Carbon-Carbon Cross Coupling Reactions in Ionic Liquids Catalysed by Palladium Metal Nanoparticles
Abstract
:Introduction
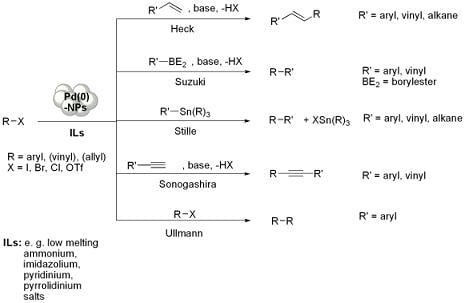
Pd-NPs Catalysed Carbon-Carbon Coupling Reactions in ILs
Preparation of Pd-NPs in ILs

Mizoroki-Heck Reaction with Pd-NPs in ILs


 | |||||
|---|---|---|---|---|---|
| Entry | Olefin | Ar | Product | Time (h) | Yield (%) b,c |
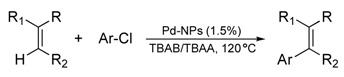
| 1 |  | 4-CF3C6H4 | 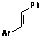 | 3 | 95 |
| 2 | C6H5 | 3 | 88 | ||
| 3 | 4-CH3OC6H4 | 2 | 95 | ||
| 4 |  | C6H5 |  | 5 | 78 |
| 5 | 4-CH3OC6H4 | 5 | 85 | ||
| 6 |  | C6H5 |  | 3 | 95 |
| 7 | 4-CH3OC6H4 | 3 | 90 | ||
| 8 | 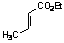 | C6H5 |  | 5 | 80d |
| 9 | 4-CH3OC6H4 | 5 | 92 | ||
Suzuki-Miyaura Reaction with Pd-NPs in ILs
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | X | IL | Base (aq) | T (°C) | t (h) | Conv (%)b | Yield (%)c |
| 1 | Br | TBAB | Na2CO3 | 110 | 0.5 | >99 | 95 |
| 2 | Br | TBAB | Na2CO3 | 60 | 16 | <1 | --- |
| 3 | Cl | TBAB | Na2CO3 | 140 | 16 | 15 | --- |
| 4 | Cl | TBAB | KOH | 90 | 16 | 36 | 20 |
| 5 | Cl | TBAB | NBu4OH | 90 | 3 | 93 | 86 |
| 6 | Cl | THeptAB | NBu4OH | 90 | 3 | 98 | 92 |
| 7 | Cl | TBAB | NBu4OH | 70 | 4.5 | 57 | 45 |
| 8 | Cl | THeptAB | NBu4OH | 70 | 4.5 | 89 | 83 |
| 9 | Cl | THeptAB | NBu4OH | 60 | 16 | <1 | --- |
| 10 | Br | THeptAB | NBu4OH | 60 | 1.5 | >99 | 93 |
Stille Reaction





Sonogashira Reaction


Ullmann Reaction




Summary and Outlook
Acknowledgements
References and Notes
- Beletskaya, I.P.; Cheprakov, A.V. The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.S.; Liu, W.; Wan, Q.X.; Wu, H.H.; Gao, G.H. Transition-Metal Catalyzed Carbon-Carbon Couplings Mediated with Functionalized Ionic Liquids, Supported-Ionic Liquid Phase, or Ionic Liquid Media. Curr. Org. Chem. 2009, 13, 1322–1346. [Google Scholar] [CrossRef]
- Moreno-Manas, M.; Pleixats, R. Formation of Carbon-Carbon Bonds under Catalysis by Transition-Metal Nanoparticles. Acc. Chem. Res. 2003, 36, 638–643. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Van Der Sluys, M.; Jones, C.W. On the Nature of the Active Species in Palladium Catalyzed Mizoroki-Heck and Suzuki-Miyaura Couplings - Homogeneous or Heterogeneous Catalysis, a Critical Review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Yin, L.X.; Liebscher, J. Carbon-Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar]
- Bedford, R.B. Palladacyclic Catalysts in C-C and C-Heteroatom Bond-Forming Reactions. Chem. Commun. 2003, 1787–1796. [Google Scholar] [CrossRef]
- de Vries, A.H.M.; Mulders, J.; Mommers, J.H.M.; Henderickx, H.J.W.; de Vries, J.G. Homeopathic Ligand-Free Palladium as a Catalyst in the Heck Reaction. A Comparison with a Palladacycle. Org. Lett. 2003, 5, 3285–3288. [Google Scholar] [CrossRef]
