Cell Proliferation of HaCaT Keratinocytes on Collagen Films Modified by Argon Plasma Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Surface Spectroscopic Analysis
| Sample | C1s% | N1s% | O1s% | S2p% | N1s/C1s | O1s/C1s |
|---|---|---|---|---|---|---|
| Untreated samples | 66.0 | 14.1 | 19.3 | 0.4 | 0.21 | 0.29 |
| Argon plasma treatment | 58.1 | 11.3 | 25.0 | 0.4 | 0.19 | 0.43 |
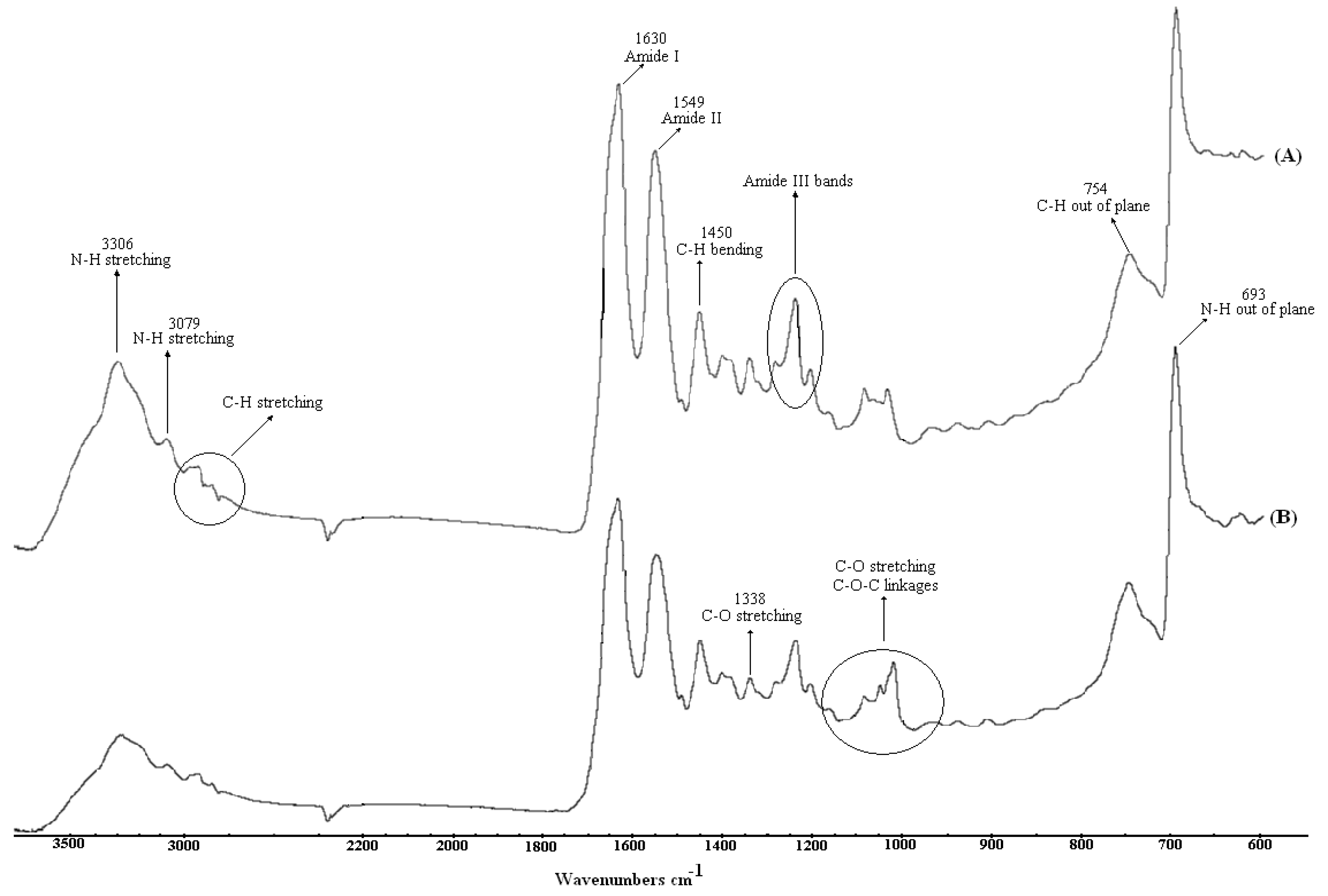
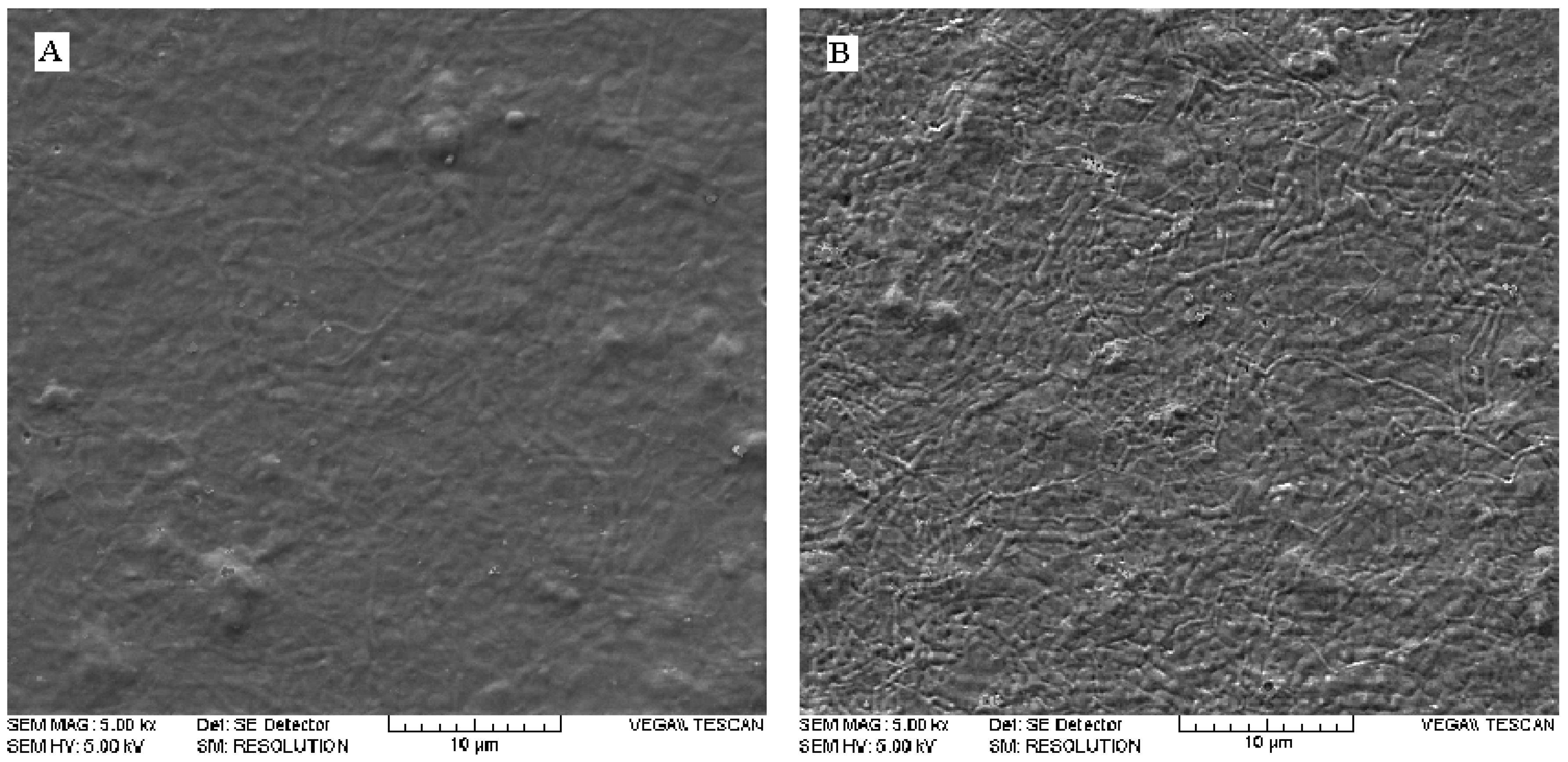
2.2. Effects of argon plasma upon surface topography
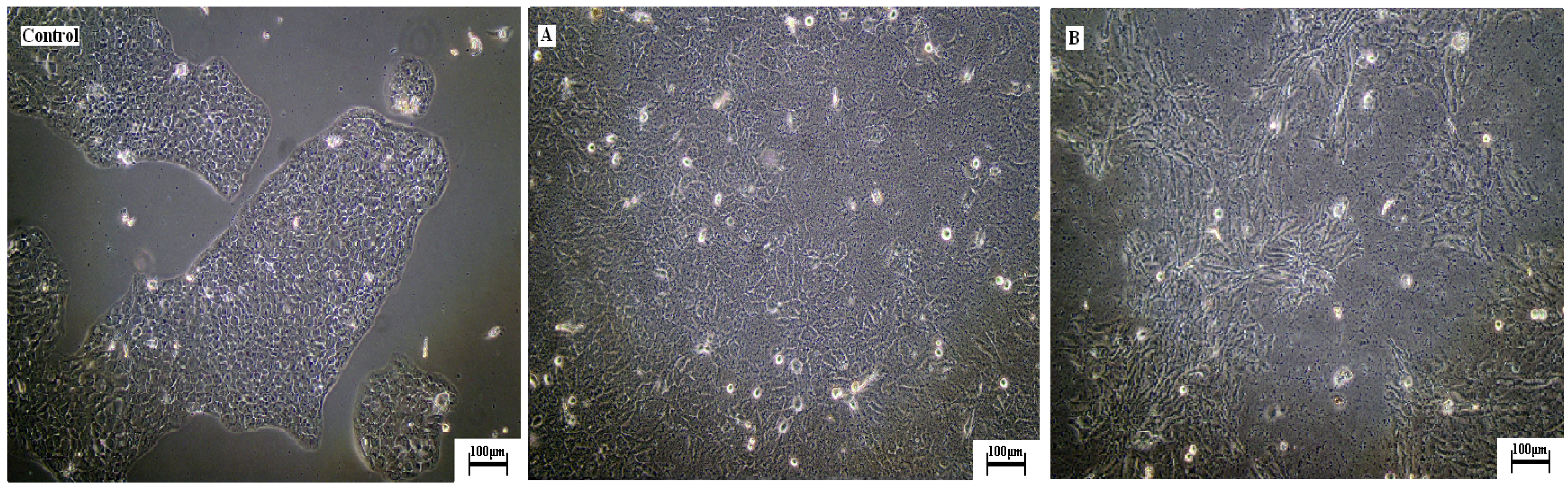
2.3. Effects of argon plasma treatment on cellular behaviour of HaCaT on collagen films

3. Experimental
3.1. Materials
3.2. Preparations of collagen films
3.3. Plasma treatment
3.4. Spectroscopic techniques
3.5. Microscopic techniques
3.6. Cell culture
3.7. Cell proliferation
4. Conclusions
Acknowledgements
- Sample Availability: Contact the authors.
References and Notes
- Prockopk, D.J.; Kivirikko, K.I. Collagens: Molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 1995, 64, 403–434. [Google Scholar] [CrossRef]
- Ji, Y.; Li, X.-T.; Chen, G.-Q. Interactions between a poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) terpolyester and human keratinocytes. Biomaterials 2008, 29, 3807–3814. [Google Scholar] [CrossRef]
- Lehmann, B. HaCaT cell line as a model system for vitamin D3 metabolism in human skin. J. Invest. Dermatol. 1997, 108, 78–82. [Google Scholar]
- Boelsma, E.; Verhoeven, M.C.; Ponec, M. Reconstruction of a human skin equivalent using a spontaneously transformed keratinocyte cell line (HaCaT). J. Invest. Dermatol. 1999, 112, 489–498. [Google Scholar] [CrossRef]
- Maas-Szabowski, M.; Stärker, A.; Fusenig, N.E. Epidermal tissue regeneration and stromal interaction in HaCaT cells is initiated by TGF-α. J. Cell Sci. 2003, 116, 2937–2948. [Google Scholar]
- Meineke, V.; Müller, K.; Ridi, R.; Cordes, N.; Köhn, F.M.; Mayerhofer, A.; Ring, J.; Van-Beuningen, D. Development and evaluation of a skin organ model for the analysis of radiation effects. Strahlenthe Onkol 2004, 108, 102–108. [Google Scholar]
- Pattrick, R.A.D. Noble Gases in Geochemistry and Cosmochemistry; Porcelli, D., Ballentine, C.J., Wieler, R., Eds.; Geochemical Society and Mineralogical Society of America: Washington, DC, USA, 2002; p. 844. [Google Scholar]
