Observation of Different Catalytic Activity of Various 1-Olefins during Ethylene/1-Olefin Copolymerization with Homogeneous Metallocene Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Homo- and co-polymerization activities
| Run number | Olefin type | Polymerization time (s) | Polymer yielda (g) | Catalytic activityb (×10-4kgPol/molZr h) |
|---|---|---|---|---|
| 1 | - | 115 | 0.8703 | 1.8 |
| 2 | 1-C6 | 124 | 1.4781 | 2.9 |
| 3 | 1-C8 | 97 | 1.5529 | 3.8 |
| 4 | 1-C10 | 115 | 1.6783 | 3.5 |
| 5 | 1-C12 | 109 | 1.6134 | 3.6 |
| 6 | 1-C14 | 89 | 1.3704 | 3.7 |
| 7 | 1-C18 | 123 | 2.3157 | 4.5 |
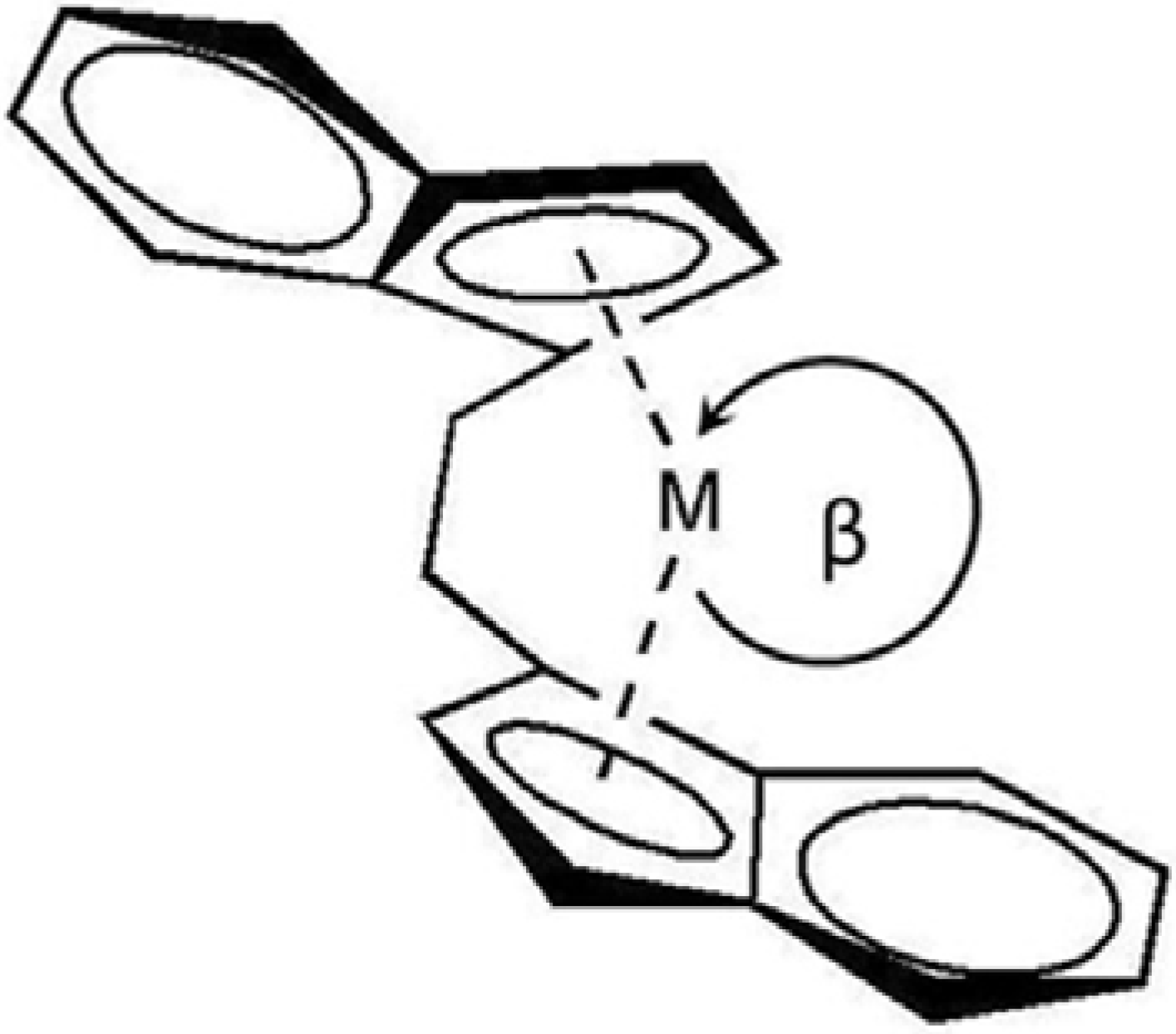
2.2. Polymer properties
2.2.1. SEM measurements

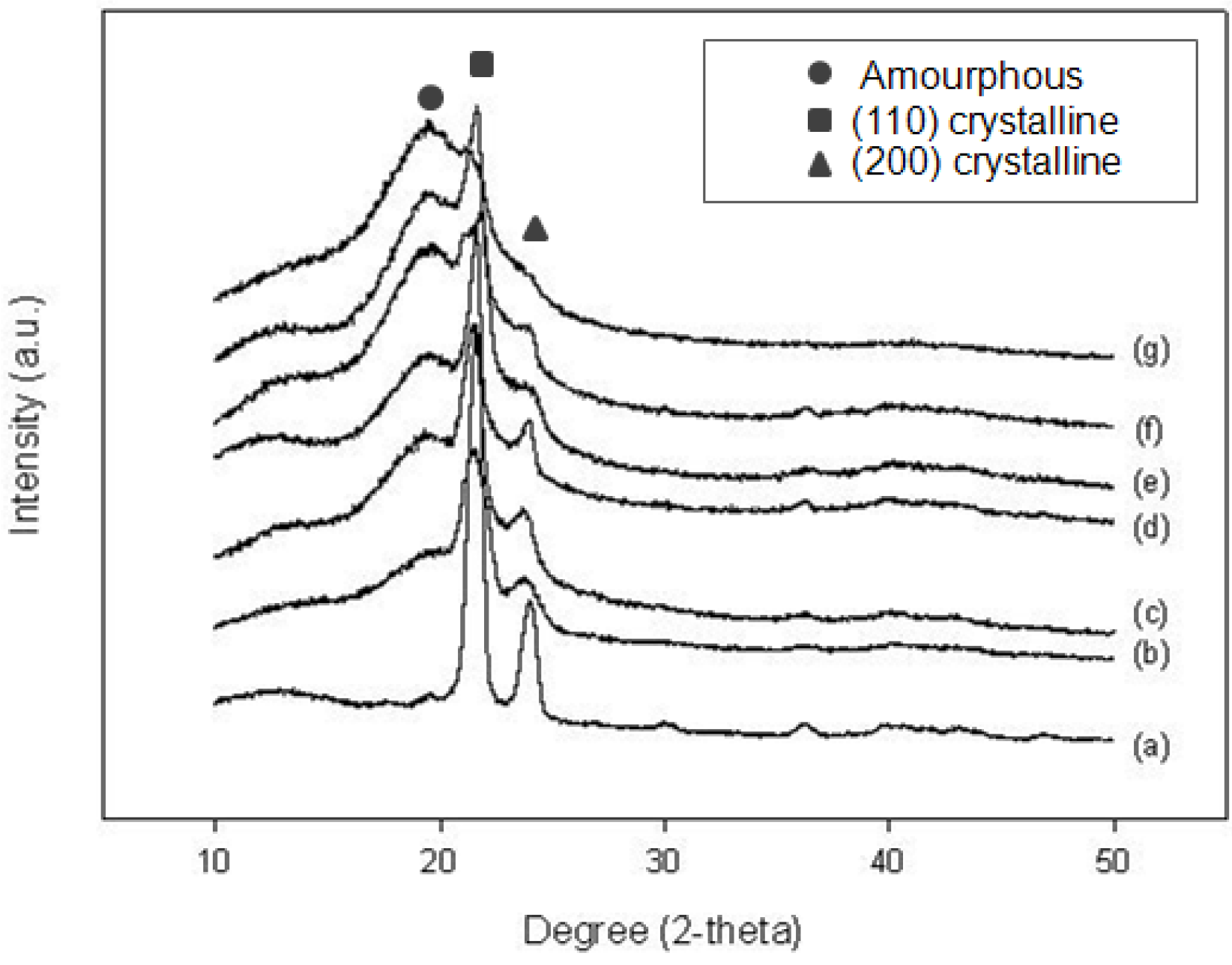
2.2.2. X-ray Diffraction (XRD) analysis
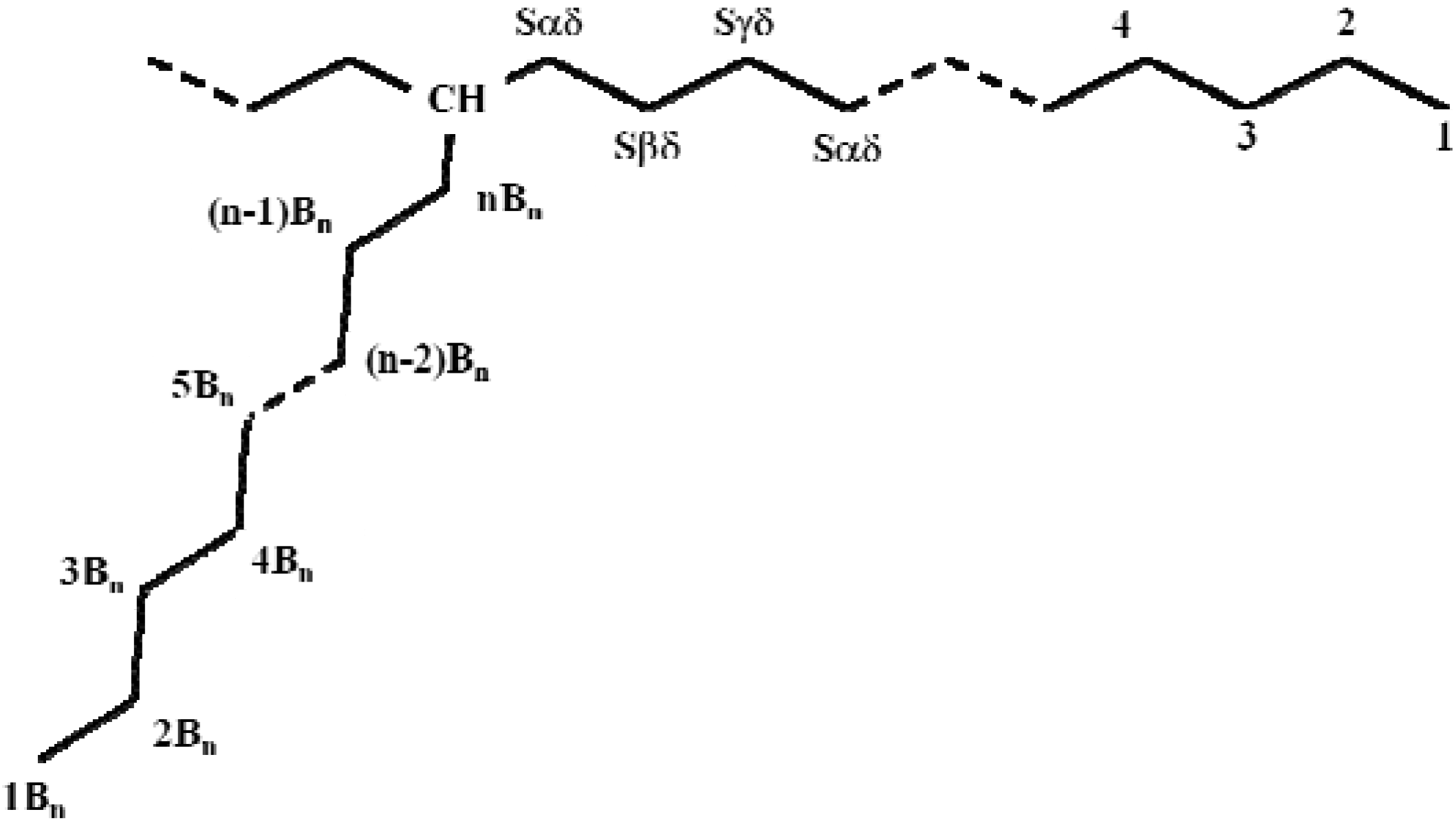
2.2.3. NMR analysis
| Carbon typea | Chemical shiftb (ppm) | ||
|---|---|---|---|
| ethylene/1-dodecene (1-C12) | ethylene/1-tetradecene (1-C14) | ethylene/1-octadecene (1-C18) | |
| 1Bn | 14.10 | 14.10 | 14.10 |
| 2 Bn | 22.80 | 22.80 | 22.80 |
| 3 Bn | 32.13 | 32.13 | 32.13 |
| 4 Bn | 29.50 | 29.50 | 29.50 |
| 5 Bn | 29.85 | 29.85 | 29.85 |
| 6 Bn | 29.90c | 29.90 c | 29.90 c |
| 7 Bn | 29.90 c | 29.90 c | 29.90 c |
| 8 Bn | 30.36 | 29.90 c | 29.90 c |
| 9 Bn | 27.18 | 29.90 c | 29.90 c |
| 10 Bn | 34.45 | 30.37 | 29.90 c |
| 11 Bn | - | 27.18 | 29.90 c |
| 12 Bn | - | 34.46 | 29.90 c |
| 13 Bn | - | - | 29.90 c |
| 14 Bn | - | - | 30.37 |
| 15 Bn | - | - | 27.18 |
| 16 Bn | - | - | 34.46 |
| CH | 38.11 | 38.11 | 38.11 |
| Sαδ | 34.49 | 34.49 | 34.49 |
| Sβδ | 27.20 | 27.20 | 27.20 |
| Sγδ | 30.38 | 30.38 | 30.38 |
| Sδδ | 29.90 | 29.90 | 29.90 |