- de Vries, A.H.M.; Mulders, J.; Willans, C.E.; Schmieder-van de Vondervoort, L.; Parlevliet, F.J.; de Vries, J.G. Heck Reactions with Homeopathic Palladium. Abstr. Paper. Am. Chem. Soc. 2003, 225, U287. [Google Scholar]
- de Vries, A.H.M.; Parlevliet, F.J.; Schmieder-van de Vondervoort, L.; Mommers, J.H.M.; Henderickx, H.J.W.; Walet, M.A.M.; de Vries, J.G. A Practical Recycle of a Ligand-Free Palladium Catalyst for Heck Reactions. Adv. Synth. Catal. 2002, 344, 996–1002. [Google Scholar] [CrossRef]
- Reetz, M.T.; de Vries, J.G. Ligand-Free Heck Reactions Using Low Pd-Loading. Chem. Commun. 2004, 1559–1563. [Google Scholar]
- Reetz, M.T.; Westermann, E.; Lohmer, R.; Lohmer, G. A Highly Active Phosphine-Free Catalyst System for Heck Reactions of Aryl Bromides. Tetrahedron Lett. 1998, 39, 8449–8452. [Google Scholar]
- Dupont, J.; Consorti, C.S.; Spencer, J. The Potential of Palladacycles: More Than Just Precatalysts. Chem. Rev. 2005, 105, 2527–2571. [Google Scholar] [CrossRef]
- Cassol, C.C.; Umpierre, A.P.; Machado, G.; Wolke, S.I.; Dupont, J. The Role of Pd Nanoparticles in Ionic Liquid in the Heck Reaction. J. Am. Chem. Soc. 2005, 127, 3298–3299. [Google Scholar]
- Migowski, P.; Dupont, J. Catalytic Applications of Metal Nanoparticles in Imidazolium Ionic Liquids. Chem. Eur. J. 2007, 13, 32–39. [Google Scholar] [CrossRef]
- Reetz, M.T.; Westermann, E. Phosphane-Free Palladium-Catalyzed Coupling Reactions: The Decisive Role of Pd Nanoparticles. Angew. Chem. Int. Ed. 2000, 39, 165–166. [Google Scholar] [CrossRef]
- Rocaboy, C.; Gladysz, J.A. Thermomorphic Fluorous Imine and Thioether Palladacycles as Precursors for Highly Active Heck and Suzuki Catalysts; Evidence for Palladium Nanoparticle Pathways. New J. Chem. 2003, 27, 39–49. [Google Scholar] [CrossRef]
- Tromp, M.; Sietsma, J.R.A.; van Bokhoven, J.A.; van Strijdonck, G.P.F.; van Haaren, R.J.; van der Eerden, A.M.J.; van Leeuwen, P.; Koningsberger, D.C. Deactivation Processes of Homogeneous Pd Catalysts Using in Situ Time Resolved Spectroscopic Techniques. Chem. Commun. 2003, 128–129. [Google Scholar]
- Prechtl, M.H.G.; Scariot, M.; Scholten, J.D.; Machado, G.; Teixeira, S. R.; Dupont, J. Nanoscale Ru(0) Particles: Arene Hydrogenation Catalysts in Imidazolium Ionic Liquids. Inorg. Chem. 2008, 47, 8995–9001. [Google Scholar]
- Prechtl, M.H.G.; Scholten, J.D.; Dupont, J. Tuning the Selectivity of Ruthenium Nanoscale Catalysts with Functionalised Ionic Liquids: Hydrogenation of Nitriles. J. Mol. Catal. A 2009, 313, 74–78. [Google Scholar] [CrossRef]
- Hart, R.; Pollet, P.; Hahne, D.J.; John, E.; Llopis-Mestre, V.; Blasucci, V.; Huttenhower, H.; Leitner, W.; Eckert, C.A.; Liotta, C.L. Benign Coupling of Reactions and Separations with Reversible Ionic Liquids. Tetrahedron 2010, 66, 1082–1090. [Google Scholar]
- Jessop, P.G.; Heldebrant, D.J.; Li, X.W.; Eckert, C.A.; Liotta, C.L. Green Chemistry - Reversible Nonpolar-to-Polar Solvent. Nature 2005, 436, 1102–1102. [Google Scholar]