- Milinchuk, V.K. Photoradiation chemistry of polymers. Nucl. Instrum. Methods Phys. Res. 1995, B105, 24–29. [Google Scholar]
- Prat, R.; Shi, M.-K.; Clouet, F. Interactions of cold plasmas with polymers and their model molecules: Degradation vs. functionalzation. J. Macromol. Sci. A Pure Appl. Chem. 1997, 34, 471–488. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.V.; Wang, L.P.; Huang, N. Plasma-surface modifications of biomaterials. Mater. Sci. Eng. Res. 2002, R36, 143–206. [Google Scholar]
- Coen, M.C.; Dietler, G.; Kasas, S.; Groening, P. AFM Measurements of the topography and the roughness of ECR plasma treated polypropylene. Appl. Surf. Sci. 1996, 103, 27–34. [Google Scholar] [CrossRef]
- Coen, M.C.; Lehmann, R.; Groening, P.; Schlapbach, L. Modification of the micro and nanotopography of several polymers by plasma treatments. Appl. Surf. Sci. 2003, 207, 276–286. [Google Scholar] [CrossRef]
- Ruddy, A.C.; McNally, G.M.; Nersisyan, G.; Graham, W.G.; Murphy, W.R. The effect of atmospheric glow discharge (APGD) treatment on polyetherimide, polybutyleneterephthalate, and polyamides. J. Plast. Film Sheeting 2006, 22, 103–119. [Google Scholar] [CrossRef]
- Ru, L.; Jie-Rong, C. Studies on wettability of medical poly(vinyl chloride) by remote argon plasma. Appl. Surf. Sci. 2006, 252, 5076–5082. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Study of the ageing behavior of polymer films treated with a dielectric barrier discharge in air, helium and argon at medium pressure. Surf. Coat. Technol. 2007, 201, 7847–7854.s. [Google Scholar]
- Kotál, V.; Stopka, P.; Sajdl, P.; Švorčík, V. Thin surface layer of plasma treated polyethylene. Strength Mater. 2008, 40, 86–89. [Google Scholar] [CrossRef]
- Olifirenko, A.S.; Novak, I.; Rozova, E.Y.; Saprykina, N.N.; Mitilineos, A.G.; Elyashevich, G.K. Hydrophilization of porous polyethylene films by cold plasma of different types. Polym. Sci. Ser. B Polym. Chem. 2009, 51, 247–255. [Google Scholar] [CrossRef]
- Krishnarmurthy, V.; Kamel, I.L. Argon plasma treatment of glass surfaces. J. Mater. Sci. 1989, 24, 3345–3352. [Google Scholar] [CrossRef]
