Efficient Lewis Acid Ionic Liquid-Catalyzed Synthesis of the Key Intermediate of Coenzyme Q10 under Microwave Irradiation
Abstract
:1. Introduction
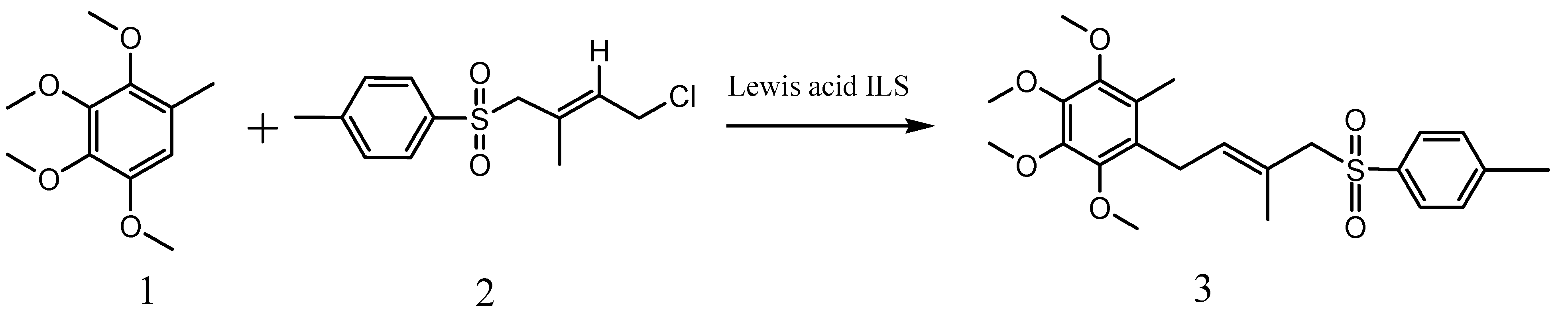
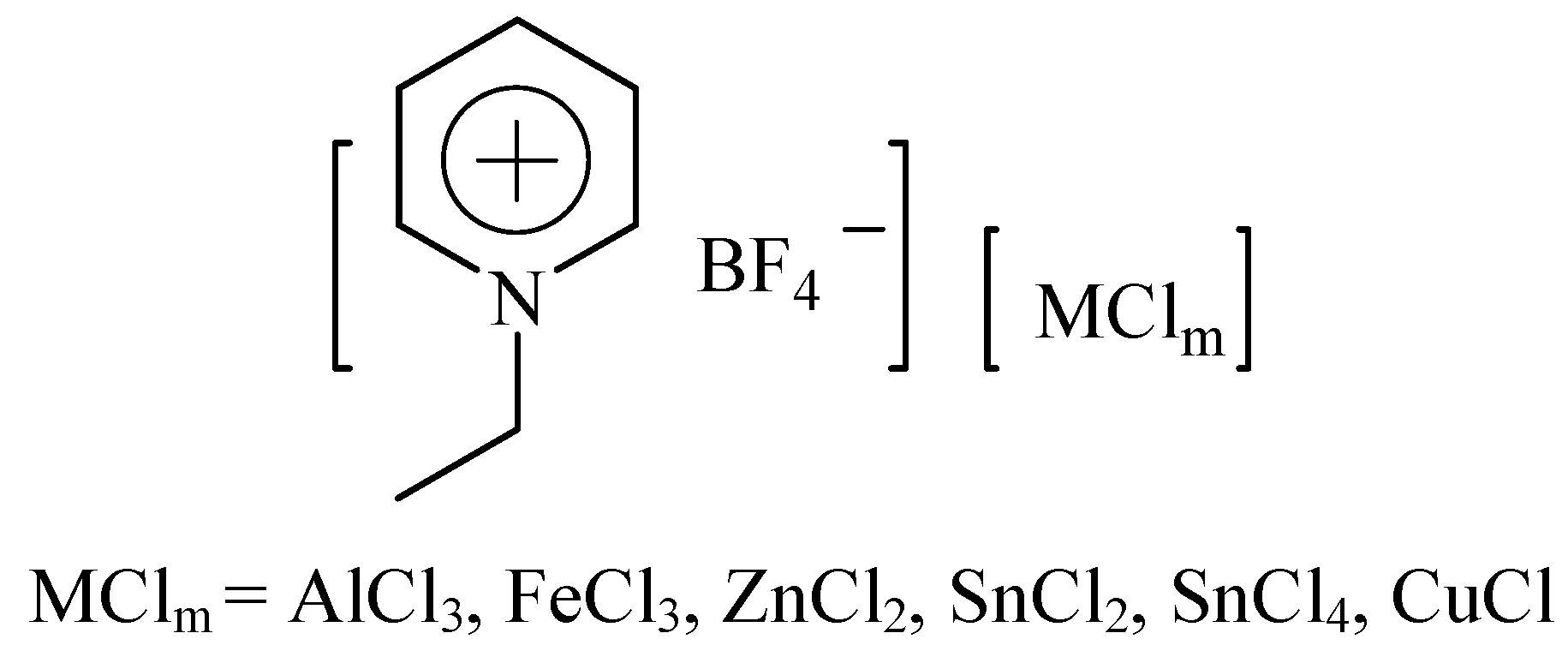
2. Results and Discussion
2.1. FT-IR characterization of Lewis acid ionic liquids
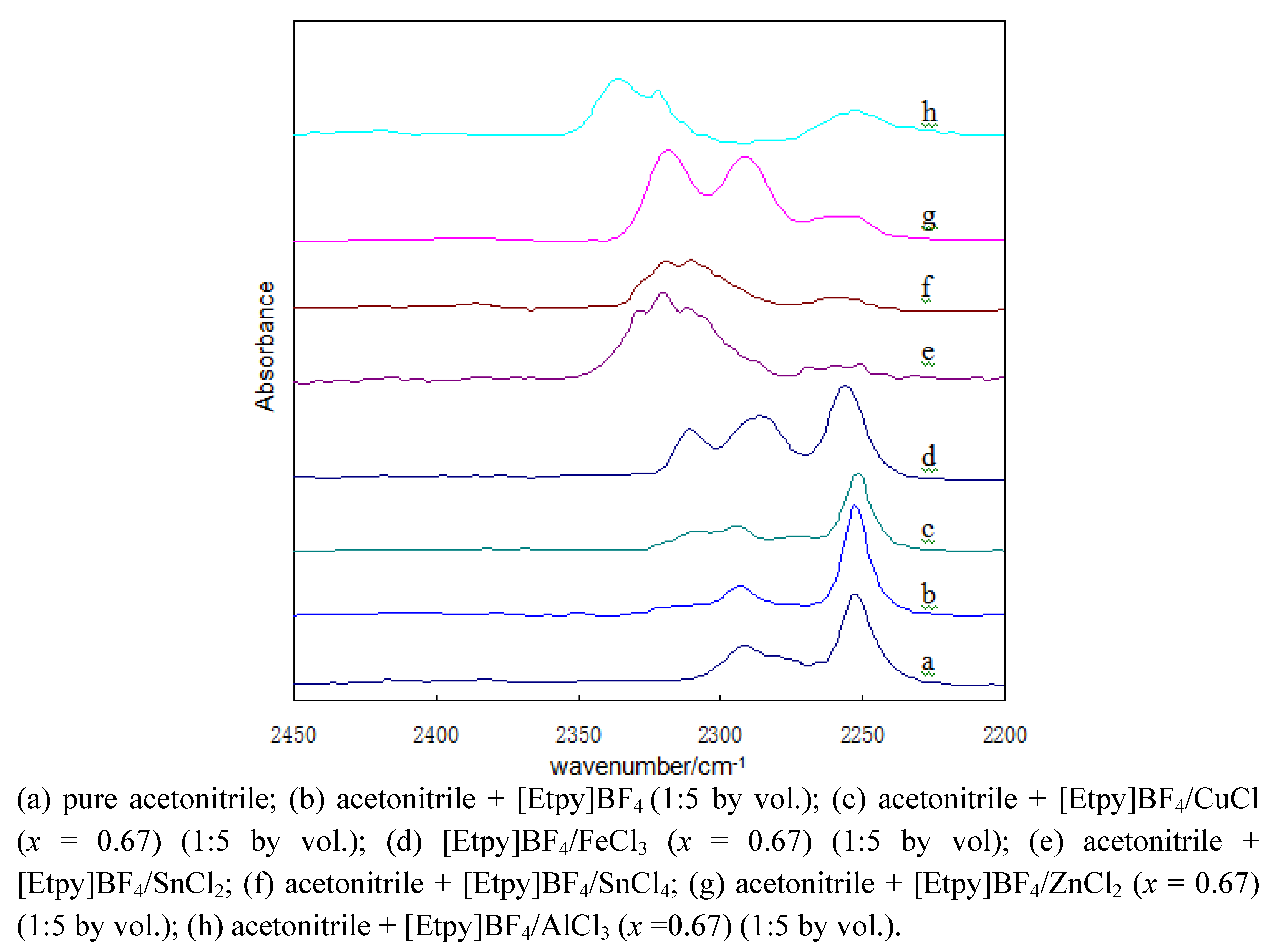
2.2. Comparison of the Friedel–Crafts reaction in organic solvents with in ionic liquids
| Entry | Catalyst/Solvent | Reaction Temperature(°C ) | Reaction Time (hrs) | Yield c (%) |
|---|---|---|---|---|
| 1 | FeCl3/C2H2Cl2 a | 80 | 7 | 51 |
| 2 | ZnCl2/C2H2Cl2 a | 80 | 8 | 75 |
| 3 | [Etpy]BF4-FeCl3b | 60 | 2 | 60 |
| 4 | [Etpy]BF4-ZnCl2 b | 60 | 2 | 89 |
2.3. Effect of molar fraction of Lewis acid in ionic liquid on the reaction yields
| Entry | x(ZnCl2) | n(ZnCl2):n([Etpy]BF4) | Yield b (%) | |
|---|---|---|---|---|
| ZnCl2 | FeCl3 | |||
| 1 | 0.33 | 0.5:1.0 | 0 | 0 |
| 2 | 0.50 | 1.0:1.0 | 51 | 0 |
| 3 | 0.60 | 1.5:1.0 | 75 | 50 |
| 4 | 0.67 | 2.0:1.0 | 89 | 60 |
| 5 | 0.71 | 2.5:1.0 | 86 | 58 |
2.4. Catalytic activities of various Lewis acid ionic liquids
| Entry | Ionic Liquid | Reaction Time (hrs) | Yield c (%) |
|---|---|---|---|
| 1 | [Etpy]BF4 | 20 | 0 |
| 2 | [Etpy]BF4-CuCl | 20 | 0 |
| 3 | [Etpy]BF4-FeCl3 | 2 | 60 |
| 4 | [Etpy]BF4-SnCl2 | 8 | 85 |
| 5 | [Etpy]BF4-SnCl4 | 4 | 86 |
| 6 | [Etpy]BF4-ZnCl2 | 2 | 89 |
| 7 | [Etpy]BF4-AlCl3 b | 1 | 0 |