- Liu, Y.X.; Jessop, P.G.; Cunningham, M.; Eckert, C.A.; Liotta, C.L. Switchable Surfactants. Science 2006, 313, 958–960. [Google Scholar] [CrossRef]
- Takahash, Y.; Ito, T.; Sakai, S.; Ishii, Y. A Novel Palladium(O) Complex - Bis(Dibenzylideneacetone)Palladium(O). J. Chem. Soc. D Chem. Commun. 1970, 1065–1066. [Google Scholar]
- Bonnemann, H.; Brijoux, W.; Brinkmann, R.; Dinjus, E.; Joussen, T.; Korall, B. Formation of Colloidal Transition-Metals in Organic Phases and Their Application in Catalysis. Angew. Chem. Int. Ed. 1991, 30, 1312–1314. [Google Scholar] [CrossRef]
- Bonnemann, H.; Brijoux, W.; Joussen, T. The Preparation of Finely Divided Metal and Alloy Powders. Angew. Chem. Int. Ed. 1990, 29, 273–275. [Google Scholar] [CrossRef]
- Reetz, M.T.; Lohmer, G. Propylene Carbonate Stabilized Nanostructured Palladium Clusters as Catalysts in Heck Reactions. Chem. Commun. 1996, 1921–1922. [Google Scholar] [CrossRef]
- Reetz, M.T.; Breinbauer, R.; Wanninger, K. Suzuki and Heck Reactions Catalyzed by Preformed Palladium Clusters and Palladium/Nickel Bimetallic Clusters. Tetrahedron Lett. 1996, 37, 4499–4502. [Google Scholar] [CrossRef]
- Reetz, M.T.; Quaiser, S.A. A New Method for the Preparation of Nanostructured Metal-Clusters. Angew. Chem. Int. Ed. 1995, 34, 2240–2241. [Google Scholar]
- Reetz, M.T.; Helbig, W. Size-Selective Synthesis of Nanostructured Transition-Metal Clusters. J. Am. Chem. Soc. 1994, 116, 7401–7402. [Google Scholar] [CrossRef]
- Deshmukh, R.R.; Rajagopal, R.; Srinivasan, K.V. Ultrasound Promoted C-C Bond Formation: Heck Reaction at Ambient Conditions in Room Temperature Ionic Liquids. Chem. Commun. 2001, 1544–1545. [Google Scholar]
- Xu, L.J.; Chen, W.P.; Xiao, J.L. Heck Reaction in Ionic Liquids and the in Situ Identification of N-Heterocyclic Carbene Complexes of Palladium. Organometallics 2000, 19, 1123–1127. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Detomaso, A.; Iliade, P. Pd Nanoparticle Catalyzed Heck Arylation of 1,1-Disubstituted Alkenes in Ionic Liquids. Study on Factors Affecting the Regioselectivity of the Coupling Process. Organometallics 2003, 22, 4193–4197. [Google Scholar] [CrossRef]
- Umpierre, A.P.; Machado, G.; Fecher, G.H.; Morais, J.; Dupont, J. Selective Hydrogenation of 1,3-Butadiene to 1-Butene by Pd(0) Nanoparticles Embedded in Imidazolium Ionic Liquids. Adv. Synth. Catal. 2005, 347, 1404–1412. [Google Scholar] [CrossRef]
- Dupont, J.; Scholten, J.D. On the Structural and Surface Properties of Transition-Metal Nanoparticles in Ionic Liquids. Chem. Soc. Rev. 2010, 39, 1780–1804. [Google Scholar] [CrossRef]
- Yang, X.; Fei, Z.F.; Zhao, D.B.; Ang, W.H.; Li, Y.D.; Dyson, P.J. Palladium Nanoparticles Stabilized by an Ionic Polymer and Ionic Liquid: A Versatile System for C-C Cross-Coupling Reactions. Inorg. Chem. 2008, 47, 3292–3297. [Google Scholar] [CrossRef]
