidine-H), 8.09 (d, 2H, aromatic-H), 8.16 (d, 2H, aromatic-H), 11.17 (s, 1H, exchangeable NH) and 14.22 (s, 1H, exchangeable NH); MS, m/z (%): 524 (98, M+), 495 (50, M+-HN2), 419 (77, M+-C7H7N), 417 (100, M+-C6H7N2), 339 (28, M+-C7H7BrN), 312 (22, M+-C13H14N3), 285 (19, M+-C14H13N3O−), 243 (11, M+-C15H15N5O), 234 (16, M+-C14H14BrN2), 165 (6, M+-C16H15BrN4O), 145 (14, M+-C19H13BrN3O), 140 (6, M+-C22H20N6O), 139 (28, M+-C17H15BrN5O), 88 (45, M+-C22H20BrN4O), 76 (95, M+-C21H16BrN6O) and 62 (29, M+-C23H20BrN5O); Anal. Calc. for C27H19BrN6O (523.38): C, 61.96; H, 3.66; Br, 15.27; N, 16.06%, found: C, 61.89; H, 3.63; Br, 15.18; N, 15.83%.
(Z)-3-{2-(3-Bromo-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}-5-chloroindolin-2-one (4c) Yield 94%; m.p. 304–306 °C; Rf 0.75 (3:1 benzene-EtOAc); IR (cm−1): 3451 (NH), 1684 (indole ring C=O), 1630 (pyrazole ring C=N), 1560 (pyrimidine ring C=N) and 1447 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δ, ppm): 7.03 (d, 1H, aromatic-H), 7.19 (s, 1H, aromatic-H), 7.42–7.57 (m, 6H, aromatic-H), 7.69 (d, 1H, aromatic-H), 7.93 (s, 1H, pyrimidine-H), 8.11 (d, 2H, aromatic-H), 8.25 (d, 2H, aromatic-H), 11.36 (s, 1H, exchangeable NH) and 14.20 (s, 1H, exchangeable NH); MS, m/z (%): 544 (53, M+), 515 (27, M+-HN2), 439 (10, M+-C7H7N), 437 (100, M+-C6H7N2), 359 (13, M+-C7H7BrN), 312 (11, M+-C12H11ClN3), 305 (1, M+-C14H13N3O−), 243 (18, M+-C14H12ClN5O), 234 (2, M+-C13H11BrClN2), 165 (15, M+-C15H12BrClN4O), 140 (3, M+-C21H17ClN6O), 139 (20, M+-C16H12BrClN5O), 88 (14, M+-C21H17BrClN4O), 76 (53, M+-C20H13BrClN6O) and 62 (13, M+-C22H17BrClN5O); Anal. Calc. for C26H16BrClN6O (543.80): C, 57.42; H, 2.97; Br, 14.69; Cl, 6.52; N, 15.45%, found: C, 57.38; H, 2.95; Br, 14.51; Cl, 6.31; N, 15.23%.
3.2.4. (E)-3-{2-(3-Bromo-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-ones 9a–c
A solution of 3-bromo-2,5-diphenyl-7-hydrazinopyrazolo[1,5-c]pyrimidine [45] (10, 0.46 g, 0.0012 mol) in dioxane (10 mL) was stirred with isatin (or isatin derivatives) 2 (0.0015 mol) for 24 h at room temperature. The products that separated out were filtered off, washed with methanol and dried.
(E)-3-{2-(3-Bromo-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-one (9a). Yield 81%; m.p. 312–314 °C; Rf 0.27 (3:1 benzene-EtOAc); IR (cm−1): 3442 (NH), 1707 (indole ring C=O), 1626 (pyrazole ring C=N), 1555 (pyrimidine ring C=N) and 1453 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δ, ppm): 7.87–8.05 (m, 10H, aromatic-H), 7.74 (s, 1H, pyrimidine-H), 8.08 (d, 2H, aromatic-H), 8.25 (d, 2H, aromatic-H), 11.21, 11.29 (s, 1H, exchangeable NH, OH) and 10.92, 14.16 (s, 1H, exchangeable NH); MS, m/z (%): 510 (35, M+), 481 (28, M+-HN2), 405 (81, M+-C7H7N), 403 (100, M+-C6H7N2), 325 (33, M+-C7H7BrN), 312 (15, M+-C12H12N3), 271 (3, M+-C14H13N3O−), 243 (13, M+-C14H13N5O), 234 (16, M+-C13H12BrN2), 165 (7, M+-C15H13BrN4O), 140 (4, M+-C21H18N6O), 139 (22, M+-C16H13BrN5O), 131 (7, M+-C19H13BrN3O), 88 (47, M+-C21H18BrN4O), 76 (73, M+-C20H14BrN6O) and 62 (18, M+-C22H18BrN5O); Anal. Calc. for C26H17BrN6O (509.36): C, 61.31; H, 3.36; Br, 15.69; N, 16.50%, found: C, 61.27; H, 3.30; Br, 15.46; N, 16.38%.