| Entry | Olefin type | Triad distributiona | Cn (mol%)b | Tm (oC)c | χc (%)d | de (g/mL) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [EEE] | [XEE] | [EXE] | [XEX] | [EXX] | [XXX] | ||||||
| 2 | 1-C6 | 0.775 | 0.142 | 0.052 | 0.017 | 0.014 | 0.000 | 7 | 121.64 | 7.36 | 0.88 |
| 3 | 1-C8 | 0.412 | 0.379 | 0.164 | 0.045 | 0.000 | 0.000 | 16 | 113.62 | 2.44 | 0.88 |
| 4 | 1-C10 | 0.471 | 0.310 | 0.176 | 0.043 | 0.000 | 0.000 | 18 | 116.25 | 2.33 | 0.88 |
| 5 | 1-C12 | 0.499 | 0.331 | 0.135 | 0.035 | 0.000 | 0.000 | 13 | 117.61 | 6.21 | 0.89 |
| 6 | 1-C14 | 0.604 | 0.154 | 0.091 | 0.087 | 0.064 | 0.000 | 20 | 112.98 | 1.33 | 0.87 |
| 7 | 1-C18 | 0.591 | 0.214 | 0.152 | 0.043 | 0.000 | 0.000 | 15 | 117.58 | 4.34 | 0.88 |
2.2.4. Differential scanning calorimetric analysis
3. Experimental
3.1. Materials
3.2. Homo- and co-polymerization
3.3. Polymer characterization
4. Conclusions
Acknowledgements
References
- Chum, P.S.; Swogger, K.W. Olefin polymer technologies-History and recent progress at The Dow Chemical Company. Prog. Polym. Sci. 2008, 33, 797–819. [Google Scholar] [CrossRef]
- Cano, J.; Kunz, K. How to synthesize a constrained geometry catalyst (CGC) - A survey. J. Organomet. Chem. 2007, 692, 4519–4527. [Google Scholar] [CrossRef]
- Bensason, S.; Minick, J.; Moet, A.; Chum, S.; Hiltner, A.; Baer, E. Classification of homogeneous ethylene-octene copolymers based on comonomer content. J. Polym. Sci., Part A: Polym. Phys. 1996, 34, 1301–1315. [Google Scholar] [CrossRef]
- Ali, E.M.; Abasaeed, A.E.; Al-Zahrani, S.M. Optimization and control of industrial gas-phase ethylene polymerization reactors. Ind. Eng. Chem. Res. 1998, 37, 3414–3423. [Google Scholar] [CrossRef]
- Halterman, R.L. Synthesis and applications of chiral cyclopentadienylmetal complexes. Chem. Rev. 1992, 92, 965. [Google Scholar]