2.5. Catalytic activities of various ionic liquids in microwave heating
| Entry | Catalyst/Solvent | Reaction Time (s) | Yield b(%) |
|---|---|---|---|
| 1 | [Etpy]BF4-FeCl3 | 150 | 60 |
| 2 | [Etpy]BF4-SnCl2 | 180 | 85 |
| 3 | [Etpy]BF4-SnCl4 | 180 | 84 |
| 4 | [Etpy]BF4-ZnCl2 | 150 | 89 |
2.6. Recycling and reuse of [Etpy]BF4-ZnCl2
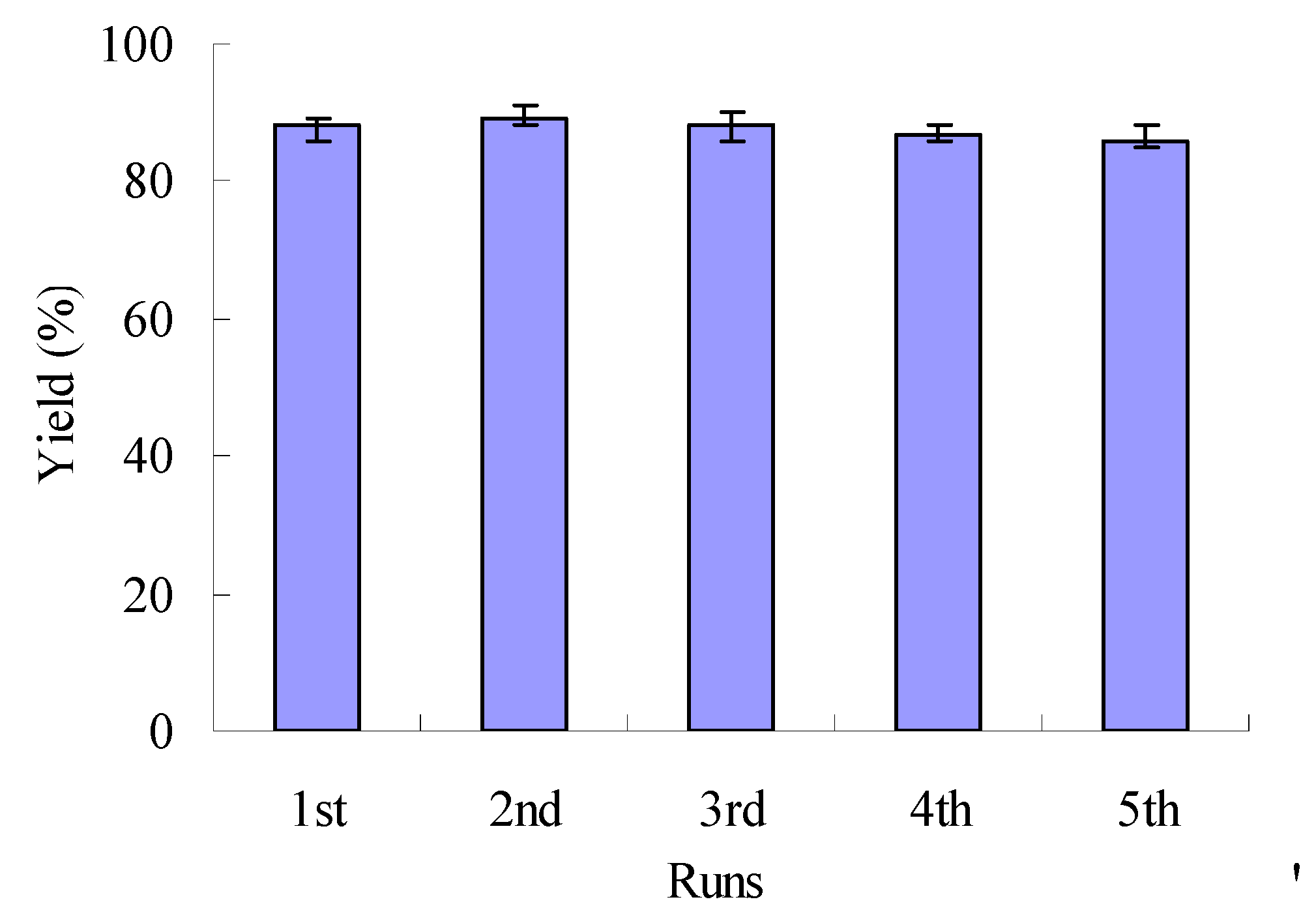
3. Experimental
3.1. General
3.2. Preparation and FT-IR characterization of Lewis acid ionic liquids
3.3. Synthesis of 1-[(E)-3-methyl-4-benzenesulfonyl-3-methylbut-2-enyl]-2,3,4,5-tetra-methoxy-6-methylbenzene (3) and analysis
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Thomson, R.H. Naturally Occurring Quinones; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Mitchell, P.; Moyle, J. Coenzyme Q; Lenaz, G., Ed.; Wiley: Chichester, UK, 1985. [Google Scholar]
- Melvyn, G. Pigments of fungi(Macromycetes). Nat. Prod. Rep. 1999, 16, 301–317. [Google Scholar] [CrossRef]
- Folkers, K. Coenzyme Q: Biochemistry, Bioenergetics and Clinical Applications of Ubiquinone; Lenaz, G., Ed.; Wiley-Interscience: New York, NY, USA, 1985. [Google Scholar]
- Frederick, L. Crane Discovery of ubiquinone (coenzyme Q) and an overview of function. Mitochondrion 2007, 7, S2–S7. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Mollard, P.; Pfeiffer, S.S.; Chrisman, W. A Short, Highly Efficient Synthesis of Coenzyme Q10. J. Am. Chem. Soc. 2002, 124, 14282–14283. [Google Scholar] [CrossRef]