(E)-3-{2-(3-Bromo-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}-5-methylindolin-2-one (9b). Yield 79%; m.p. 300–302 °C; Rf 0.43 (3:1 benzene-EtOAc); IR (cm−1): 3451 (NH), 1698 (indole ring C=O), 1630 (pyrazole ring C=N), 1543 (pyrimidine ring C=N) and 1460 (pyrimidine ring C=C); MS, m/z (%): 524 (81, M+), 495 (40, M+-HN2), 419 (69, M+-C7H7N), 417 (86, M+-C6H7N2), 339 (32, M+-C7H7BrN), 312 (17, M+-C13H14N3), 285 (26, M+-C14H13N3O−), 243 (15, M+-C15H15N5O), 234 (16, M+-C14H14BrN2), 165 (7, M+-C16H15BrN4O), 145 (11, M+-C19H13BrN3O), 140 (12, M+-C22H20N6O), 139 (30, M+-C17H15BrN5O), 88 (38, M+-C22H20BrN4O), 76 (100, M+-C21H16BrN6O) and 62 (21, M+-C23H20BrN5O); Anal. Calc. for C27H19BrN6O (523.38): C, 61.96; H, 3.66; Br, 15.27; N, 16.06%, found: C, 61.81; H, 3.51; Br, 15.05; N, 15.76%.
(E)-3-{2-(3-Bromo-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}-5-chloroindolin-2-one (9c). Yield 79%; m.p. 308–310 °C; Rf 0.42 (3:1 benzene-EtOAc); IR (cm−1): 3464 (NH), 1700 (indole ring C=O), 1629 (pyrazole ring C=N), 1533 (pyrimidine ring C=N) and 1449 (pyrimidine ring C=C); MS, m/z (%): 544 (100, M+), 515 (44, M+-HN2), 439 (91, M+-C7H7N), 437 (94, M+-C6H7N2), 359 (29, M+-C7H7BrN), 312 (18, M+-C12H11ClN3), 305 (1, M+-C14H13N3O−), 243 (18, M+-C14H12ClN5O), 234 (9, M+-C13H11BrClN2), 165 (12, M+-C15H12BrClN4O), 140 (5, M+-C21H17ClN6O), 139 (36 M+-C16H12BrClN5O), 88 (24, M+-C21H17BrClN4O), 76 (98, M+-C20H13BrClN6O) and 62 (35, M+-C22H17BrClN5O); Anal. Calc. for C26H16BrClN6O (543.80): C, 57.42; H, 2.97; Br, 14.69; Cl, 6.52; N, 15.45%, found: C, 57.21; H, 2.82; Br, 14.48; Cl, 6.25; N, 15.27%.
3.2.5. (Z)-3-{2-(3-Iodo-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-ones 5a–c
A solution of iodine monochloride (0.20 g, 0.0012 mol) in acetic acid (10 mL) was gradually added to a suspension of (E or Z)-3a–c (0.001 mol) in acetic acid (10 mL) with stirring for three hours at room temperature. The reaction mixture was then poured onto crushed ice and the products that separated out were filtered off, washed with water, dried and crystallized from dioxan as orange needles.
(Z)-3-{2-(3-Iodo-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-one (5a). Yield 92%; m.p. 280–282 °C; IR (cm−1): 3454 (NH), 1684 (indole ring C=O), 1622 (pyrazole ring C=N), 1561 (pyrimidine ring C=N) and 1455 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δ, ppm): 6.96 (d, 1H aromatic-H) 7.12 (t, 1H, aromatic-H), 7.36–7.69 (m, 8H, aromatic-H), 7.61 (s, 1H, pyrimidine-H), 8.00 (d, 2H, aromatic-H), 8.27 (d, 2H, aromatic-H), 11.27 (s, 1H, exchangeable NH) and 14.12 (s, 1H, exchangeable NH); MS, m/z (%): 557 (95, M+), 529 (24, M+-N2), 452 (100, M+-C7H7N), 374 (1, M+-C13H11O), 360 (22, M+-C12H11N3), 325 (41, M+-C7H7IN), 320 (1, M+-C14H11N3O), 257 (5, M+-C19H16N4−), 234 (8, M+-C13H12IN2), 188 (10, M+-C21H17N6O), 165 (7, M+-C15H13IN4O), 139 (41, M+-C16H13IN5O), 131 (8, M+-C19H13IN3O), 88 (7, M+-C21H18IN4O), 76 (96, M+-C20H14IN6O) and 62 (35, M+-C22H18IN5O); Anal. Calc. for C26H17IN6O (556.36): C, 56.13; H, 3.08; I, 22.81; N, 15.11%, found: C, 55.89; H, 2.99; I, 22.45; N, 14.78%.
