Farnesol, a Potential Efflux Pump Inhibitor in Mycobacterium smegmatis
Abstract
:Introduction
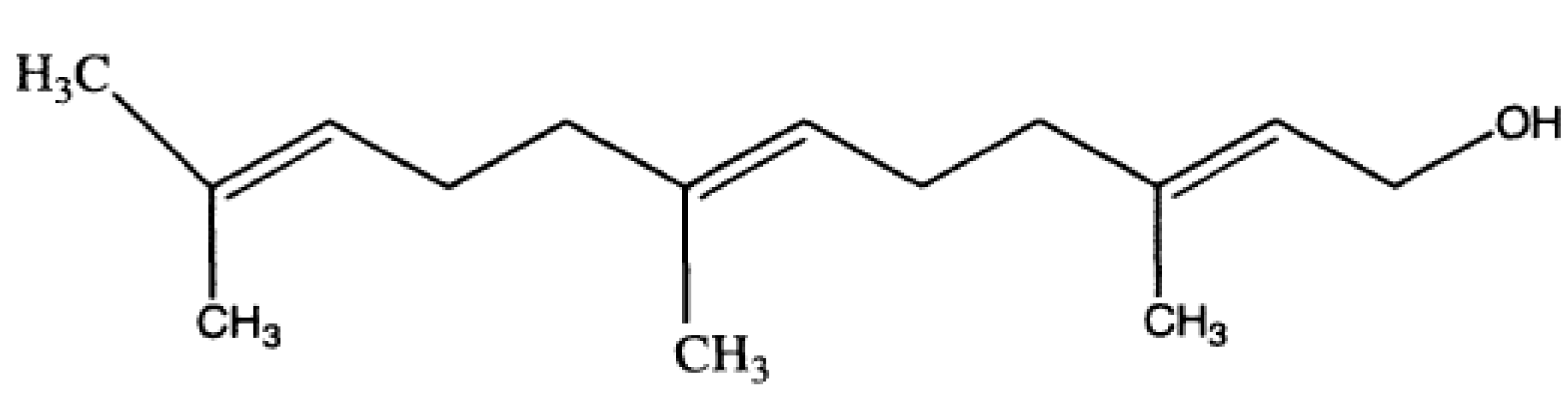
Results
MIC results
| Compound | MIC (μg/mL) | Concentration as modulator (μg/mL) | Modulation factor (EtBr) | FICI |
|---|---|---|---|---|
| Farnesol | 64 | 8 | 2 | 0.625 |
| 16 | 8 | 0.375 | ||
| 32 | 8 | 0.625 | ||
| Chlorpromazine | 32 | 8 | 1 | 1.25 |
| 16 | 4 | 0.75 | ||
| Reserpine | 256 | 32 | 2 | 0.625 |
| 64 | 4 | 0.5 | ||
| Verapamil | 300 | 32 | 1 | 1.107 |
| 64 | 2 | 0.713 | ||
| CCCP | 25 | 16 | 2 | 1.14 |
Checkerboard synergy assay results
EtBr efflux inhibition
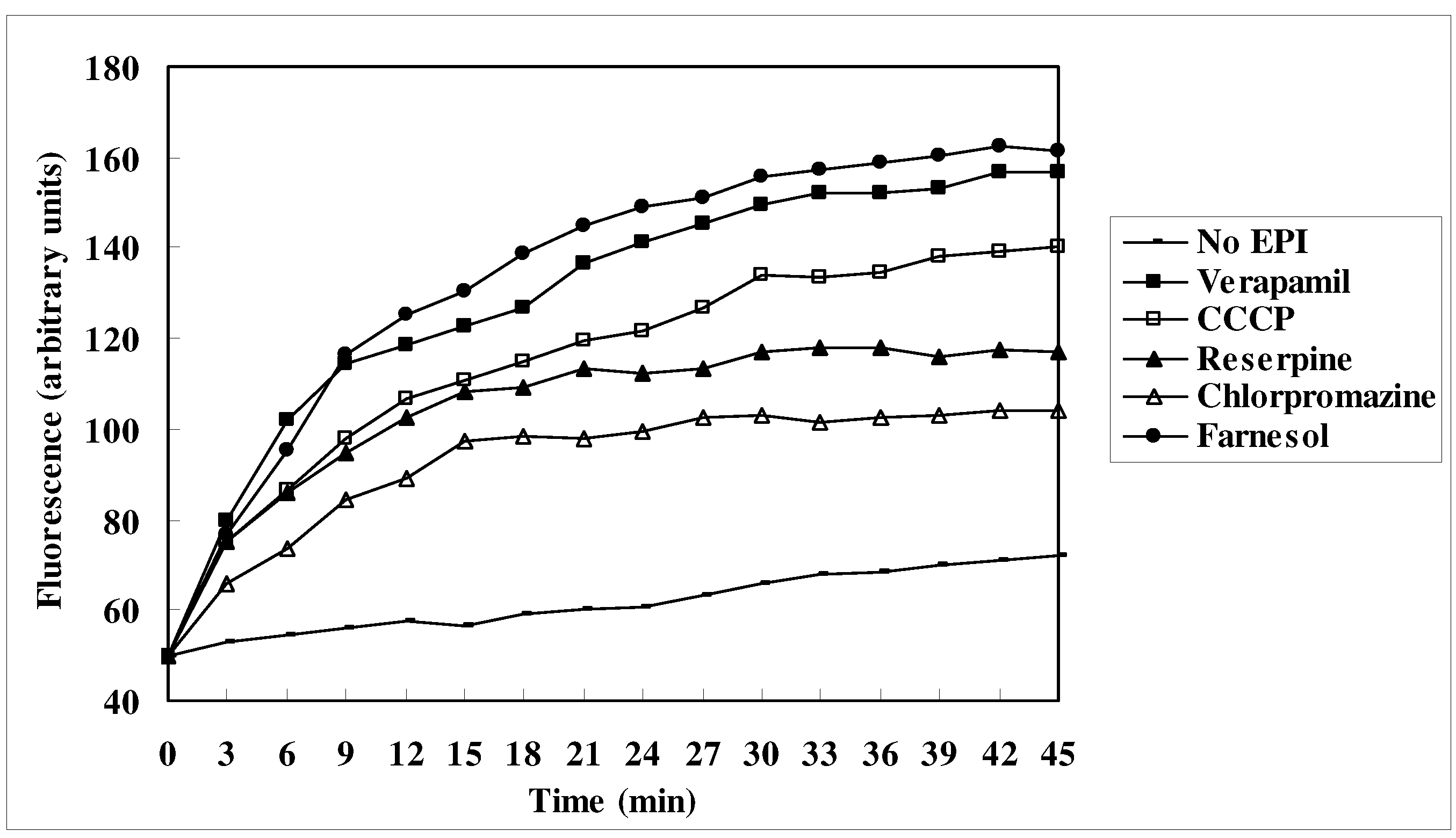

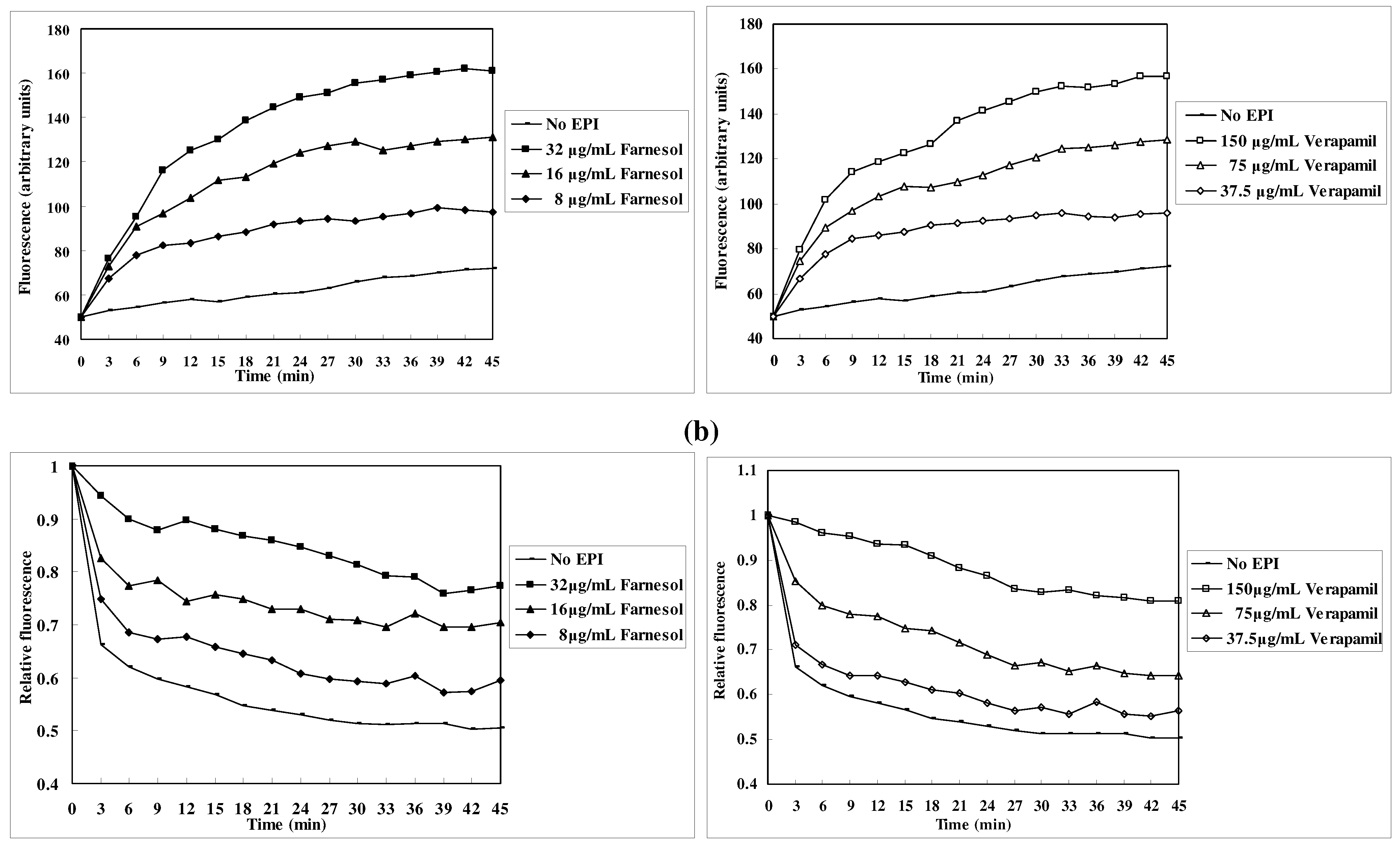
Discussion
Experimental
Chemicals
Strain and culture conditions
Determination of antibacterial susceptibility
Checkerboard synergy assay
Measurement of EtBr accumulation
Measurement of EtBr efflux
Conclusions
Acknowledgements
- Samples Availability: Samples of the compound farnesol is available from the authors.
References
- Han, X.Y.; Dé, I.; Jacobson, K.L. Rapidly growing mycobacteria: Clinical and microbiologic studies of 115 cases. Am. J. Clin. Pathol. 2007, 128, 612–621. [Google Scholar] [CrossRef]
- World Health Organization, Global Tuberculosis Control: Epidemiology, strategy, financing; WHO/HTM/TB: Geneva, Switzerland, 2009; p. 411.
- Bloom, B.R.; Murray, C.J. Tuberculosis: Commentary on a reemergent killer. Science 1992, 257, 1055–1064. [Google Scholar]
- German, N.; Kaatz, G.W.; Kerns, R.J. Synthesis and evaluation of PSSRI-based inhibitors of Staphylococcus aureus multidrug efflux pumps. Bioorg. Med. Chem. Lett. 2008, 18, 1368–1373. [Google Scholar] [CrossRef]
- De Rossi, E.; Aínsa, J.A.; Riccardi, G. Role of mycobacterial efflux transporters in drug resistance: an unresolved question. FEMS Microbiol. Rev. 2006, 30, 36–52. [Google Scholar] [CrossRef]