- Mohri, M.; Kinoshita, H. Palladium-catalyzed Regio- and Stereoselective reduction of allylic compounds with LiHBEt3. Chem. Lett. 1986, 5, 1177–1179. [Google Scholar]
- Sato, K.; Myamoto, O.; Inoue, S.; Yamamoto, T.; Hirasawa, Y. An efficient stereoselective synthesis of coenzyme Q10. J. Chem. Soc. Chem. Commun. 1982, 1, 153–154. [Google Scholar]
- Min, J.H.; Lee, J.S.; Yang, J.D.; Koo, S. The Friedel-Crafts Allylation of a Prenyl Group Stabilized by a Sulfone Moiety: Expeditious Syntheses of Ubiquinones and Menaquinones. J. Org. Chem. 2003, 68, 7925–7927. [Google Scholar] [CrossRef]
- Malhotra, S.V. Ionic Liquids in Organic Synthesis; ACS: Washington, DC, USA, 2007; ACS Symposium Series 950. [Google Scholar]
- Chiappe, C.; Pieraccini, D. Ionic liquids: Solvent properties and organic reactivity. J. Phys. Org. Chem. 2005, 18, 275–297. [Google Scholar] [CrossRef]
- Handy, S.T. Room Temperature Ionic Liquids: Different Classes and Physical Properties. Curr. Org. Chem. 2005, 9, 959–988. [Google Scholar] [CrossRef]
- Blanco, C.G.; Banciella, D.C.; Azpiroz, M.D.G. Alkylation of naphthalene using three different ionic liquids. J. Mol. Catal. A 2006, 253, 203–206. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Wang, G.; Qiao, W.; Cheng, L. Friedel–Crafts alkylation of 2-methylnaphthalene in room temperature ionic liquids. Appl. Catal. A 2004, 262, 69–73. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.; Zhao, Z. Efficient synthesis of benzophenone derivatives in Lewis acid ionic liquids. Catal. Commun. 2007, 8, 1834–1837. [Google Scholar] [CrossRef]
