Free-Radical Scavenging Properties and Reducing Power of Grape Cane Extracts from 11 Selected Grape Cultivars Widely Grown in China
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yields (EY), Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)

| TPC | TFC | DPPH• | ABTS•+ | O2•− | OH• | H2O2 | Reducing Power | |
|---|---|---|---|---|---|---|---|---|
| TPC | 1 | 0.930 ** | −0.866 ** | −0.893 ** | −0.896 ** | −0.885 ** | −0.918 ** | −0.804 ** |
| TFC | 1 | −0.855 ** | −0.888 ** | −0.766 ** | −0.754 ** | −0.872 ** | −0.749 ** |
2.2. Antioxidant Activities of Grape Cane Extracts
2.2.1. DPPH Radical-Scavenging Activity
2.2.2. ABTS Radical-Scavenging Activity
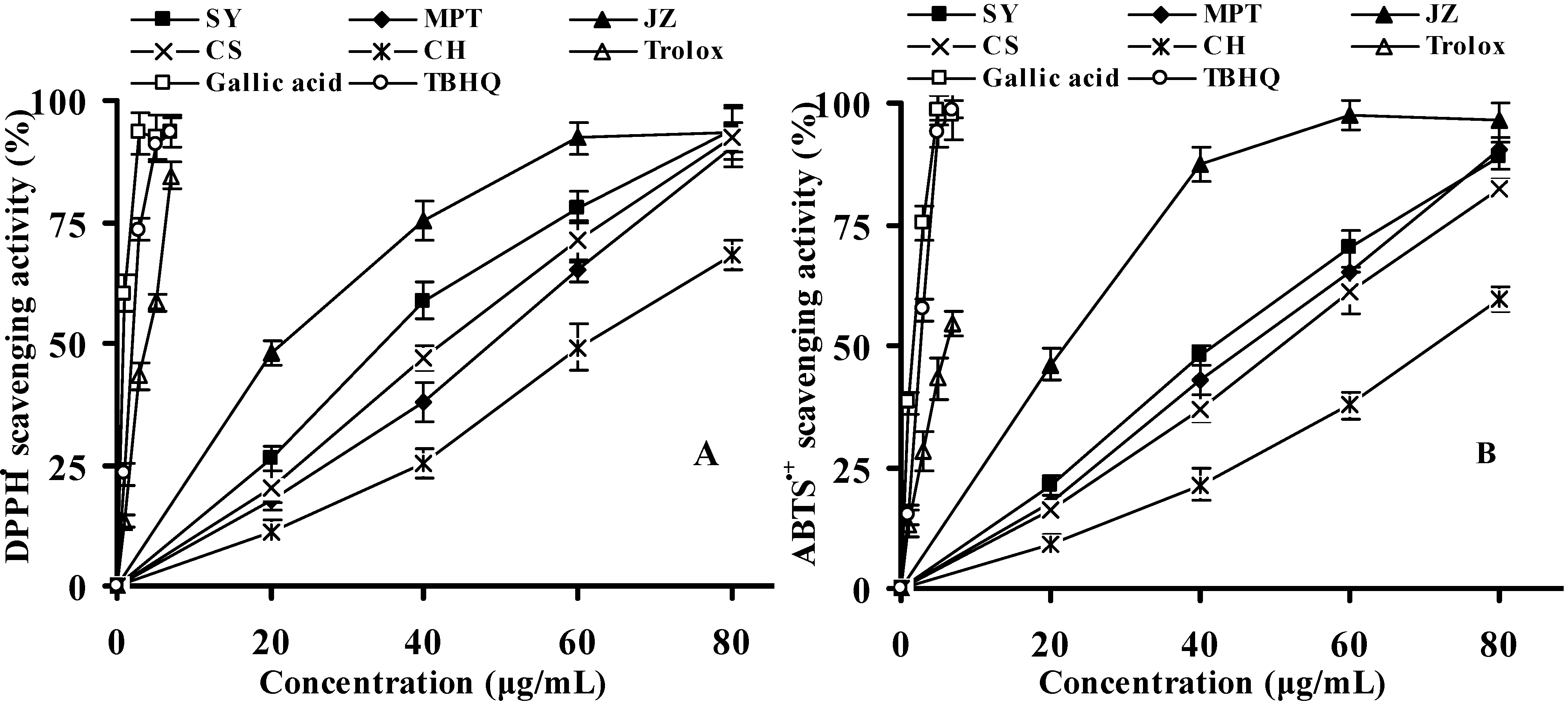
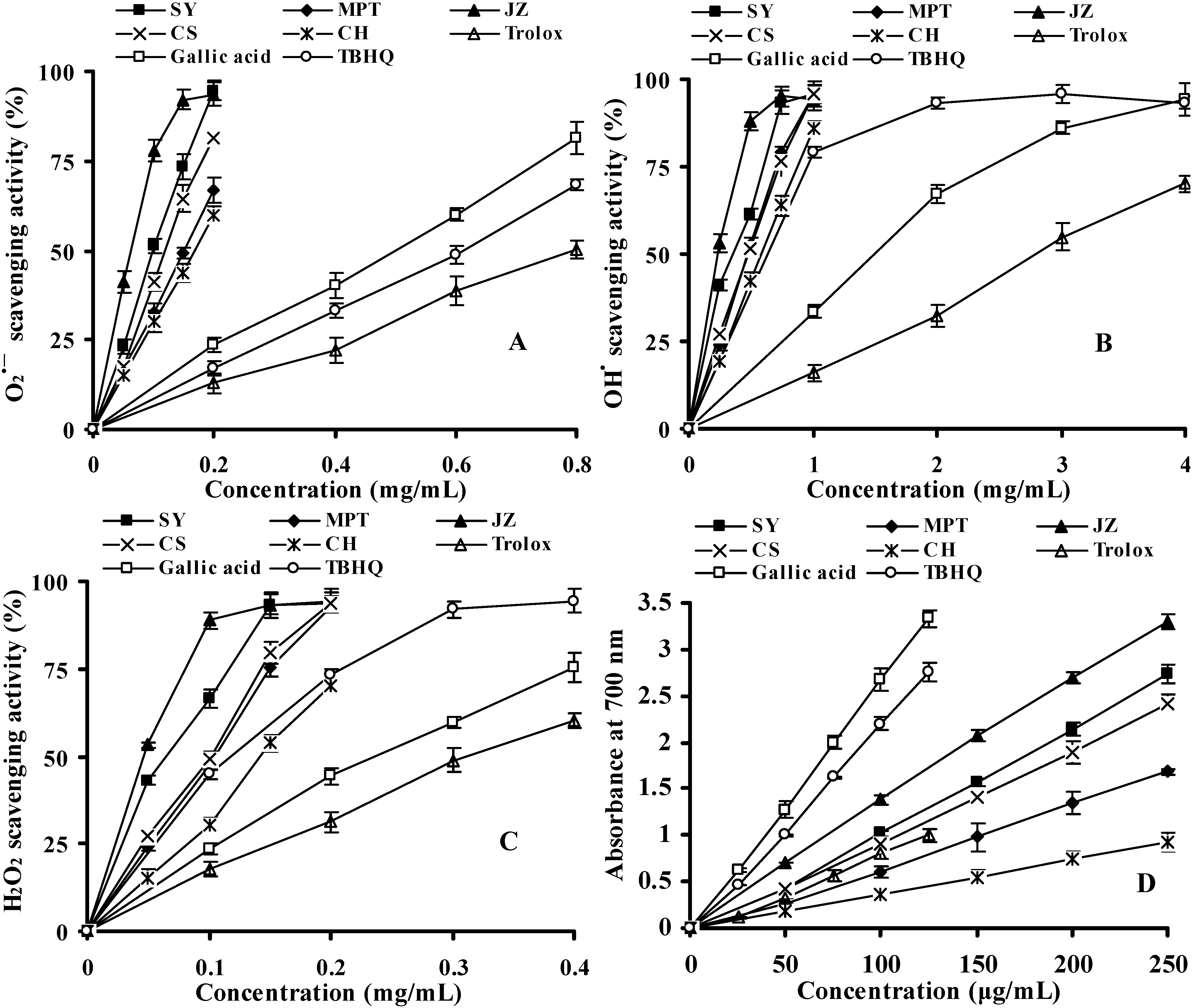
| Species/cultivars | DPPH• EC50 | ABTS•+ EC50 | H2O2 EC50 | O2•− EC50 | OH• EC50 | RP EC50 |
|---|---|---|---|---|---|---|
| (μg/mL) b | (μg/mL) b | (mg/mL) b | (mg/mL) b | (mg/mL) b | (μg/mL) c | |
| V. amurensis | ||||||
| Shuangyou | 36.40 ± 1.03 e | 42.73 ± 0.55 e | 0.07 ± 0.00 b | 0.10 ± 0.00 b | 0.31 ± 0.02 b | 62.14 ± 2.60 d |
| Shuanghong | 49.80 ± 0.93 i | 52.47 ± 2.55 hi | 0.10 ± 0.01 cde | 0.13 ± 0.01 cde | 0.40 ± 0.02 d | 70.32 ± 3.65 fg |
| Beibinghong | 43.09 ± 1.45 fg | 45.76 ± 0.80 f | 0.09 ± 0.00 bcd | 0.12 ± 0.00 bc | 0.36 ± 0.02 c | 64.27 ± 3.25 de |
| V. pentagona | ||||||
| Maoputao | 49.06 ± 1.22 i | 50.06 ± 1.13 g | 0.12 ± 0.01 efg | 0.16 ± 0.00 fg | 0.48 ± 0.02 f | 82.44 ± 1.75 h |
| V. davidii | ||||||
| Junzi | 21.97 ± 0.93 c | 23.64 ± 0.58 c | 0.04 ± 0.00 a | 0.07 ± 0.00 a | 0.21 ± 0.01 a | 33.24 ± 2.26 c |
| Baiyu w | 30.70 ± 0.89 d | 33.70 ± 1.11 d | 0.08 ± 0.00 bc | 0.12 ± 0.00 cd | 0.39 ± 0.01 cd | 65.32 ± 4.07 def |
| V. vinifera | ||||||
| Cabernet Sauvignon | 44.00 ± 1.43 gh | 54.00 ± 1.43 i | 0.10 ± 0.01 def | 0.15 ± 0.01 ef | 0.48 ± 0.01 f | 65.83 ± 2.43 def |
| Hongmeigui | 52.30 ± 1.39 j | 60.97 ± 1.25 j | 0.10 ± 0.01 def | 0.12 ± 0.01 cd | 0.43 ± 0.02 e | 62.28 ± 1.71 d |
| Pinot Noir | 45.51 ± 1.11 h | 50.17 ± 1.91 g | 0.12 ± 0.01 efg | 0.14 ± 0.00 def | 0.44 ± 0.01 e | 69.25 ± 2.01 efg |