- Gupta, A.K.; Chauhan, D.S.; Srivastava, K.; Das, R.; Batra, S.; Mittal, M.;, Goswami; Singhal, N.; Sharma, V.D.; Venkatesan, K.; Hasnain, S.E.; Katoch, V.M. Estimation of efflux mediated multi-drug resistance and its correlation with expression levels of two major efflux pumps in mycobacteria. J. Commun. Dis. 2006, 38, 246–254. [Google Scholar]
- Marquez, B. Bacterial efflux systems and efflux pumps inhibitors. Biochimie 2005, 87, 1137–1147. [Google Scholar] [CrossRef]
- Lechner, D.; Gibbons, S.; Bucar, F. Plant phenolic compounds as ethidium bromide efflux inhibitors in Mycobacterium smegmatis. J. Antimicrob. Chemother. 2008, 62, 345–348. [Google Scholar] [CrossRef]
- Rodrigues, L.; Wagner, D.; Viveiros, M.; Sampaio, D.; Couto, I.; Vavra, M.; Kern, W.V.; Amaral, L. Thioridazine and chlorpromazine inhibition of ethidium bromide efflux in Mycobacterium avium and Mycobacterium smegmatis. J. Antimicrob. Chemother. 2008, 61, 1076–1082. [Google Scholar] [CrossRef]
- Gibbons, S.; Oluwatuyi, O.; Kaatz, G.W. A novel inhibitor of multidrug efflux pumps in Staphylococcus aureus. J. Antimicrob. Chemother. 2003, 51, 13–17. [Google Scholar]
- Piddock, L.J. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacterial. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef]
- Kuroda, M.; Nagasaki, S.; Ohta, T. Sesquiterpene farnesol inhibits recycling of the C55 lipid carrier of the murein monomer precursor contributing to increased susceptibility to beta-lactams in methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2007, 59, 425–432. [Google Scholar] [CrossRef]
- Brehm-Stecher, B.F.; Johnson, E.A. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and aprito. Antimicrob. Agents Chemother. 2003, 47, 3357–3360. [Google Scholar] [CrossRef]
- Jabra-Rizk, M.A.; Meiller, T.F.; James, C.E. Shirtliff, M.E. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob. Agents Chemother. 2006, 50, 1463–1469. [Google Scholar] [CrossRef]