- Brintzinger, H.; Beck, S.; Leclerc, M.; Stehling, U.; Röll, W. Reaction Mechanisms in Metallocene-Catalyzed Olefin Polymerization. Stud. Surf. Sci. Catal. 1994, 89, 193–200. [Google Scholar] [CrossRef]
- Chien, J.C.W.; Nozaki, T. Ethylene-hexene copolymerization by heterogeneous and homogeneous Ziegler-Natta catalysts and the “comonomer effect”. J. Polym. Sci., Part A: Polym. Chem. 1993, 31, 227–237. [Google Scholar] [CrossRef]
- Simanke, A.G.; Galland, G.B.; Freitas, L.; Da Jornada, J.A.H.; Quijada, R.; Mauler, R.S. Influence of the comonomer content on the thermal and dynamic mechanical properties of metallocene ethylene/1-octene copolymers. Polymer 1999, 40, 5489–5495. [Google Scholar] [CrossRef]
- Shan, C.L.P.; Soares, J.B.P.; Penlidis, A. Ethylene/1-octene copolymerization studies with in situ supported metallocene catalysts: Effect of polymerization parameters on the catalyst activity and polymer microstructure. J. Polym. Sci., Part A: Polym. Chem. 2002, 40, 4426–4454. [Google Scholar] [CrossRef]
- Jaber, I.A.; Ray, W.H. Polymerization of olefins through heterogeneous catalysis. XIII. The influence of comonomer in the solution copolymerization of ethylene. J. Appl. Polym. Sci. 1993, 49, 1709–1724. [Google Scholar] [CrossRef]
- Kim, I.; Woo, S.I. Homo- and co-polymerization of ethylene with highly active Ti/Mg bimetallic complexes - Effect of crystallization conditions on structure and productivity. Polym. Bull. 1989, 22, 239–246. [Google Scholar] [CrossRef]
- Koivumäki, J.; Fink, G.; Seppälä, J.V. Copolymerization of ethene/1-dodecene and ethene/1-octadecene with the stereorigid zirconium catalyst systems iPr[FluCp]ZrCl2/ MAO and Me2Si[Ind]2ZrCl2/MAO: Influence of the comonomer chain length. Macromolecules 1994, 27, 6254–6258. [Google Scholar] [CrossRef]