| Chardonnay w | 60.88 ± 1.53 k | 71.55 ± 1.80 k | 0.15 ± 0.01 h | 0.17 ± 0.01 g | 0.59 ± 0.01 g | 138.8 ± 4.13 j |
| Victoria Blanc w | 42.00 ± 1.38 f | 51.67 ± 1.64 gh | 0.12 ± 0.01 fg | 0.13 ± 0.01 cde | 0.40 ± 0.03 d | 95.94 ± 2.15 i |
| Positive controls | ||||||
| Gallic acid | 0.85 ± 0.03 a | 1.35 ± 0.09 a | 0.25 ± 0.02 i | 0.52 ± 0.02 h | 1.48 ± 0.02 h | 21.36 ± 3.93 a |
| Trolox | 3.86 ± 0.04 b | 6.50 ± 0.56 b | 0.33 ± 0.03 j | 0.79 ± 0.02 j | 2.79 ± 0.02 i | 73.25 ± 2.65 g |
| TBHQ | 1.90 ± 0.07 a | 2.53 ± 0.18 a | 0.13 ± 0.01 gh | 0.62 ± 0.02 i | 0.59 ± 0.01 g | 26.81 ± 1.92 b |
2.2.3. Reactive Oxygen Species (ROS) Scavenging Activity
2.2.4. Reducing Power (RP) Assay
2.3. Identification and Determination of Phenolic Constituents in Extracts
| Species/cultivars | GA a | PA | VA | SYA | CAT | EC | RES |
|---|---|---|---|---|---|---|---|
| V. amurensis | |||||||
| Shuangyou | 0.28 ± 0.01 c | 1.19 ± 0.05 g | 0.42 ± 0.02 f | 0.93 ± 0.04 c | 5.64 ± 0.22 b | 5.98 ± 0.23 c | 10.95 ± 0.43 b |
| Shuanghong | 0.42 ± 0.02 f | 0.89 ± 0.03 c | 0.39 ± 0.02 de | 0.84 ± 0.03 b | 6.88 ± 0.27 d | 7.02 ± 0.28 d | 9.88 ± 0.39 b |
| Beibinghong | 0.37 ± 0.01 e | 0.98 ± 0.04 de | 0.37 ± 0.01 cd | 0.88 ± 0.03 bc | 7.24 ± 0.28 de | 7.55 ± 0.30 e | 12.32 ± 0.48 c |
| V. pentagona | |||||||
| Maoputao | 0.41 ± 0.03 f | 0.91 ± 0.04 c | 0.40 ± 0.02 ef | 0.58 ± 0.02 a | 6.21 ± 0.24 c | 6.03 ± 0.24 c | 14.33 ± 0.56 ef |
| V. davidii | |||||||
| Junzi | 0.43 ± 0.03 f | 1.32 ± 0.05 h | 0.45 ± 0.02 g | 1.07 ± 0.04 d | 12.34 ± 0.48 g | 11.22 ± 0.44 g | 7.33 ± 0.28 a |
| Baiyu w | 0.44 ± 0.02 f | 1.03 ± 0.04 ef | 0.39 ± 0.02 de | 0.89 ± 0.03 bc | 9.32 ± 0.37 f | 9.01 ± 0.35 f | 6.58 ± 0.26 a |
| V. vinifera | |||||||
| Cabernet Sauvignon | 0.41 ± 0.01 f | 0.93 ± 0.04 cd | 0.42 ± 0.02 f | 1.22 ± 0.05 e | 6.32 ± 0.25 c | 6.15 ± 0.24 c | 13.58 ± 0.53 de |
| Hongmeigui | 0.33 ± 0.03 d | 0.68 ± 0.03 b | 0.37 ± 0.01 cd | 0.88 ± 0.03 bc | 7.03 ± 0.28 de | 6.87 ± 0.27 d | 15.42 ± 0.60 f |