- Astruc, D. Palladium Nanoparticles as Efficient Green Homogeneous and Heterogeneous Carbon-Carbon Coupling Precatalysts: A Unifying View. Inorg. Chem. 2007, 46, 1884–1894. [Google Scholar]
- Dupont, J.; de Souza, R.F.; Suarez, P.A.Z. Ionic Liquid (Molten Salt) Phase Organometallic Catalysis. Chem. Rev. 2002, 102, 3667–3691. [Google Scholar] [CrossRef]
- Dupont, J. On the Solid, Liquid and Solution Structural Organization of Imidazolium Ionic Liquids. J. Braz. Chem. Soc. 2004, 15, 341–350. [Google Scholar] [CrossRef]
- Gozzo, F.C.; Santos, L.S.; Augusti, R.; Consorti, C.S.; Dupont, J.; Eberlin, M.N. Gaseous Supramolecules of Imidazolium Ionic Liquids: "Magic" Numbers and Intrinsic Strengths of Hydrogen Bonds. Chem. Eur. J. 2004, 10, 6187–6193. [Google Scholar] [CrossRef]
- Hardacre, C.; Holbrey, J.D.; McMath, S.E.J.; Bowron, D.T.; Soper, A.K. Structure of Molten 1,3-Dimethylimidazolium Chloride Using Neutron Diffraction. J. Chem. Phys. 2003, 118, 273–278. [Google Scholar]
- Tsuzuki, S.; Tokuda, H.; Hayamizu, K.; Watanabe, M. Magnitude and Directionality of Interaction in Ion Pairs of Ionic Liquids: Relationship with Ionic Conductivity. J. Phys. Chem. B 2005, 109, 16474–16481. [Google Scholar]
- Mathews, C.J.; Smith, P.J.; Welton, T.; White, A.J.P.; Williams, D.J. In Situ Formation of Mixed Phosphine-Imidazolylidene Palladium Complexes in Room-Temperature Ionic Liquids. Organometallics 2001, 20, 3848–3850. [Google Scholar] [CrossRef]
- Dupont, J.; Spencer, J. On the Noninnocent Nature of 1,3-Dialkylimidazolium Ionic Liquids. Angew. Chem. Int. Ed. 2004, 43, 5296–5297. [Google Scholar] [CrossRef]
- Lebel, H.; Janes, M.K.; Charette, A.B.; Nolan, S.P. Structure and Reactivity of "Unusual" N-Heterocyclic Carbene (Nhc) Palladium Complexes Synthesized from Imidazolium Salts. J. Am. Chem. Soc. 2004, 126, 5046–5047. [Google Scholar]
- Wang, R.H.; Zeng, Z.; Twamley, B.; Piekarski, M.M.; Shreeve, J.M. Synthesis and Characterization of Pyrazolyl-Functionalized Imidazolium-Based Ionic Liquids and Hemilabile (Carbene)Palladium(Ii) Complex Catalyzed Heck Reaction. Eur. J. Org. Chem. 2007, 655–661. [Google Scholar]
- Ye, C.F.; Xiao, X.C.; Twamley, B.; LaLonde, A.D.; Norton, M.G.; Shreeve, J.M. Basic Ionic Liquids: Facile Solvents for Carbon-Carbon Bond Formation Reactions and Ready Access to Palladium Nanoparticles. Eur. J. Org. Chem. 2007, 5095–5100. [Google Scholar]
- Scholten, J.D.; Ebeling, G.; Dupont, J. On the Involvement of Nhc Carbenes in Catalytic Reactions by Iridium Complexes, Nanoparticle and Bulk Metal Dispersed in Imidazolium Ionic Liquids. Dalton Trans. 2007, 5554–5560. [Google Scholar]