- Karol, F.J.; Kao, S.-C.; Cann, K.J. Comonomer effects with high-activity titanium- and vanadium-based catalysts for ethylene polymerization. J. Polym. Sci., Part A: Polym. Chem. 1993, 31, 2541–2553. [Google Scholar] [CrossRef]
- Bialek, M.; Czaja, K. The effect of the comonomer on the copolymerization of ethylene with long chain α-olefins using Ziegler-Natta catalysts supported on MgCl2(THF)2. Polymer 2000, 41, 7899–7904. [Google Scholar] [CrossRef]
- Awudza, J.A.M.; Tait, P.J.T. The "comonomer effect" in ethylene/α-olefin copolymerization using homogeneous and silica-supported Cp2ZrCl2/MAO catalyst systems: Some insights from the kinetics of polymerization, active center studies, and polymerization temperature. J. Polym. Sci., Part A: Polym. Chem. 2008, 46, 267–277. [Google Scholar] [CrossRef]
- McKnight, A.L.; Waymouth, R.M. Group 4 ansa-cyclopentadienyl-amido catalysts for olefin polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef]
- Kaminsky, W.; Piel, C.; Scharlach, K. Polymerization of ethene and longer chained olefins by metallocene catalysis. Macromol. Symp. 2005, 226, 25–34. [Google Scholar] [CrossRef]
- Braunschweig, H.; Breitling, F.M. Constrained geometry complexes-synthesis and applications. Coord. Chem. Rev. 2006, 250, 2691–2720. [Google Scholar] [CrossRef]
- Pérez, E.; Benavente, R.; Quijada, R.; Narváez, A.; Barrera Galland, G. Structure characterization of copolymers of ethylene and 1-octadecene. J. Polym. Sci., Part B: Polym. Phys. 2000, 38, 1440–1448. [Google Scholar] [CrossRef]
- Li, K.-T.; Dai, C.-L.; Kuo, C.-W. Ethylene polymerization over a nano-sized silica supported Cp2ZrCl2/MAO catalyst. Catal. Commun. 2007, 8, 1209–1213. [Google Scholar] [CrossRef]