(Z)-3-{2-(3-Iodo-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}-5-methylindolin-2-one (5b). Yield 98%; m.p. 314–316 °C; IR (cm−1): 3443 (NH), 1684 (indole ring C=O), 1630 (pyrazole ring C=N), 1564 (pyrimidine ring C=N) and 1460 (pyrimidine ring C=C); MS, m/z (%): 571 (67, M+ ), 543 (14, M+-N2), 466 (44, M+-C7H7N), 388 (2, M+-C13H11O), 360 (3, M+-C13H13N3), 339 (25, M+-C7H7IN), 334 (8, M+-C14H11N3O), 257 (4, M+-C20H18N4−), 234 (3, M+-C14H14IN2), 188 (5, M+-C22H19N6O), 165 (5, M+-C16H15IN4O), 145 (7, M+-C19H13IN3O), 139 (11, M+-C17H15IN5O), 88 (11, M+-C22H20IN4O), 76 (100, M+-C21H16IN6O) and 62 (13, M+-C23H20IN5O); Anal. Calc. for C27H19IN6O (570.38): C, 56.85; H, 3.36; I, 22.25; N, 14.73%, found: C, 56.73; H, 3.24; I, 21.83; N, 14.47%.
(Z)-3-{2-(3-Iodo-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}-5-chloroindolin-2-one (5c). Yield 97%; m.p. 306–308 °C; IR (cm−1): 3466 (NH), 1677 (indole ring C=O), 1625 (pyrazole ring C=N), 1558 (pyrimidine ring C=N) and 1447 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δ, ppm): 7.01 (d, 1H, aromatic-H), 7.16 (s, 1H, aromatic-H), 7.39–7.66 (m, 7H, aromatic-H), 7.89 (s, 1H, pyrimidine-H), 8.11 (d, 2H, aromatic-H), 8.24 (d, 2H, aromatic-H), 11.33 (s, 1H, exchangeable NH) and 14.17 (s, 1H, exchangeable NH); MS, m/z (%): 591 (33, M+), 563 (12, M+-N2), 486 (44, M+-C7H7N), 408 (1, M+-C13H11O), 360 (19, M+-C12H10ClN3), 359 (63, M+-C7H7IN), 354 (10, M+-C14H11N3O), 257 (9, M+-C19H15ClN4−), 234 (20, M+-C13H11ClIN2), 188 (10, M+-C21H16ClN6O), 165 (12, M+-C15H12ClIN4O), 139 (43, M+-C16H12ClIN5O), 88 (32, M+-C21H17ClIN4O), 76 (100, M+-C20H13ClIN6O) and 62 (27, M+-C22H17ClIN5O); Anal. Calc. for C26H16ClIN6O (590.80): C, 52.86; H, 2.73; Cl, 6.00; I, 21.48; N, 14.22%, found: C, 52.77; H, 2.62; Cl, 4.68; I, 21.20; N, 13.93%.
3.2.6. (Z)-3-{2-(3-Nitro-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-ones 6a–c
A mixture of nitric acid (d 1.14, 1 mL) and sulfuric acid (d 1.84, 1 mL) in glacial acetic acid (10 mL) was added gradually to a suspension of (E or Z)-3a–c (0.001 mol) in acetic acid (10 mL) with stirring for three hours at room temperature. The reaction mixture was then poured onto crushed ice and the products that separated out were filtered off, washed with water, dried and crystallized from dioxane as orange needles.
(Z)-3-{2-(3-Nitro-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-one (6a). Yield 91%; m.p. 268–270 °C; IR (cm−1): 3460 (NH), 1708 (indole ring C=O), 1624 (pyrazole ring C=N), 1560 (pyrimidine ring C=N), 1473 (pyrimidine ring C=C), and 1416, 1341 (NO2); MS, m/z (%): 476 (1, M+), 447 (1, M+-HN2), 371 (5, M+-C7H7N), 370 (2, M+-C6H6N2), 325 (5, M+-C7H7N2O2), 291 (3, M+-C13H13O), 279 (3, M+-C12H11N3−), 237 (3, M+-C14H13N3O), 234 (5, M+-C13H12N3O2), 210 (3, M+-C14H12N5O), 165 (5, M+-C15H13N5O3), 139 (3, M+-C16H13N6O3), 131 (5, M+-C19H13N4O3), 107 (9, M+-C21H17N6O), 88 (8, M+-C21H18N5O3), 76 (7, M+-C20H14N7O3) and 62 (4, M+-C22H18N6O3); Anal. Calc. for C26H17N7O3 (475.46): C, 65.68; H, 3.60; N, 20.62%, found: C, 65.56; H, 3.57; N, 20.40%.
(Z)-3-{2-(3-Nitro-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}-5-methylindolin-2-one (6b). Yield 95%; m.p. 318–320 °C; IR (cm−1): 3465 (NH), 1697 (indole ring C=O), 1635 (pyrazole ring C=N), 1564 (pyrimidine ring C=N), 1487 (pyrimidine ring C=C), and 1419, 1381 (NO2); MS, m/z (%): 490 (20, M+), 461 (7, M+-HN2), 385 (35, M+-C7H7N), 384 (3, M+-C6H6N2), 339 (5, M+-C7H7N2O2), 305 (1, M+-C13H13O), 279 (1, M+-C13H13N3−), 251 (4, M+-C14H13N3O), 234 (8, M+-C14H14N3O2), 210 (2, M+-C15H14N5O), 165 (5, M+-C16H15N5O3), 145 (28, M+-C19H13N4O3), 139 (8, M+-C17H15N6O3), 107 (24, M+-C22H19N6O), 88 (11, M+-C22H20N5O3), 76 (85, M+-C21H16N7O3) and 62 (16, M+-C23H20N6O3); Anal. Calc. for C27H19N7O3 (489.48): C, 66.25; H, 3.91; N, 20.03%, found: C, 66.02; H, 3.72; N, 19.84%.