- Nakahara, M.; Sanada, Y. Effect of Plasma treatment on graphitic surface structure. Carbon 1995, 33, 735–736. [Google Scholar] [CrossRef]
- Surdu-Bob, C.C.; Saied, S.O.; Sullivan, J.L. An X-ray photoelectron spectroscopy study of the oxides of GaAs. Appl. Surf. Sci. 2001, 183, 126–136. [Google Scholar] [CrossRef]
- De Iorio, I.; Leone, C.; Nele, L.; Tagliaferri, V. Plasma treatment of polymeric materials and Al alloys for adhesive bonding. J. Mater. Process. Technol. 1997, 68, 179–183. [Google Scholar] [CrossRef]
- Quast, M.; Stock, H.-R.; Mayr, P. Plasma-assisted nitriding of aluminum-alloy parts. Met. Sci. Heat Treat. 2004, 46, 299–304. [Google Scholar] [CrossRef]
- Tsafack, M.J.; Levalois-Grutzmacher, J. Flame retardancy of cotton textiles by plasma-induced graft-polymerization (PIGP). Surf. Coat. Technol. 2006, 201, 2599–2610. [Google Scholar]
- Wei, Q.; Wang, Y.; Yang, Q.; Yu, L. Functionalization of textile materials by plasma enhanced modification. J. Ind. Text. 2007, 36, 301–309. [Google Scholar] [CrossRef]
- Karahan, H.A.; Özdogan, E. Improvements of surface functionality of cotton fibers by atmospheric plasma treatment. Fibers Polym. 2008, 9, 21–26. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, J.-C.; Yao, W.-H. Argon-plasma treatment for improving the physical properties of crosslinked cotton fabrics with dimethyloldihydroxyethyleneurea-acrylic acid. Text. Res. J. 2009. [Google Scholar] [CrossRef]
- Stanford, C.M.; Keller, J.C.; Solursh, M. Bone cell expression on titanium surfaces is altered by sterilization treatments. J. Dent. Res. 1994, 73, 1061–1071. [Google Scholar]
- Ayhan, F.; Ayhan, H.; Piskin, E. Sterilization of sutures by low temperature argon plasma. J. Bioact. Compat. Polym. 1998, 13, 65–72. [Google Scholar]
- Baxter, H.C.; Campbell, G.A.; Richardson, P.R.; Jones, A.C.; Whittle, I.R.; Casey, M.; Whittaker, A.G.; Baxter, R.L. Surgical instrument decontamination: Efficacy of introducing argon: Oxygen RF gas-plasmacleaning step as part of the cleaning cycle for stainless steel. IEEE Trans. Plasma Sci. 2006, 34, 1337–1344. [Google Scholar]
- Everaert, E.P.J.M.; van de Belt-Gritter, B.; van Der Mei, H.C.; Busscher, H.J.; Verkerke, G.J.; Dijk, J.; Mahieu, F. H. F.; Reitsma, A. In vitro and in vivo microbial adhesion and growth on argon plasma-treated silicone rubber voice prostheses. J. Mater. Sci. Mater. Med. 1998, 9, 147–157. [Google Scholar]