- Krimm, S.; Tobolsky, A.V. Quantitative x-ray studies of order in amorphous and crystalline polymers. Quantitative x-ray determination of crystallinity in polyethylene. J. polym. Sci. 1951, 7, 57–76. [Google Scholar] [CrossRef]
- Hong, H.; Zhang, Z.; Chung, T.C.M.; Lee, R.W. Synthesis of new 1-decene-based LLDPE resins and comparison with the corresponding 1-octene- and 1-hexene-based LLDPE resins. J. Polym. Sci., Part A: Polym. Chem. 2007, 45, 639–649. [Google Scholar]
- Camurati, I.; Cavicchi, B.; Dall'Occo, T.; Piemontesi, F. Synthesis and characterization of ethylene/1-olefin copolymers obtained by "single centre" catalysis. Macromol. Chem. Phys. 2001, 202, 701–709. [Google Scholar] [CrossRef]
- Koivumäki, J. Properties of ethylene/1-octene, 1-tetradecene and 1-octadecene copolymers obtained with Cp2ZrCl2/MAO catalyst: Effect of composition and comonomer chain length. Polym. Bull. 1996, 36, 7–12. [Google Scholar] [CrossRef]
- Starck, P.; Löfgren, B. Thermal properties of ethylene/long chain α-olefin copolymers produced by metallocenes. Eur. Polym. J. 2002, 38, 97–107. [Google Scholar] [CrossRef]
- Randall, J.C. Carbon-13 NMR of ethylene-1-olefin copolymers: extension to the short-chain branch distribution in a low-density polyethylene. J. Polym. Sci., Part A-2: Polym. Phys. 1973, 11, 275–287. [Google Scholar] [CrossRef]
- Kim, C.; Kim, H. Copolymerization of propylene with various higher α-olefins using silica-supported rac-Me2Si(Ind)2ZrCl2. J. Polym. Sci., Part A: Polym. Chem. 2001, 39, 3294–3303. [Google Scholar] [CrossRef]
- Quijada, R.; Galland, G.B.; Mauler, R.S. The influence of the comonomer in the copolymerization of ethylene with α-olefins using C2H4[Ind]2ZrCl2/ methylaluminoxane as catalyst system. Macromol. Chem. Phys. 1996, 197, 3091–3098. [Google Scholar] [CrossRef]