(Z)-3-{2-(3-Nitro-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}-5-chloroindolin-2-one (6c). Yield 97%; m.p. 330–332 °C; IR (cm−1): 3460 (NH), 1693 (indole ring C=O), 1629 (pyrazole ring C=N), 1559 (pyrimidine ring C=N), 1487 (pyrimidine ring C=C), and 1416, 1363 (NO2); MS, m/z (%): 510 (1, M+), 481 (3, M+-HN2), 405 (1, M+-C7H7N), 404 (1, M+-C6H6N2), 359 (1, M+-C7H7N2O2), 325 (5, M+-C13H13O), 279 (1, M+-C12H10ClN3−), 271 (1, M+-C14H13N3O), 234 (1, M+-C13H11ClN3O2), 210 (1, M+-C14H11ClN5O), 165 (2, M+-C15H12ClN5O3), 139 (3, M+-C16H12ClN6O3), 107 (1, M+-C21H16ClN6O), 88 (3, M+-C21H17ClN5O3), 76 (100, M+-C20H13ClN7O3) and 62 (13, M+-C22H17ClN6O3); Anal. Calc. for C26H16ClN7O3 (509.90): C, 61.24; H, 3.16; Cl, 6.95; N, 19.23%, found: C, 61.19; H, 3.02; Cl, 6.62; N, 18.91%.
3.2.7. (Z)-3-{2-(3-Phenyldiazenyl-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-ones 7a–c
An aqueous sodium hydroxide solution (10%, 8 mL) was added to a suspension of (E or Z)-3a–c (0.001 mol) in ethanol (15 mL). The reaction mixture was cooled to 5 °C and gradually treated with a solution of benzendiazonium chloride (prepared from 1 mL of aniline) with stirring for one hour. The target products that separated out were collected by filtration and crystallized from dioxan as reddish-brown needles.
(Z)-3-{2-(3-Phenyldiazenyl-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}indolin-2-one (7a). Yield 94%; m.p. 298–300 °C; IR (cm−1): 3463 (NH), 1693 (indole ring C=O), 1625 (pyrazole ring C=N), 1557 (pyrimidine ring C=N) and 1458 (pyrimidine ring C=C); MS, m/z (%): 534(1, M+), 506 (1, M+-N2), 431 (29, M+-C7H5N), 430 (2, M+-C6H4N2), 353 (1, M+-C13H9O), 339 (1, M+-C12H9N3), 325 (69, M+-C13H11N3), 298 (1, M+-C14H10N3O), 270 (7, M+-C14H10N5O−), 234 (14, M+-C19H16N4), 167 (6, M+-C21H15N6O), 165 (5, M+-C21H17N6O), 139 (17, M+-C22H17N7O), 131 (6, M+-C25H17N5O), 88 (44, M+-C27H22N6O), 76 (100, M+-C26H18N8O) and 62 (21, M+-C28H22N7O); Anal. Calc. for C32H22N8O (534.57): C, 71.90; H, 4.15; N, 20.96%, found: C, 71.79; H, 3.97; N, 20.62%.
(Z)-3-{2-(3-Phenydiazenyl-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}-5-methyl-indolin-2-one (7b). Yield 94%; m.p. 338–340 °C; IR (cm−1): 3460 (NH), 1689 (indole ring C=O), 1629 (pyrazole ring C=N), 1557 (pyrimidine ring C=N) and 1455 (pyrimidine ring C=C); MS, m/z (%): 548 (1, M+), 520 (3, M+-N2), 445 (40, M+-C7H5N), 444 (3, M+-C6H4N2), 367 (1, M+-C13H9O), 339 (100, M+-C13H11N3), 312 (2, M+-C14H10N3O), 270 (11, M+-C15H12N5O−), 234 (29, M+-C20H18N4), 167 (4, M+-C22H17N6O), 165 (3, M+-C22H19N6O), 145 (6, M+-C25H17N5O), 139 (16, M+-C23H19N7O), 88 (52, M+-C28H24N6O), 76 (7, M+-C27H20N8O) and 62 (8, M+-C29H24N7O); Anal. Calc. for C33H24N8O (548.60): C, 72.25; H, 4.41; N, 20.43%, found: C, 72.12; H, 4.20; N, 20.22%.
(Z)-3-{2-(3-Phenyldiazenyl-2,5-diphenylpyrazolo[1,5-c]pyrimidin-7-yl)hydrazono}-5-chloro-indolin-2-one (7c). Yield 94%; m.p. 328–330 °C; IR (cm−1): 3460 (NH), 1690 (indole ring C=O), 1626 (pyrazole ring C=N), 1553 (pyrimidine ring C=N) and 1451 (pyrimidine ring C=C); MS, m/z (%): 568 (2, M+), 540 (3, M+-N2), 465 (45, M+-C7H5N), 464 (1, M+-C6H4N2), 387 (5, M+-C13H9O), 359 (100, M+-C13H11N3), 339 (5, M+-C12H8ClN3), 332 (4, M+-C14H10N3O), 270 (17, M+-C14H9ClN5O−), 234 (46, M+-C19H15ClN4), 167 (8, M+-C21H14ClN6O), 165 (4, M+-C21H16ClN6O), 139 (18, M+-C22H16ClN7O), 88 (12, M+-C27H21ClN6O), 76 (50, M+-C26H17ClN8O) and 62 (13, M+-C28H21ClN7O); Anal. Calc. for C32H21ClN8O (569.02): C, 67.55; H, 3.72; Cl, 6.23; N, 19.69%, found: C, 67.36; H, 3.61; Cl, 5.82; N, 19.42%.