| Pinot Noir | 0.35 ± 0.03 de | 1.08 ± 0.04 f | 0.35 ± 0.01 c | 1.02 ± 0.04 d | 7.52 ± 0.30 e | 7.12 ± 0.28 de | 18.99 ± 0.75 g |
| Chardonnay w | 0.17 ± 0.01 a | 0.53 ± 0.02 a | 0.29 ± 0.01 b | 0.93 ± 0.04 c | 4.29 ± 0.17 a | 4.11 ± 0.17 a | 12.64 ± 0.50 cd |
| Victoria Blanc w | 0.24 ± 0.01 b | 0.69 ± 0.03 b | 0.25 ± 0.01 a | 0.88 ± 0.03 bc | 5.31 ± 0.21 b | 4.98 ± 0.20 b | 13.33 ± 1.45 cd |
3. Experimental
3.1. Plant Materials and Chemicals
3.2. Extraction
3.3. Determination of Total Phenolic and Total Flavonoid Contents
3.4. Determination of Free Radicals and Hydrogen Peroxide Scavenging Activities

3.5. Determination of Reducing Power
3.6. HPLC Analysis of Grape Cane Extracts
3.7. Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Ellis, A.; Triggle, C.R. Endothelium-derived reactive oxygen species: Their relationship to endothelium-dependent hyperpolarization and vascular tone. Can. J. Physiol. Pharmacol. 2003, 81, 1013–1028. [Google Scholar] [CrossRef]
- Reddy, S.V.; Suchitra, M.M.; Reddy, Y.M.; Reddy, P.E. Beneficial and detrimental actions of free radicals: A review. J. Global Pharma Technol. 2010, 2, 3–11. [Google Scholar]
- Kourounakis, A.P.; Galanakis, D.; Tsiakitzis, K.; Rekka, E.A.; Kourounakis, P.N. Synthesis and pharmacological evaluation of novel derivatives of anti-inflammatory drugs with increased antioxidant and anti-inflammatory activities. Drug Dev. Res. 1999, 47, 9–16. [Google Scholar] [CrossRef]
- Gulcin, I.; Buyukokuroglu, M.E.; Oktay, M.; Kufrevioglu, O.I. On the in vitro antioxidative properties of melatonin. J. Pineal Res. 2002, 33, 167–171. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: London, UK, 1999; pp. 608–610. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar]
- Halliwell, B. Antioxidants and human disease: A general introduction. Nutr. Rev. 1997, 5, 544–552. [Google Scholar]
- Ito, N.; Fukushima, S.; Tsuda, H. Carcinogenicity and modification of the carcinogenic response by BHA, BHT and other antioxidants. Crit. Rev. Toxicol. 1985, 15, 109–150. [Google Scholar]
