Palladium(II) Complexes of NS Donor Ligands Derived from Steroidal Thiosemicarbazones as Antibacterial Agents
Abstract
:1. Introduction
2. Results and Discussion


2.1. IR spectral studies
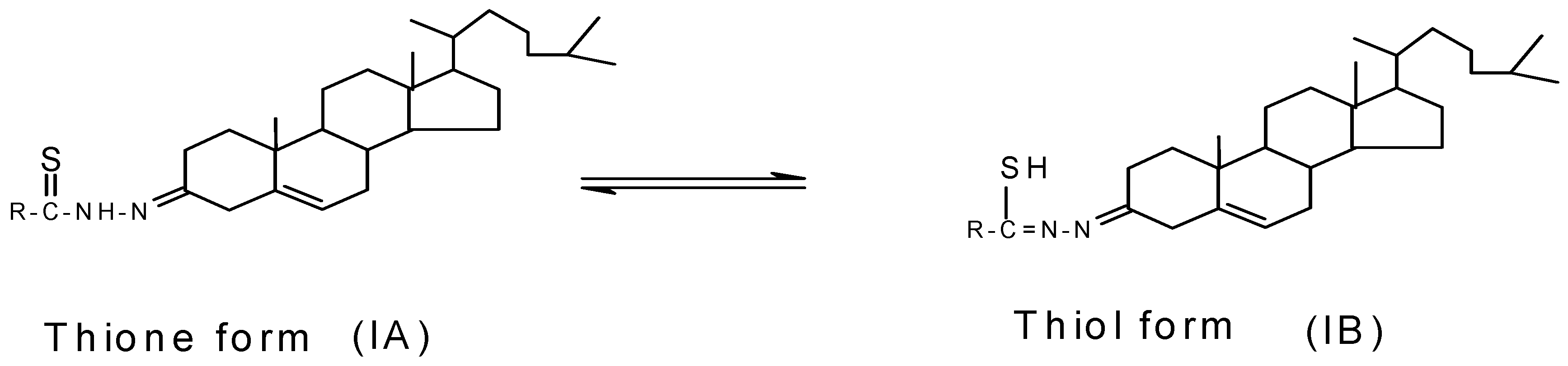

2.2. NMR spectral analysis
2.3. Thermogravimetric analysis
2.4. FAB mass analysis
2.5. In-vitro anti-bacterial activity
| Compound | Corresponding effect on microorganisms | |||
|---|---|---|---|---|
| S. aureus | S. Pyogenes | S. typhimurium | E. coli | |
| 1 | 15.2 ± 0.4 | 16.8 ± 0.4 | 16.4 ± 0.2 | 14.2 ± 0.5 |
| 2 | 13.4 ± 0.4 | 12.2 ± 0.5 | 11.6 ± 0.4 | 13.2 ± 0.5 |
| 3 | 11.2 ± 0.4 | 13.5 ± 0.3 | 10.9 ± 0.5 | 12.2 ± 0.2 |
| 1a | 21.5 ± 0.5 | 23.2± 0.4 | 25.6 ± 0.2 | 21.6 ± 0.4 |
| 2a | 16.6 ± 0.4 | 15.4 ± 0.2 | 16.2 ± 0.2 | 15.8 ± 0.4 |
| 3a | 15.5 ± 0.3 | 16.7± 0.4 | 14.5 ± 0.5 | 16.8 ± 0.5 |
| A. | 21.0 ± 0.5 | 22.2 ± 0.4 | 25.2 ± 0.8 | 20.0 ± 0.2 |
| DMSO | - | - | - | - |
3. Experimental
3.1. General
3.2. Synthesis of thiosemicarbazones: A general method
3.3. Preparation of palladium (II) complexes
3.4. Organism culture and in vitro screening
4. Conclusions
Acknowledgements
References
- Gupta, P.; Basuli, F.; Peng, S.M.; Lee, G.H.; Bhattacharya, S. Unprecedented Chemical Transformation of Benzaldehyde Semicarbazone Mediated by Osmium. Inorg. Chem. 2003, 42, 2069–2074. [Google Scholar] [CrossRef]
- Lobana, T.S.; Kumari, P.; Hundal, G.; Butcher, R.J. Metal derivatives of N1-substituted thiosemicarbazones with divalent metal ions (Ni, Cu): Synthesis and structures. Polyhedron 2010, 29, 1130–1136. [Google Scholar] [CrossRef]
- Singh, N.K.; Srivastava, A. In vitro and in vivo antitumour studies of a new thiosemicarbazide derivative and its complexes with 3d-metal ions. Trans. Met. Chem. 2000, 25, 133. [Google Scholar] [CrossRef]
- Rosu, T.; Pahontu, E.; Pasculescu, S.; Georgescu, R.; Stanica, N.; Curaj, A.; Popescu, A.; Leabu, M. Synthesis, characterization antibacterial and antiproliferative activity of novel Cu(II) and Pd(II) complexes with 2-hydroxy-8-R-tricyclo[7.3.1.0.2,7]tridecane-13-one thiosemicarbazone. Eur. J. Med. Chem. 2010, 45, 1627–1634. [Google Scholar] [CrossRef]
- Chandra, S.; Kumar, U. Spectral and magnetic studies on manganese(II), cobalt(II) and nickel(II) complexes with Schiff bases. Spect. Chim. Acta A. 2005, 61, 219–224. [Google Scholar] [CrossRef]