3.2.8. 2,5-Diphenylindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazines 13a–c
A solution of (E)-3a–c (0.0023 mol) in phosphorus oxychloride (15 mL) was heated at 70–80 °C for two hours. The mixture was cooled, poured onto crushed ice and made alkaline (pH = 9) with potassium hydrogen carbonate. The target productswere filtered off, washed with water, dried and crystallized from dimethylformamide as reddish-brown needles.
2,5-Diphenylindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazines (13a). Yield 93%; m.p. 308–310 °C; IR (cm−1): 1647 (indole ring C=N), 1624 (pyrazole ring C=N), 1535 (triazine ring C=N) and 1470 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δ, ppm): 7.24 (d, 1H, aromatic-H), 7.53 (s, 1H, pyrazole-H), 7.33–7.55 (m, 9H, aromatic-H), 7.91 (s, 1H, pyrimidine-H), 8.13 (d, 2H, aromatic-H), 8.20 (d, 2H, aromatic-H); MS, m/z (%): 412 (64, M+), 335 (2, M+-C6H5), 307 (15, M+-C6H5N2), 281 (13, M+-C8H7N2), 253 (8, M+-C8H7N4), 228 (7, M+-C12H12N2−), 217 (8, M+-C13H11N2), 191 (4, M+-C14H11N3), 176 (7, M+-C14H12N4), 150 (12, M+-C15H12N5), 114 (17, M+-C20H16N3), 88 (27, M+-C21H16N4), 76 (100, M+-C20H12N6), 62 (11, M+-C22H16N5) and 50 (34, M+-C23H16N5); Anal. Calc. for C26H16N6 (412.45): C, 75.71; H, 3.91; N, 20.38%, found: C, 75.49; H, 3.76; N, 20.08%.
2,5-Diphenyl-10-methylindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazines (13b). Yield 96%; m.p. 286–288 °C; IR (cm−1): 1648 (indole ring C=N), 1604 (pyrazole ring C=N), 1542 (triazine ring C=N) and 1474 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δ, ppm): 2.44 (s, 3H, CH3), 7.11 (d, 1H, aromatic-H), 7.42 (s, 1H, pyrazole-H), 7.33–7.53 (m, 8H, aromatic-H), 7.55 (s, 1H, pyrimidine-H), 8.01 (d, 2H, aromatic-H), 8.13 (d, 2H, aromatic-H); MS, m/z (%): 426 (100, M+), 349 (2, M+-C6H5), 321 (2, M+-C6H5N2), 295 (2, M+-C8H7N2), 267 (7, M+-C8H7N4), 242 (12, M+-C12H12N2−), 217 (5, M+-C14H13N2), 191 (5, M+-C15H13N3), 190 (10, M+-C14H12N4), 164 (9, M+-C15H12N5), 114 (35, M+-C21H18N3), 88 (25, M+-C22H18N4), 76 (71, M+-C21H14N6), 62 (17, M+-C23H18N5) and 50 (33, M+-C24H18N5); Anal. Calc. for C27H18N6 (426.47): C, 76.04; H, 4.25; N, 19.71%, found: C, 75.90; H, 4.15; N, 19.36%.
2,5-Diphenyl-10-chloroindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazines (13c). Yield 94%; m.p. 276–278 °C; IR (cm−1): 1645 (indole ring C=N), 1619 (pyrazole ring C=N), 1563 (triazine ring C=N) and 1453 (pyrimidine ring C=C); MS, m/z (%): 446 (2, M+ ), 369 (1, M+-C6H5), 341 (2, M+-C6H5N2), 315 (5, M+-C8H7N2), 287 (10, M+-C8H7N4), 262 (8, M+-C12H12N2−), 217 (4, M+-C13H10ClN2), 210 (3, M+-C14H12N4), 191 (3, M+-C14H10ClN3), 184 (2, M+-C15H12N5), 114 (4, M+-C20H15ClN3), 88 (3, M+-C21H15ClN4), 76 (100, M+-C20H11ClN6), 62 (3, M+-C22H15ClN5) and 50 (44, M+-C23H15ClN5); Anal. Calc. for C26H15ClN6 (446.89): C, 69.88; H, 3.38; Cl, 7.93; N, 18.81%, found: C, 69.69; H, 3.16; Cl, 7.70; N, 18.42%.