- Hao, P.P.; Ni, J.R.; Sun, W.L.; Huang, W. Determination of tertiary butylhydroquinone in edible vegetable oil by liquid chromatography/ion trap mass spectrometry. Food Chem. 2007, 105, 1732–1737. [Google Scholar] [CrossRef]
- Fiorentino, A.; D’Abrosca, B.; Pacifico, S.; Mastellone, C.; Piscopo, V.; Caputo, R.; Monaco, P. Isolation and structure elucidation of antioxidant polyphenols from quince (Cydonia vulgaris) Peels. J. Agric. Food Chem. 2008, 56, 2660–2667. [Google Scholar]
- Zhang, A.; Fang, Y.; Li, X.; Meng, J.; Wang, H.; Li, H.; Zhang, Z.; Guo, Z. Occurrence and estimation of trans-resveratrol in one-year-old canes from seven major Chinese grape producing regions. Molecules 2011, 16, 2846–2861. [Google Scholar] [CrossRef]
- Luque-Rodríguez, J.M.; Pérez-Juan, P.; Luque de Castro, M.D. Extraction of Polyphenols from vine shoots of Vitis vinifera by superheated ethanol-water mixtures. J. Agric. Food Chem. 2006, 54, 8775–8781. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, Y.L.; Wang, H.; Song, J.Q.; Zhang, Y.L.; Song, S.R. Simultaneous determination of individual phenolics in grape tissues by switching detection wavelength in high performance liquid chromatography. Chin. J. Anal. Chem. 2007, 35, 1614–1618. [Google Scholar]
- Karacabey, E.; Mazza, G. Optimisation of antioxidant activity of grape cane extracts using response surface methodology. Food Chem. 2010, 119, 343–348. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure-activity insight. Innov. Food Sci. Emerg. 2010, 11, 210–218. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Garg, V.K.; Gupta, R. Vermicomposting of agro-industrial processing waste. In Biotechnology for Agro-Industrial Residues Utilisation; Nigam, P.S., Pandey, A., Eds.; Springer Press: Dordrecht, The Netherlands, 2009; pp. 431–432. [Google Scholar]
- Wang, Y.Q.; Schuchardt, F. Effect of C/N ratio on the composting of vineyard pruning residues. Landbauforsch. vTI Agric. For. Res. 2010, 60, 131–138. [Google Scholar]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Hussein, L.; Fattah, M.; Salem, E. Characterization of pure anthocyanidins isolated from the hulls of faba beans. J. Agric. Food Chem. 1990, 38, 95–98. [Google Scholar] [CrossRef]