- Inoue, Y.; Shiraishi, A.; Hada, T.; Hirose, K.; Hamashima, H.; Shimada, J. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol. Lett. 2004, 237, 325–331. [Google Scholar]
- Oluwatuyi, M.; Kaatz, G.W.; Gibbons, S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry 2004, 65, 3249–3254. [Google Scholar] [CrossRef]
- Stavri, M.; Piddock, L.J.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007, 59, 1247–1260. [Google Scholar] [CrossRef]
- Jacobs, W.R., Jr. Mycobacterium Tuberculosis: A Once Genetically Intractable Organism. In Molecular Genetics of Mycobacteria; Hatfull, G.F., Jacobs, W.R., Jr., Eds.; ASM Press: Washington, DC, USA, 2000; pp. 1–36. [Google Scholar]
- Coldham, N.G.; Webber, M.; Woodward, M.J.; Piddock, L.J. A 96-well plate fluorescence assay for assessment of cellular permeability and active efflux in Salmonella enterica serovar Typhimurium and Escherichia coli. J. Antimicrob. Chemother. 2010, 65, 1655–1663. [Google Scholar] [CrossRef]
- Viveiros, M.; Leandro, C.; Amaral, L. Mycobacterial efflux pumps and chemotherapeutic implications. Int. J. Antimicrob. Agents 2003, 22, 274–278. [Google Scholar] [CrossRef]
- Li, X.Z.; Zhang, L.; Nikaido, H. Efflux pump-mediated intrinsic drug resistance in Mycobacterium smegmatis. Antimicrob. Agents Chemother. 2004, 48, 2415–2423. [Google Scholar] [CrossRef]
- Kuroda, M.; Nagasaki, S.; Ito, R.; Ohta, T. Sesquiterpene farnesol as a competitive inhibitor of lipase activity of Staphylococcus aureus. FEMS Microbiol. Lett. 2007, 273, 28–34. [Google Scholar] [CrossRef]
- Ge, F.; Zeng, F.; Liu, S.; Guo, N.; Ye, H.; Song, Y.; Fan, J.; Wu, X.; Wang, X.; Deng, X.; Jin, Q.; Yu, L. In vitro synergistic interactions of oleanolic acid in combination with isoniazid, rifampicin or ethambutol against Mycobacterium tuberculosis. J. Med. Microbiol. 2010, 59, 567–572. [Google Scholar] [CrossRef]