- Clas, S.D.; Mcfaddin, D.C.; Russell, K.E.; Scammell-Bullock, M.V.; Peat, I.R. Melting points of homogeneous random copolymers of ethylene and 1-alkenes. J. Polym. Sci., Part A: Polym. Chem. 2000, 41, 7899–7904. [Google Scholar]
- Jongsomjit, B.; Kaewkrajang, P.; Shiono, T.; Praserthdam, P. Supporting effects of silica-supported methylaluminoxane (MAO) with zirconocene catalyst on ethylene/1-olefin copolymerization behaviors for linear low-density polyethylene (LLDPE) production. Ind. Eng. Chem. Res. 2004, 43, 7959–7963. [Google Scholar] [CrossRef]
- Carlini, C.; D'Alessio, A.; Giaiacopi, S.; Po, R.; Pracella, M.; Raspolli Galletti, A.; Sbrana, G. Linear low-density polyethylenes by co-polymerization of ethylene with 1-hexene in the presence of titanium precursors and organoaluminium co-catalysts. Polymer 2007, 48, 1185–1192. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds (LLDPEs) are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wannaborworn, M.; Praserthdam, P.; Jongsomjit, B. Observation of Different Catalytic Activity of Various 1-Olefins during Ethylene/1-Olefin Copolymerization with Homogeneous Metallocene Catalysts. Molecules 2011, 16, 373-383. https://doi.org/10.3390/molecules16010373
Wannaborworn M, Praserthdam P, Jongsomjit B. Observation of Different Catalytic Activity of Various 1-Olefins during Ethylene/1-Olefin Copolymerization with Homogeneous Metallocene Catalysts. Molecules. 2011; 16(1):373-383. https://doi.org/10.3390/molecules16010373
Chicago/Turabian StyleWannaborworn, Mingkwan, Piyasan Praserthdam, and Bunjerd Jongsomjit. 2011. "Observation of Different Catalytic Activity of Various 1-Olefins during Ethylene/1-Olefin Copolymerization with Homogeneous Metallocene Catalysts" Molecules 16, no. 1: 373-383. https://doi.org/10.3390/molecules16010373