- Cao, F.; Tian, L.; Luo, N.; Fang, D.; Ying, W.; Wang, J. FT-IR characterization of [bupy]BF4-MCl3 (M = Al, Fe) ionic liquids using new molecular probe and their alkylation performance. Catal. Commun. 2009, 10, 1310–1312. [Google Scholar] [CrossRef]
- Landge, S.M.; Török, B. Synthesis of Condensed Benzo[N,N]-Heterocycles byMicrowave-Assisted Solid Acid Catalysis. Catal. Lett. 2008, 122, 338–343. [Google Scholar] [CrossRef]
- Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis- a review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Bazin, M.A.; Kihel, L.E.; Lancelot, J.C.; Rault, S. Original one-pot microwave-promoted Hunsdiecker-Suzuki strategy: stra$ightforward access to trans-1,2-diarylethenes from cinnamic acids. Tetrahedron Letters. 2007, 48, 4347–4351. [Google Scholar] [CrossRef]
- Hakala, U.; Wähälä, K. Microwave-promoted synthesis of polyhydroxydeoxy-benzoins in ionic liquids. Tetrahedron Lett. 2006, 47, 8375–8378. [Google Scholar] [CrossRef]
- Kantevari, S.; Mahankhali, V.C.; Das, A.P.R.; Srinivasu, V.N.; Vuppalapati, N. L. Catalysis by an ionic liquid: Highly efficient solvent-free synthesis of aryl-14H-dibenzo [a.j] xanthenes by molten tetrabutylammonium bromide under conventional and microwave heating. Catal. Commun. 2008, 9, 1575–1578. [Google Scholar] [CrossRef]