3.2.9. 4-Bromo-2,5-diphenylindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazines 14a–c
A solution of bromine (0.06 mL, 0.0012 mol) in acetic acid (10 mL) was gradually added to a suspension of 13a–c (0.001 mol) in acetic acid (10 mL) with stirring for three hours at room temperature. The reaction mixture was then poured onto crushed ice. The products that separated out were filtered off, washed with water, dried and crystallized from dimethylformamide as brown needles.
4-Bromo-2,5-diphenylindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazine (14a). Yield 91%; m.p. 282–284 °C; IR (cm−1): 1659 (indole ring C=N), 1616 (pyrazole ring C=N), 1551 (triazine ring C=N) and 1462 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δ, ppm): 7.26 (d, 1H, aromatic-H), 7.33 (s, 1H, pyrazole-H), 7.36–7.63 (m, 9H, aromatic-H), 8.05 (d, 2H, aromatic-H), 8.20 (d, 2H, aromatic-H); MS, m/z (%): 491 (1, M+), 307 (1, M+-C7H6BrN), 306 (1, M+-C6H5BrN2), 281 (1, M+-C8H6BrN2), 253 (1, M+-C8H6BrN4), 228 (2, M+-C12H11BrN2−), 217 (1, M+-C13H10BrN2), 191 (1, M+-C14H10BrN3), 176 (2, M+-C14H11BrN4), 150 (2, M+-C15H11BrN5), 114 (2, M+-C20H15BrN3), 88 (5, M+-C21H15BrN4), 76 (100, M+-C20H11BrN6), 62 (5, M+-C22H15BrN5) and 50 (33, M+-C23H15BrN5); Anal. Calc. for C26H15BrN6 (491.34): C, 63.56; H, 3.08; Br, 16.26; N, 17.10%, found: C, 63.41; H, 2.86; Br, 15.94; N, 16.72%.
4-Bromo-2,5-diphenyl-10-methylindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazine (14b). Yield 92%; m.p. 308–310 °C; IR (cm−1): 1674 (indole ring C=N), 1631 (pyrazole ring C=N), 1562 (triazine ring C=N) and 1477 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δ, ppm): 2.43 (s, 3H, CH3), 7.11 (d, 1H, aromatic-H), 7.28 (s, 1H, pyrazole-H), 7.34-7.64 (m, 7H, aromatic-H), 7.92 (d, 2H, aromatic-H), 7.99 (s, 1H, aromatic-H), 8.13 (d, 2H, aromatic-H); MS, m/z (%): 505 (8, M+ ), 321 (1, M+-C7H6BrN), 320 (5, M+-C6H5BrN2), 295 (1, M+-C8H6BrN2), 267 (1, M+-C8H6BrN4), 242 (1, M+-C12H11BrN2−), 217 (1, M+-C14H12BrN2), 191 (1, M+-C15H12BrN3), 190 (6, M+-C14H11BrN4), 164 (3, M+-C15H11BrN5), 114 (19, M+-C21H17BrN3), 88 (17, M+-C22H17BrN4), 76 (100, M+-C21H13BrN6), 62 (8, M+-C23H17BrN5) and 50 (27, M+-C24H17BrN5); Anal. Calc. for C27H17BrN6 (505.37): C, 64.17; H, 3.39; Br, 15.81; N, 16.63%, found: C, 64.07; H, 3.24; Br, 15.53; N, 16.29%.
4-Bromo-2,5-diphenyl-10-chloroindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazine (14c). Yield 92%; m.p. 240–242 °C; IR (cm−1): 1667 (indole ring C=N), 1616 (pyrazole ring C=N), 1550 (triazine ring C=N) and 1449 (pyrimidine ring C=C); MS, m/z (%): 525 (1, M+), 341 (2, M+-C7H6BrN), 340 (1, M+-C6H5BrN2), 315 (1, M+-C8H6BrN2), 287 (13, M+-C8H6BrN4), 262 (3, M+-C12H11BrN2−), 217 (1, M+-C13H9BrClN2), 210 (1, M+-C14H11BrN4), 191 (1, M+-C14H9BrClN3), 184 (2, M+-C15H11BrN5), 114 (2, M+-C20H14BrClN3), 88 (8, M+-C21H14BrClN4), 76 (100, M+-C20H10BrClN6), 62 (14, M+-C22H14BrClN5) and 50 (59, M+-C23H14BrClN5); Anal. Calc. for C26H14BrClN6 (525.79): C, 59.39; H, 2.68; Br, 15.20; Cl, 6.74; N, 15.98%, found: C, 59.16; H, 2.57; Br, 14.73; Cl, 6.34; N, 15.71%.
3.2.10. 3-Bromo-2,5-diphenylindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazines 17a–c
A solution of (Z)-4a–c (0.0005 mol) in phosphorus oxychloride (5 mL) was heated at 70–80 °C for two hours. The mixture was cooled and poured onto crushed ice and basified with potassium hydrogen carbonate to pH = 9. The products were filtered off, washed with water, dried and crystallized from dimethylformamide.