- Rafat, M.; Griffith, M.; Hakim, M.; Muzakare, L.; Li, F.; Khulbe, K.C.; Matsuura, T. Plasma surface modification and characterization of collagen-based artificial cornea for enhanced epithelialisation. J. Appl. Polym. Sci. 2007, 106, 2056–2064. [Google Scholar]
- Lim, H.R.; Bael, H.S.; Lee, M.H.; Woo, Y.I.; Han, D.-W.; Han, M.H.; Baik, H.K.; Choi, W.S.; Park, K.D.; Chung, K.-H.; Park, J.-C. Surface modification for enhancing behaviors of vascular endothelial cells onto polyurethane films by microwave-induced argon plasma. Surf. Coat. Technol. 2008, 202, 5768–5772. [Google Scholar]
- Baek, H.S.; Park, Y.H.; Ki, C.S.; Park, J.-C.; Rah, D.K. Enhanced chondrogenic responses of articular chondrocytes onto porous silk fibroin scaffolds treated with microwave-induced argon plasma. Surf. Coat. Technol. 2008, 202, 5794–5797. [Google Scholar] [CrossRef]
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar]
- Hauser, J.; Zietlow, J.; Köller, M.; Esenwein, S.A.; Halfmann, H.; Awakowicz, P.; Steinau, H.U. Enhanced cell adhesion to silicone implant material through plasma surface modification. J. Mater. Sci. Mater. Med. 2009, 20, 2541–2548. [Google Scholar]
- Sullivan, J.L.; Yu, W.; Saied, S.O. A study of the compositional changes in chemically etched, Ar ion bombarded and reactive ion etched GaAs(100) surfaces by means of ARXPS and LEISS. Appl. Surf. Sci. 1995, 90, 309–319. [Google Scholar] [CrossRef]
- Surdu-Bob, C.C.; Sullivan, J.L.; Saied, S.O.; Layberry, R.; Aflori, M. Surface compositional changes in GaAs subjected to argon plasma treatment. Appl. Surf. Sci. 2002, 202, 183–198. [Google Scholar] [CrossRef]
- Pascu, M.; Vasile, C.; Gheorghiu, M. Modification of polymer blend properties by argon/electron bean treatment: Surface properties. Mater. Chem. Phys. 2003, 80, 548–554. [Google Scholar] [CrossRef]
- Ye, R.; Kagohashi, T.; Zheng, W. Investigation of surface treatment of conductive wire in cylindrical atmospheric pressure plasmas. Thin Solid Films 2009, 518, 971–975. [Google Scholar] [CrossRef]