- Spingo, G.; Pizzorno, T.; De Faveri, D.M. Cellulose and hemicelluloses recovery from grape stalks. Bioresource Technol. 2008, 99, 4329–4337. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Spencer, J.P.E. Flavonoids: Modulators of brain function? Brit. J. Nutr. 2008, 99, 60–77. [Google Scholar]
- Ruberto, G.; Renda, A.; Daquino, C.; Amico, V.; Spatafora, C.; Tringali, C.; De Tommasi, N. Polyphenol constituents and antioxidant activity of grape pomace extracts from five Sicilian red grape cultivars. Food Chem. 2007, 100, 203–210. [Google Scholar] [CrossRef]
- Yang, J.; Martinson, T.E.; Liu, R.H. Phytochemical profiles and antioxidant activities of wine grapes. Food Chem. 2009, 116, 332–339. [Google Scholar] [CrossRef]
- de Campos Luanda, M.A.S.; Fernanda, V.L.; Rozangela, C.P.; Sandra, R.S. Free radical scavenging of grape pomace extracts from Cabernet sauvingnon (Vitis vinifera). Bioresour. Technol. 2008, 99, 8413–8420. [Google Scholar] [CrossRef]
- Sun, T.; Ho, C.T. Antioxidant activities of buckwheat extracts. Food Chem. 2005, 90, 743–749. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moya, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
- Ao, C.; Deba, F.; Tako, M.; Tawata, S. Biological activity and composition of extract from aerial root of Ficus microcarpa L. fil. Int. J. Food Sci. Technol. 2009, 44, 349–358. [Google Scholar] [CrossRef]
- Lissi, E.A.; Modak, B.; Torres, R.; Escobar, J.; Urzua, A. Total antioxidant potential of resinous exudates from Heliotropium sp. A comparison of ABTS and DPPH methods. Free Radic. Res. 1999, 30, 471–477. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem. Toxicol. 2006, 44, 198–206. [Google Scholar] [CrossRef]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Fu, W.; Chen, J.; Cai, Y.; Lei, Y.; Chen, L.; Pei, L.; Zhou, D.; Liang, X.; Ruan, J. Antioxidant, free radical scavenging, anti-inflammatory and hepatoprotective potential of the extract from Parathelypteris nipponica (Franch. et Sav.) Ching. J. Ethnopharmacol. 2010, 130, 521–528. [Google Scholar]