3-Bromo-2,5-diphenylindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazine (17a). Yield 90%; m.p. 302–304 °C; IR (cm−1): 1641 (indole ring C=N), 1619 (pyrazole ring C=N), 1561 (triazine ring C=N) and 1474 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δ, ppm): 6.93 (d, 1H, aromatic-H), 7.01–7.92 (m, 9H, aromatic-H), 7.94 (s, 1H, pyrimidine-H), 8.05 (d, 2H, aromatic-H), 8.28 (d, 2H, aromatic-H); MS, m/z (%): 491 (1, M+), 307 (13, M+-C7H6BrN), 306 (1, M+-C6H5BrN2), 281 (4, M+-C8H6BrN2), 253 (13, M+-C8H6BrN4), 228 (18, M+-C12H11BrN2−), 217 (6, M+-C13H10BrN2), 191 (5, M+-C14H10BrN3), 176 (5, M+-C14H11BrN4), 150 (10, M+-C15H11BrN5), 114 (31, M+-C20H15BrN3), 88 (2, M+-C21H15BrN4), 76 (2, M+-C20H11BrN6), 62 (1, M+-C22H15BrN5) and 50 (100, M+-C23H15BrN5); Anal. Calc. for C26H15BrN6 (491.34): C, 63.56; H, 3.08; Br, 16.26; N, 17.10%, found: C, 63.41; H, 2.92; Br, 15.83; N, 16.79%.
3-Bromo-2,5-diphenyl-10-methylindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazine (17b). Yield 90%; m.p. 200–202 °C; IR (cm−1): 1667 (indole ring C=N), 1619 (pyrazole ring C=N), 1567 (triazine ring C=N) and 1454 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δ, ppm): 2.34 (s, 3H, CH3), 7.12 (d, 1H, aromatic-H), 7.19–7.67 (m, 8H, aromatic-H), 7.69 (s, 1H, pyrimidine-H), 8.00 (d, 2H, aromatic-H), 8.09 (d, 2H, aromatic-H); MS, m/z (%): 505 (1, M+), 321 (1, M+-C7H6BrN), 320 (1, M+-C6H5BrN2), 295 (1, M+-C8H6BrN2), 267 (1, M+-C8H6BrN4), 242 (1, M+-C12H11BrN2−), 217 (1, M+-C14H12BrN2), 191 (1, M+-C15H12BrN3), 190 (4, M+-C14H11BrN4), 164 (2, M+-C15H11BrN5), 114 (30, M+-C21H17BrN3), 88 (12, M+-C22H17BrN4), 76 (100, M+-C21H13BrN6), 62 (19, M+-C23H17BrN5) and 50 (69, M+-C24H17BrN5); Anal. Calc. for C27H17BrN6 (505.37): C, 64.17; H, 3.39; Br, 15.81; N, 16.63%, found: C, 63.70; H, 3.24; Br, 15.52; N, 16.20%.
3-Bromo-2,5-diphenyl-10-chloroindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazine (17c). Yield 90%; m.p. 248–250 °C; IR (cm−1): 1667 (indole ring C=N), 1625 (pyrazole ring C=N), 1564 (triazine ring C=N) and 1456 (pyrimidine ring C=C); MS, m/z (%): 525 (2, M+), 341 (1, M+-C7H6BrN), 340 (1, M+-C6H5BrN2), 315 (1, M+-C8H6BrN2), 287 (2, M+-C8H6BrN4), 262 (1, M+-C12H11BrN2−), 217 (3, M+-C13H9BrClN2), 210 (2, M+-C14H11BrN4), 191 (4, M+-C14H9BrClN3), 184 (5, M+-C15H11BrN5), 114 (8, M+-C20H14BrClN3), 88 (15, M+-C21H14BrClN4), 76 (100, M+-C20H10BrClN6), 62 (12, M+-C22H14BrClN5) and 50 (36, M+-C23H14BrClN5); Anal. Calc. for C26H14BrClN6 (525.79): C, 59.39; H, 2.68; Br, 15.20; Cl, 6.74; N, 15.98%, found: C, 59.12; H, 2.49; Br, 14.72; Cl, 6.31; N, 15.54%.
3.2.11. 4-Iodo-2,5-diphenylindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazines 15a,b
A solution of iodine monochloride (0.20 g, 0.0012 mol) in acetic acid (10 mL) was gradually added to a suspension of 13a,b (0.001 mol) in acetic acid (10 mL) with stirring for three hours at room temperature. The reaction mixture was then poured onto crushed ice and the products that separated out were filtered off, washed with water, dried and crystallized from dimethylformamide.
4-Iodo-2,5-diphenylindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazine (15a). Yield 90%; m.p. 314–316 °C; IR (cm−1): 1652 (indole ring C=N), 1620 (pyrazole ring C=N), 1567 (triazine ring C=N) and 1438 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δ, ppm): 7.22 (d, 1H, aromatic-H), 7.37 (t, 1H, aromatic-H), 7.41 (s, 1H, pyrazole-H), 7.42-7.59 (m, 8H, aromatic-H), 8.16 (d, 2H, aromatic-H), 8.20 (d, 2H, aromatic-H); MS, m/z (%): 538 (1, M+), 307 (3, M+-C7H6IN), 306 (2, M+-C6H5IN2), 281 (2, M+-C8H6IN2), 253 (1, M+-C8H6IN4), 228 (1, M+-C12H11IN2−), 217 (1, M+-C13H10IN2), 191 (1, M+-C14H10IN3), 176 (1, M+-C14H11IN4), 150 (1, M+-C15H11IN5), 114 (4, M+-C20H15IN3), 88 (4, M+-C21H15IN4), 76 (100, M+-C20H11IN6), 62 (7, M+-C22H15IN5) and 50 (29, M+-C23H15IN5); Anal. Calc. for C26H15IN6 (538.34): C, 58.01; H, 2.81; I, 23.57; N, 15.61%, found: C, 57.82; H, 2.66; I, 23.19; N, 15.14%.