- Ferraz, K.S.O.; Ferandes, L.; Carrilho, D.; Pinto, M.C.X.; Leite, M.F.; Souza–Fagundes, E.M.; Speziali, N.L.; Mendes, I.C.; Beraldo, H. 2-Benzoylpyridine-N(4)-tolyl thiosemicarbazones and their palladium(II) complexes: Cytotoxicity against leukemia cells. Bioorg. Med. Chem. 2009, 17, 7138–7144. [Google Scholar] [CrossRef]
- Saha, N.C.; Butcher, R.J.; Chaudhuri, S.; Saha, N. Synthesis and spectroscopic characterisation of cobalt(III) and nickel(II) complexes with 5-methyl-3-formylpyrazole-N(4)-dibutylthiosemicarbazone (HMPzNBu2): X-ray crystallography of [Co(MPzNBu2)2]NO3·H2O (I) and [Ni(HMPzNBu2)2](ClO4)2 (II). Polyhedron 2003, 22, 383–390. [Google Scholar] [CrossRef]
- West, D.X.; Ives, J.S.; Krejci, J.; Salberg, M.M.; Zumbahlen, T.L.; Bain, G.A.; Liberta, A.E.; Valdes-Martinez, J.; Hernandez-Ortiz, S.; Toscano, R.A. Copper(II) complexes of 2-benzoylpyridine 4N-substituted thiosemicarbazones. Polyhedron 1995, 14, 2189–2200. [Google Scholar]
- Khan, S.A. Synthesis, characterization and in vitro antibacterial activity of new steroidal 5-en-3-oxazolo and thiazoloquinoxaline. Eur. J. Med. Chem. 2008, 43, 2040–2044. [Google Scholar] [CrossRef]
- Singh, S.; Athar, F.; Maurya, M.R.; Azam, A. Cyclooctadiene Ru(II) complexes of thiophene-2-carboxaldehyde-derived thiosemicarbazones: synthesis, characterization and antiamoebic activity. Eur. J. Med. Chem. 2006, 41, 592–598. [Google Scholar] [CrossRef]
- Budakoti, A.; Abid, A.; Azam, A. Syntheses, characterization and in vitro antiamoebic activity of new Pd(II) complexes with 1-N-substituted thiocarbamoyl-3,5-diphenyl-2-pyrazoline derivatives. Eur. J. Med. Chem. 2007, 42, 544–551. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, Z.; Saleem, K. Synthesis, structure elucidation and antibacterial evaluation of new steroidal -5-en-7-thiazoloquinoxaline derivative. Eur. J. Med. Chem. 2008, 43, 2257–2261. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Asiri, A.M.; Khan, S.A. Palladium(II) Complexes of NS Donor Ligands Derived from Steroidal Thiosemicarbazones as Antibacterial Agents. Molecules 2010, 15, 4784-4791. https://doi.org/10.3390/molecules15074784
Asiri AM, Khan SA. Palladium(II) Complexes of NS Donor Ligands Derived from Steroidal Thiosemicarbazones as Antibacterial Agents. Molecules. 2010; 15(7):4784-4791. https://doi.org/10.3390/molecules15074784
Chicago/Turabian StyleAsiri, Abdullah M., and Salman A. Khan. 2010. "Palladium(II) Complexes of NS Donor Ligands Derived from Steroidal Thiosemicarbazones as Antibacterial Agents" Molecules 15, no. 7: 4784-4791. https://doi.org/10.3390/molecules15074784