- Xia, M.; Lu, Y. A novel neutral ionic liquid-catalyzed solvent-free synthesis of2,4,5-trisubstituted imidazoles under microwave irradiation. J. Mol. Catal.A 2007, 265, 205–208. [Google Scholar] [CrossRef]
- Li, X.; Eli, W.; Li, G. Solvent-free synthesis of benzoicesters and benzyl esters in novel Brønsted acidic ionic liquids under microwave irradiation. Catal. Commun. 2008, 9, 2264–2268. [Google Scholar] [CrossRef]
- Thomas, A.; Zawodzinski, J.; Osteryoung, R.A. Donor-Acceptor Properties of AmbientTemperature Chloroaluminate Melts. Inorgan. Chem. 1989, 28, 1710–1715. [Google Scholar] [CrossRef]
- Hsiu, S.I.; Huang, J.F.; Sun, I.W.; Yuan, C.H.; Jantaie, S. Lewis acidity dependency of the electrochemical window of zinc chloride/1-ethyl-3-methylimidazolium chloride ionic liquids. Electrochim. Acta 2002, 47, 4367–4372. [Google Scholar] [CrossRef]
- Angell, C.A.; Bennett, P.D. Optical vs. Thermodynamic Basicities: Probe Pb2+ Ion Spectra in Thermodynamically Characterized Molten Chloroaluminate Solutions. J. Am. Chem. Soc. 1982, 104, 6304–6309. [Google Scholar] [CrossRef]
- Yang, Y.L.; Kou, Y. Determination of the Lewis acidity of ionic liquids by means of an IR spectroscopic probe. Chem. Commun. 2004, 226–227. [Google Scholar] [CrossRef]
- Yin, D.; Li, C.; Tao, L.; Yu, N.; Hu, S.; Yi, D. Synthesis of diphenylmethane derivatives in Lewis acidic ionic liquids. J. Mol. Catal. 2006, 245, 260–265. [Google Scholar] [CrossRef]