- Guo, N.; Wu, X.; Yu, L.; Liu, J.; Meng, R.; Jin, J.; Lu, H.; Wang, X.; Yan, S.; Deng, X. In vitro and in vivo interactions between fluconazole and allicin against clinical isolates of fluconazole-resistant Candida albicans determined by alternative methods. FEMS Immunol. Med. Microbiol. 2010, 58, 193–201. [Google Scholar] [CrossRef]
- Spengler, G.; Martins, A.; Schelz, Z.; Rodrigues, L.; Aagaard, L.; Martins, M.; Costa, S.S.; Couto, I.; Viveiros, M.; Fanning, S.; Kristiansen, J.E.; Molnar, J.; Amaral, L. Characterization of intrinsic efflux activity of Enterococcus faecalis ATCC29212 by a semi-automated ethidium bromide method. In Vivo 2009, 23, 81–87. [Google Scholar]
- Couto, I.; Costa, S.S.; Viveiros, M.; Martins, M.; Martins, M. Efflux-mediated response of Staphylococcus aureus exposed to ethidium bromide. J. Antimicrob. Chemother. 2008, 62, 504–513. [Google Scholar] [CrossRef]
- Li, X.Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria. Drugs 2004, 64, 159–204. [Google Scholar] [CrossRef]
- Poole, K. Efflux-mediated multiresistance in Gram negative bacteria. Clin. Microbiol. Infect. 2004, 10, 12–26. [Google Scholar] [CrossRef]
- Crowle, A.J.; Douvas, G.S.; May, M.H. Chlorpromazine: A drug potentially useful for treating mycobacterial infections. Chemotherapy 1992, 38, 410–419. [Google Scholar] [CrossRef]
- Ramón-García, S.; Martín, C.; Aínsa, J.A.; De Rossi, E. Characterization of tetracycline resistance mediated by the efflux pump Tap from Mycobacterium fortuitum. J. Antimicrob. Chemother. 2006, 57, 252–259. [Google Scholar] [CrossRef]
- Micol, V.; Mateo, C.R.; Shapiro, S.; Aranda, F.J.; Villalaín, J. Effects of (+)-totarol, a diterpenoid antibacterial agent, on phospholipid model membranes. Biochim. Biophys. Acta 2001, 1511, 281–290. [Google Scholar]
- Ong, T.P.; Heidor, R.; de Conti, A.; Dagli, M.L.; Moreno, F.S. Farnesol and geraniol chemopreventive activities during the initial phases of hepatocarcinogenesis involve similar actions on cell proliferation and DNA damage, but distinct actions on apoptosis, plasma cholesterol and HMGCoA reductase. Carcinogenesis 2006, 27, 1194–1203. [Google Scholar] [CrossRef]