4-Iodo-2,5-diphenyl-10-methylindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazine (15b). Yield 89%; m.p. 298–300 °C; IR (cm−1): 1689 (indole ring C=N), 1622 (pyrazole ring C=N), 1560 (triazine ring C=N) and 1449 (pyrimidine ring C=C); 1H-NMR (DMSO-d6, δ, ppm): 2.31 (s, 3H, CH3), 6.83 (d, 1H, aromatic-H), 7.47 (s, 1H, pyrazole-H), 7.09–7.58 (m, 8H, aromatic-H), 8.00 (d, 2H, aromatic-H), 8.25 (d, 2H, aromatic-H); MS, m/z (%): 552 (1, M+), 321 (1, M+-C7H6IN), 320 (1, M+-C6H5IN2), 295 (1, M+-C8H6IN2), 267 (1, M+-C8H6IN4), 242 (2, M+-C12H11IN2−), 217 (1, M+-C14H12IN2), 191 (1, M+-C15H12IN3), 190 (1, M+-C14H11IN4), 164 (2, M+-C15H11IN5), 114 (8, M+-C21H17IN3), 88 (4, M+-C22H17IN4), 76 (100, M+-C21H13IN6), 62 (12, M+-C23H17IN5) and 50 (37, M+-C24H17IN5); Anal. Calc. for C27H17IN6 (552.37): C, 58.71; H, 3.10; I, 22.97; N, 15.21%, found: C, 58.58; H, 2.89; I, 22.62; N, 14.94%.
3.2.12. 4-Nitro-2,5-diphenyl-10-methylindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4] triazines 16b,c
A mixture of nitric acid (d 1.14, 1 mL) and sulfuric acid (d 1.84, 1 mL) in glacial acetic acid (10 mL) was added gradually to a suspension of 13b,c (0.001 mol) in acetic acid (10 mL) with stirring for three hours at room temperature. The reaction mixture was then poured onto crushed ice and the products that separated out were filtered off, washed with water, dried and crystallized from dimethylformamide.
4-Nitro-2,5-diphenyl-10-methylindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazine (16b). Yield 92%; m.p. 312–314 °C; IR (cm−1): 1650 (indole ring C=N), 1617 (pyrazole ring C=N), 1555 (triazine ring C=N), 1449 (pyrimidine ring C=C) and 1421, 1391 (NO); MS, m/z (%): 471 (2, M+), 321 (2, M+-C7H6N2O2), 320 (2, M+-C6H5N3O2), 295 (2, M+-C8H6N3O2), 267 (2, M+-C8H6N5O2), 242 (2, M+-C12H11N3O2−), 217 (3, M+-C14H12N3O2), 191 (3, M+-C15H12N4O2), 190 (2, M+-C14H11N5O2), 164 (3, M+-C15H11N6O2), 114 (3, M+-C21H17N4O2), 88 (4, M+-C22H17N5O2), 76 (70, M+-C21H13N7O2), 62 (4, M+-C23H17N6O2) and 50 (100, M+-C24H17N6O2); Anal. Calc. for C27H17N7O2 (471.47): C, 68.78; H, 3.63; N, 20.80%, found: C, 68.49; H, 3.52; N, 20.49%.
4-Nitro-2,5-diphenyl-10-chloroindolo[2,3-e]pyrazolo[1',5':3",4"]pyrimido[2",1"-c][1,2,4]triazine (16c). Yield 92%; m.p. 226–228 °C; IR (cm−1): 1681 (indole ring C=N), 1622 (pyrazole ring C=N), 1530 (triazine ring C=N), 1442 (pyrimidine ring C=C), and 1426,1342 (NO2); MS, m/z (%): 491 (1, M+), 341 (1, M+-C7H6N2O2), 340 (1, M+-C6H5N3O2), 315 (4, M+-C8H6N3O2), 287 (2, M+-C8H6N5O2), 262 (2, M+-C12H11N3O2−), 217 (2, M+-C13H9ClN3O2), 210 (2, M+-C14H11N5O2), 191 (3, M+-C14H9ClN4O2), 184 (1, M+-C15H11N6O2), 114 (9, M+-C20H14ClN4O2), 88 (4, M+-C21H14ClN5O2), 76 (100, M+-C20H10ClN7O2), 62 (8, M+-C22H14ClN6O2) and 50 (30, M+-C23H14ClN6O2); Anal. Calc. for C26H14ClN7O2 (491.89): C, 63.49; H, 2.87; Cl, 7.21; N, 19.93%, found: C, 63.27; H, 2.71; Cl, 6.83; N, 19.54%.