- Seddon, K.R. Ionic liquids for clean technology. J. Chem. Tech. Biotechnol. 1997, 68, 351–356. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic liquids—new “solutions” for transition metal catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled Microwave Heating in Modern Organic Synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar]
- Xia, M.; Lu, Y. A novel neutral ionic liquid-catalyzed solvent-free synthesis of 2,4,5-trisubstituted imidazoles under microwave irradiation. J. Mol. Catal. A 2007, 265, 205–208. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, Y.; Zu, Y.; Fu, Y.; Zhang, X.; Yu, P.; Sun, G.; Efferth, T. Efficient Lewis Acid Ionic Liquid-Catalyzed Synthesis of the Key Intermediate of Coenzyme Q10 under Microwave Irradiation. Molecules 2010, 15, 9486-9495. https://doi.org/10.3390/molecules15129486
Chen Y, Zu Y, Fu Y, Zhang X, Yu P, Sun G, Efferth T. Efficient Lewis Acid Ionic Liquid-Catalyzed Synthesis of the Key Intermediate of Coenzyme Q10 under Microwave Irradiation. Molecules. 2010; 15(12):9486-9495. https://doi.org/10.3390/molecules15129486
Chicago/Turabian StyleChen, Yue, Yuangang Zu, Yujie Fu, Xuan Zhang, Ping Yu, Guoyong Sun, and Thomas Efferth. 2010. "Efficient Lewis Acid Ionic Liquid-Catalyzed Synthesis of the Key Intermediate of Coenzyme Q10 under Microwave Irradiation" Molecules 15, no. 12: 9486-9495. https://doi.org/10.3390/molecules15129486