- Oyaizu, M. Studies on products of browning reaction—Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–4. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C.; Aruoma, O. The deoxyribose method: A simple “test tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar]
- Meir, S.; Kanner, J.; Akiri, B.; Hadas, S.P. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J. Agric. Food Chem. 1995, 43, 1813–1819. [Google Scholar] [CrossRef]
- Rayne, S.; Karacabey, E.; Mazza, G. Grape cane waste as a source of trans-resveratrol and trans-viniferin: High-value phytochemicals with medicinal and anti-phytopathogenic applications. Ind. Crop Prod. 2008, 27, 335–340. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Chang, C.; Yang, M.; Wen, H.; Chern, J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1998, 72, 1231–1237. [Google Scholar]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the extracts of 11 grape canes (V. vinifera, V. Amurensis, V. Davidii, and V. pentagona) are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, A.; Fang, Y.; Wang, H.; Li, H.; Zhang, Z. Free-Radical Scavenging Properties and Reducing Power of Grape Cane Extracts from 11 Selected Grape Cultivars Widely Grown in China. Molecules 2011, 16, 10104-10122. https://doi.org/10.3390/molecules161210104
Zhang A, Fang Y, Wang H, Li H, Zhang Z. Free-Radical Scavenging Properties and Reducing Power of Grape Cane Extracts from 11 Selected Grape Cultivars Widely Grown in China. Molecules. 2011; 16(12):10104-10122. https://doi.org/10.3390/molecules161210104
Chicago/Turabian StyleZhang, Ang, Yulin Fang, Hua Wang, Hua Li, and Zhenwen Zhang. 2011. "Free-Radical Scavenging Properties and Reducing Power of Grape Cane Extracts from 11 Selected Grape Cultivars Widely Grown in China" Molecules 16, no. 12: 10104-10122. https://doi.org/10.3390/molecules161210104