- Bernardi, F.; Scholten, J.D.; Fecher, G.H.; Dupont, J.; Morais, J. Probing the Chemical Interaction between Iridium Nanoparticles and Ionic Liquid by Xps Analysis. Chem. Phys. Lett. 2009, 479, 113–116. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Laera, S.; Cioffi, N. Pd Nanoparticles Catalyzed Stereospecific Synthesis of Beta-Aryl Cinnamic Esters in Ionic Liquids. J. Org. Chem. 2003, 68, 2929–2933. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Cotugno, P. Heck Reactions with Palladium Nanoparticles in Ionic Liquids: Coupling of Aryl Chlorides with Deactivated Olefins. Angew. Chem. Int. Ed. 2009, 48, 6101–6103. [Google Scholar] [CrossRef]
- Consorti, C.S.; Flores, F.R.; Dupont, J. Kinetics and Mechanistic Aspects of the Heck Reaction Promoted by a Cn-Palladacycle. J. Am. Chem. Soc. 2005, 127, 12054–12065. [Google Scholar]
- Collman, J.P.; Kosydar, K.M.; Bressan, M.; Lamanna, W.; Garrett, T. Polymer-Bound Substrates - a Method to Distinguish between Homogeneous and Heterogeneous Catalysis. J. Am. Chem. Soc. 1984, 106, 2569–2579. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Fornaro, A.; Sabbatini, L.; Cioffi, N.; Ditaranto, N. Heck Reaction Catalyzed by Nanosized Palladium on Chitosan in Ionic Liquids. Organometallics 2004, 23, 5154–5158. [Google Scholar] [CrossRef]
- Tao, R.T.; Miao, S.D.; Liu, Z.M.; Xie, Y.; Han, B.X.; An, G.M.; Ding, K.L. Pd Nanoparticles Immobilized on Sepiolite by Ionic Liquids: Efficient Catalysts for Hydrogenation of Alkenes and Heck Reactions. Green Chem. 2009, 11, 96–101. [Google Scholar] [CrossRef]
- Pryjomska-Ray, I.; Gniewek, A.; Trzeciak, A.M.; Ziolkowski, J.J.; Tylus, W. Homogeneous/Heterogeneous Palladium Based Catalytic System for Heck Reaction. The Reversible Transfer of Palladium between Solution and Support. Top. Catal. 2006, 40, 173–184. [Google Scholar]
- Qiao, K.; Sugimura, R.; Bao, Q.X.; Tomida, D.; Yokoyamal, C. An Efficient Heck Reaction in Water Catalyzed by Palladium Nanoparticles Immobilized on Imidazolium-Styrene Copolymers. Catal. Commun. 2008, 9, 2470–2474. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Montingelli, F. Pd Nanoparticles as Efficient Catalysts for Suzuki and Stille Coupling Reactions of Aryl Halides in Ionic Liquids. J. Org. Chem. 2005, 70, 6040–6044. [Google Scholar] [CrossRef]
- Fernandez, F.; Cordero, B.; Durand, J.; Muller, G.; Malbosc, F.; Kihn, Y.; Teuma, E.; Gomez, M. Palladium Catalyzed Suzuki C-C Couplings in an Ionic Liquid: Nanoparticles Responsible for the Catalytic Activity. Dalton Trans. 2007, 5572–5581. [Google Scholar]
- Durand, J.; Teuma, E.; Malbosc, F.; Kihn, Y.; Gomez, M. Palladium Nanoparticles Immobilized in Ionic Liquid: An Outstanding Catalyst for the Suzuki C-C Coupling. Catal. Commun. 2008, 9, 273–275. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Effect of Catalysis on the Stability of Metallic Nanoparticles: Suzuki Reaction Catalyzed by Pvp-Palladium Nanoparticles. J. Am. Chem. Soc. 2003, 125, 8340–8347. [Google Scholar] [CrossRef]
- Pathak, S.; Greci, M.T.; Kwong, R.C.; Mercado, K.; Prakash, G.K.S.; Olah, G.A.; Thompson, M.E. Synthesis and Applications of Palladium-Coated Poly(Vinylpyridine) Nanospheres. Chem. Mat. 2000, 12, 1985–1989. [Google Scholar] [CrossRef]