- Zubavichus, Y.; Shaporenko, A.; Grunze, M.; Zharnikow, M. Is X-ray absorption spectroscopy sensitive to the amino acid composition of functional proteins? J. Phys. Chem. B 2008, 112, 4478–4480. [Google Scholar]
- Wolf, K.L.; Sobral, P.J.A.; Telis, V.R.N. Physicochemical characterization of collagen fibers and collagen powder for self-composite film production. Food Hydrocolloids 2009, 23, 1886–1894. [Google Scholar]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–599. [Google Scholar] [CrossRef]
- Pelin, I.M.; Maier, S.S.; Chitanu, G.C.; Bulacovschi, V. Preparation and characterization of a hydroxyapatite-collagen composite as component for injectable bone substitute. Mater. Sci. Eng., C 2009, 29, 2188–2194. [Google Scholar]
- Almazán-Almazán, M.C.; Paredes, J.I.; Pérez-Mendoza, M.; Domingo-García, M.; López-Garzón, F.J.; Martínez-Alonso, A.; Tascón, J.M.D. Surface characterisation of plasma-modified poly(ethylene terephthalate). J. Colloid Interface Sci. 2006, 293, 353–363. [Google Scholar]
- Shi, L.-S.; Wang, L.-Y.; Wang, Y.-N. The investigation of argon plasma surface modification to polyethylene: Quantitative ATR-FTIR spectroscopic analysis. Eur. Polym. J. 2006, 42, 1625–1633. [Google Scholar]
- Schumacher, M.; Mizuno, K.; Bächinger, H.P. The crystal structure of the collagen-like polypeptide (glycyl-4(R)-hydroxyprolyl-4(R)-hydroxyprolyl)9 at 1.55 Å resolution shows up-puckering of the proline ring in the Xaa position. J. Biol. Chem. 2005, 280, 20397–20403. [Google Scholar]
- Okuyama, K.; Xu, X.; Iguchi, M.; Noguchi, K. Revision of collagen molecular structure. Biopolymers 2005, 84, 181–191. [Google Scholar]
- France, R.M.; Short, R.D. Effects of energy transfer from an argon plasma on the surface chemistry of poly(styrene), low density poly(ethylene), poly(propylene) and poly(ethylene terephthalate). J. Chem. Soc.,Faraday Trans. 1997, 93, 3173–3178. [Google Scholar] [CrossRef]
- Ishaug-Riley, S.L.; Okun, L.E.; Prado, G.; Applegate, M.A.; Ratcliffe, A. Human articular chondrocyte adhesion and proliferation on synthetic biodegradable polymer films. Biomaterials 1999, 20, 2245–2256. [Google Scholar] [CrossRef]