- Koo, H.; Schobel, B.; Scott-Anne, K.; Watson, G.; Bowen, W.H.; Cury, J.A.; Rosalen, P.L.; Park, Y.K. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J. Dent. Res. 2005, 84, 1016–1020. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Krom, B.P.; Meijering, R.A.; Peters, B.M.; Zhu, J.; Scheper, M.A.; Harris, M.L.; Jabra-Rizk, M.A. Farnesol-induced apoptosis in Candida albicans. Antimicrob. Agents Chemother. 2009, 53, 392–401. [Google Scholar]
- Luft, U.C.; Bychkov, R.; Gollasch, M.; Gross, V.; Roullet, J.B.; McCarron, D.A.; Ried, C.; Hofmann, F.; Yagil, Y.; Yagil, C.; Haller, H.; Luft, F.C. Farnesol blocks the L-type Ca2+ channel by targeting the alpha 1C subunit. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 959–966. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards, Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes: Approved Standard M24-A; NCCLS: Wayne, PA, USA, 2003.
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2000, 6, 503–508. [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the checkerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jin, J.; Zhang, J.-Y.; Guo, N.; Sheng, H.; Li, L.; Liang, J.-C.; Wang, X.-L.; Li, Y.; Liu, M.-Y.; Wu, X.-P.; et al. Farnesol, a Potential Efflux Pump Inhibitor in Mycobacterium smegmatis. Molecules 2010, 15, 7750-7762. https://doi.org/10.3390/molecules15117750
Jin J, Zhang J-Y, Guo N, Sheng H, Li L, Liang J-C, Wang X-L, Li Y, Liu M-Y, Wu X-P, et al. Farnesol, a Potential Efflux Pump Inhibitor in Mycobacterium smegmatis. Molecules. 2010; 15(11):7750-7762. https://doi.org/10.3390/molecules15117750
Chicago/Turabian StyleJin, Jing, Ji-Yu Zhang, Na Guo, Hui Sheng, Lei Li, Jun-Chao Liang, Xue-Lin Wang, Yang Li, Ming-Yuan Liu, Xiu-Ping Wu, and et al. 2010. "Farnesol, a Potential Efflux Pump Inhibitor in Mycobacterium smegmatis" Molecules 15, no. 11: 7750-7762. https://doi.org/10.3390/molecules15117750