- Ramarao, C.; Ley, S.V.; Smith, S.C.; Shirley, I.M.; DeAlmeida, N. Encapsulation of Palladium in Polyurea Microcapsules. Chem. Commun. 2002, 1132–1133. [Google Scholar]
- Liu, Y.B.; Khemtong, C.; Hu, J. Synthesis and Catalytic Activity of a Poly(N,N-Dialkylcarbodiimide)/Palladium Nanoparticle Composite: A Case in the Suzuki Coupling Reaction Using Microwave and Conventional Heating. Chem. Commun. 2004, 398–399. [Google Scholar]
- Gopidas, K.R.; Whitesell, J.K.; Fox, M.A. Synthesis, Characterization, and Catalytic Applications of a Palladium-Nanoparticle-Cored Dendrimer. Nano Lett. 2003, 3, 1757–1760. [Google Scholar] [CrossRef]
- Pittelkow, M.; Moth-Poulsen, K.; Boas, U.; Christensen, J.B. Poly(Amidoamine)-Dendrimer-Stabilized Pd(0) Nanoparticles as a Catalyst for the Suzuki Reaction. Langmuir 2003, 19, 7682–7684. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H.; Leyva, A. Catalytic Activity of Palladium Supported on Single Wall Carbon Nanotubes Compared to Palladium Supported on Activated Carbon Study of the Heck and Suzuki Couplings, Aerobic Alcohol Oxidation and Selective Hydrogenation. J. Mol. Catal. A 2005, 230, 97–105. [Google Scholar] [CrossRef]
- Kim, N.; Kwon, M.S.; Park, C.M.; Park, J. One-Pot Synthesis of Recyclable Palladium Catalysts for Hydrogenations and Carbon-Carbon Coupling Reactions. Tetrahedron Lett. 2004, 45, 7057–7059. [Google Scholar]
- Zim, D.; Nobre, S.M.; Monteiro, A.L. Suzuki Cross-Coupling Reaction Catalyzed by Sulfur-Containing Palladacycles: Formation of Palladium Active Species. J. Mol. Catal. A 2008, 287, 16–23. [Google Scholar] [CrossRef]
- Gaikwad, A.V.; Holuigue, A.; Thathagar, M.B.; ten Elshof, J.E.; Rothenberg, G. Ion- and Atom-Leaching Mechanisms from Palladium Nanoparticles in Cross-Coupling Reactions. Chem. Eur. J. 2007, 13, 6908–6913. [Google Scholar] [CrossRef]
- Thathagar, M.B.; ten Elshof, J.E.; Rothenberg, G. Pd Nanoclusters in C-C Coupling Reactions: Proof of Leaching. Angew. Chem. Int. Ed. 2006, 45, 2886–2890. [Google Scholar]
- de Vries, J.G. A Unifying Mechanism for All High-Temperature Heck Reactions. The Role of Palladium Colloids and Anionic Species. Dalton Trans. 2006, 421–429. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.B. Pd Nanoparticle Aging and Its Implications in the Suzuki Cross-Coupling Reaction. Langmuir 2005, 21, 2121–2123. [Google Scholar] [CrossRef]
- Zhao, D.B.; Fei, Z.F.; Geldbach, T.J.; Scopelliti, R.; Dyson, P.J. Nitrile-Functionalized Pyridinium Ionic Liquids: Synthesis, Characterization, and Their Application in Carbon - Carbon Coupling Reactions. J. Am. Chem. Soc. 2004, 126, 15876–15882. [Google Scholar]
- Chiappe, C.; Pieraccini, D.; Zhao, D.B.; Fei, Z.F.; Dyson, P.J. Remarkable Anion and Cation Effects on Stille Reactions in Functionalised Ionic Liquids. Adv. Synth. Catal. 2006, 348, 68–74. [Google Scholar] [CrossRef]