- Hu, S.-G.; Jou, C.-H.; Yang, M.C. Protein adsorption, fibroblast activity and antibacterial properties of poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) grafted with chitosan and chitooligosaccharide after immobilized with hyaluronic acid. Biomaterials 2003, 24, 2685–2693. [Google Scholar] [CrossRef]
- Lehocký, M.; Amaral, P.F.F.; Coelho, M.A.Z.; St’ahel, P.; Barros-Timmons, A.M.; Coutinho, J.A.P. Attachment/detachment of Saccharomyces cerevisiae on plasma deposited organosilicon thin films. Czech. J. Phys. 2006, 56, B1256–B1262. [Google Scholar]
- Peschel, G.; Dahse, H.-M.; Konrad, A.; Dieter-Wieland, G.; Mueller, P.-J.; Martin, D.P.; Roth, M. Growth of keratinocytes on porous films of poly(3-hydroxybutyrate) and poly(4-hydroxybutyrate) blended with hyaluronic acid and chitosan. J. Biomed. Mater. Res. Part A 2007, 85A, 1072–1081. [Google Scholar]
- Lehocký, M.; St’ahel, P.; Koutný, M.; Čech, J.; Institoris, J.; Mráček, A. Adhesion of Rhodococcus sp S3E2 and Rhodococcus sp S3E3 to plasma prepared teflon-like and organosilicon surfaces. J. Mater. Process. Technol. 2009, 209, 2871–2875. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tsiourvas, D.; Sideratou, Z. Hydrogen bonding interactions of liposomes simulating cell-cell recognition. A review. Origins Life Evol. Biosphere 2004, 34, 195–213. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A. Normal keratinization in a spontaneously immortalized aneuploid keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
García, J.L.; Asadinezhad, A.; Pacherník, J.; Lehocký, M.; Junkar, I.; Humpolíček, P.; Sáha, P.; Valášek, P. Cell Proliferation of HaCaT Keratinocytes on Collagen Films Modified by Argon Plasma Treatment. Molecules 2010, 15, 2845-2856. https://doi.org/10.3390/molecules15042845
García JL, Asadinezhad A, Pacherník J, Lehocký M, Junkar I, Humpolíček P, Sáha P, Valášek P. Cell Proliferation of HaCaT Keratinocytes on Collagen Films Modified by Argon Plasma Treatment. Molecules. 2010; 15(4):2845-2856. https://doi.org/10.3390/molecules15042845
Chicago/Turabian StyleGarcía, Jorge López, Ahmad Asadinezhad, Jiří Pacherník, Marián Lehocký, Ita Junkar, Petr Humpolíček, Petr Sáha, and Pavel Valášek. 2010. "Cell Proliferation of HaCaT Keratinocytes on Collagen Films Modified by Argon Plasma Treatment" Molecules 15, no. 4: 2845-2856. https://doi.org/10.3390/molecules15042845