- Fei, Z.F.; Zhao, D.B.; Pieraccini, D.; Ang, W.H.; Geldbach, T.J.; Scopelliti, R.; Chiappe, C.; Dyson, P.J. Development of Nitrile-Functionalized Ionic Liquids for C-C Coupling Reactions: Implication of Carbene and Nanoparticle Catalysts. Organometallics 2007, 26, 1588–1598. [Google Scholar]
- Cui, Y.G.; Biondi, I.; Chaubey, M.; Yang, X.; Fei, Z.F.; Scopelliti, R.; Hartinger, C.G.; Li, Y.D.; Chiappe, C.; Dyson, P.J. Nitrile-Functionalized Pyrrolidinium Ionic Liquids as Solvents for Cross-Coupling Reactions Involving in Situ Generated Nanoparticle Catalyst Reservoirs. Phys. Chem. Chem. Phys. 2010, 12, 1834–1841. [Google Scholar]
- Yang, X.; Fei, Z.F.; Geldbach, T.J.; Phillips, A.D.; Hartinger, C.G.; Li, Y.D.; Dyson, P.J. Suzuki Coupling Reactions in Ether-Functionalized Ionic Liquids: The Importance of Weakly Interacting Cations. Organometallics 2008, 27, 3971–3977. [Google Scholar]
- Yan, N.; Yang, X.; Fei, Z.F.; Li, Y.D.; Kou, Y.; Dyson, P.J. Solvent-Enhanced Coupling of Sterically Hindered Reagents and Aryl Chlorides Using Functionalized Ionic Liquids. Organometallics 2009, 28, 937–939. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H.; Leyva, A. An Imidazolium Ionic Liquid Having Covalently Attached an Oxime Carbapalladacycle Complex as Ionophilic Heterogeneous Catalysts for the Heck and Suzuki-Miyaura Cross-Coupling. Tetrahedron 2004, 60, 8553–8560. [Google Scholar] [CrossRef]
- Gao, S.Y.; Zhang, H.J.; Wang, X.M.; Mai, W.P.; Peng, C.Y.; Ge, L.H. Palladium Nanowires Stabilized by Thiol-Functionalized Ionic Liquid: Seed-Mediated Synthesis and Heterogeneous Catalyst for Sonogashira Coupling Reaction. Nanotechnology 2005, 16, 1234–1237. [Google Scholar] [CrossRef]
- Calo, V.; Nacci, A.; Monopoli, A.; Cotugno, P. Palladium-Nanoparticle-Catalysed Ullmann Reactions in Ionic Liquids with Aldehydes as the Reductants: Scope and Mechanism. Chem. Eur. J. 2009, 15, 1272–1279. [Google Scholar] [CrossRef]
- Pachon, L.D.; Elsevier, C.J.; Rothenberg, G. Electroreductive Palladium-Catalysed Ullmann Reactions in Ionic Liquids: Scope and Mechanism. Adv. Synth. Catal. 2006, 348, 1705–1710. [Google Scholar] [CrossRef]
© 2010 by the authors;
Share and Cite
Prechtl, M.H.G.; Scholten, J.D.; Dupont, J. Carbon-Carbon Cross Coupling Reactions in Ionic Liquids Catalysed by Palladium Metal Nanoparticles. Molecules 2010, 15, 3441-3461. https://doi.org/10.3390/molecules15053441
Prechtl MHG, Scholten JD, Dupont J. Carbon-Carbon Cross Coupling Reactions in Ionic Liquids Catalysed by Palladium Metal Nanoparticles. Molecules. 2010; 15(5):3441-3461. https://doi.org/10.3390/molecules15053441
Chicago/Turabian StylePrechtl, Martin H. G., Jackson D. Scholten, and Jairton Dupont. 2010. "Carbon-Carbon Cross Coupling Reactions in Ionic Liquids Catalysed by Palladium Metal Nanoparticles" Molecules 15, no. 5: 3441-3461. https://doi.org/10.3390/molecules15053441