Synthesis and Characterization of a Ru(II) Complex with Functionalized Phenanthroline Ligands Having Single-Double Linked Anthracenyl and 1-Methoxy-1-buten-3-yne Moieties
Abstract
:1. Introduction
2. Results and Discussion
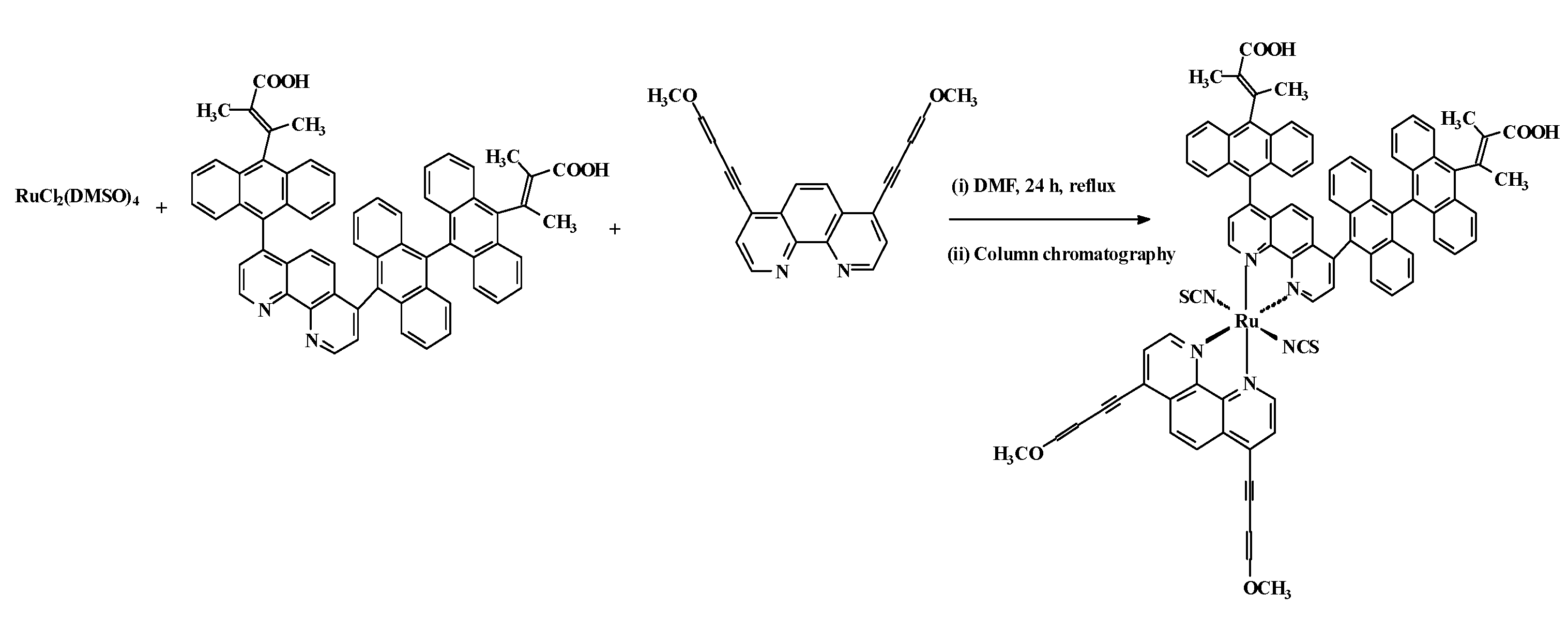
2.1. Chemistry

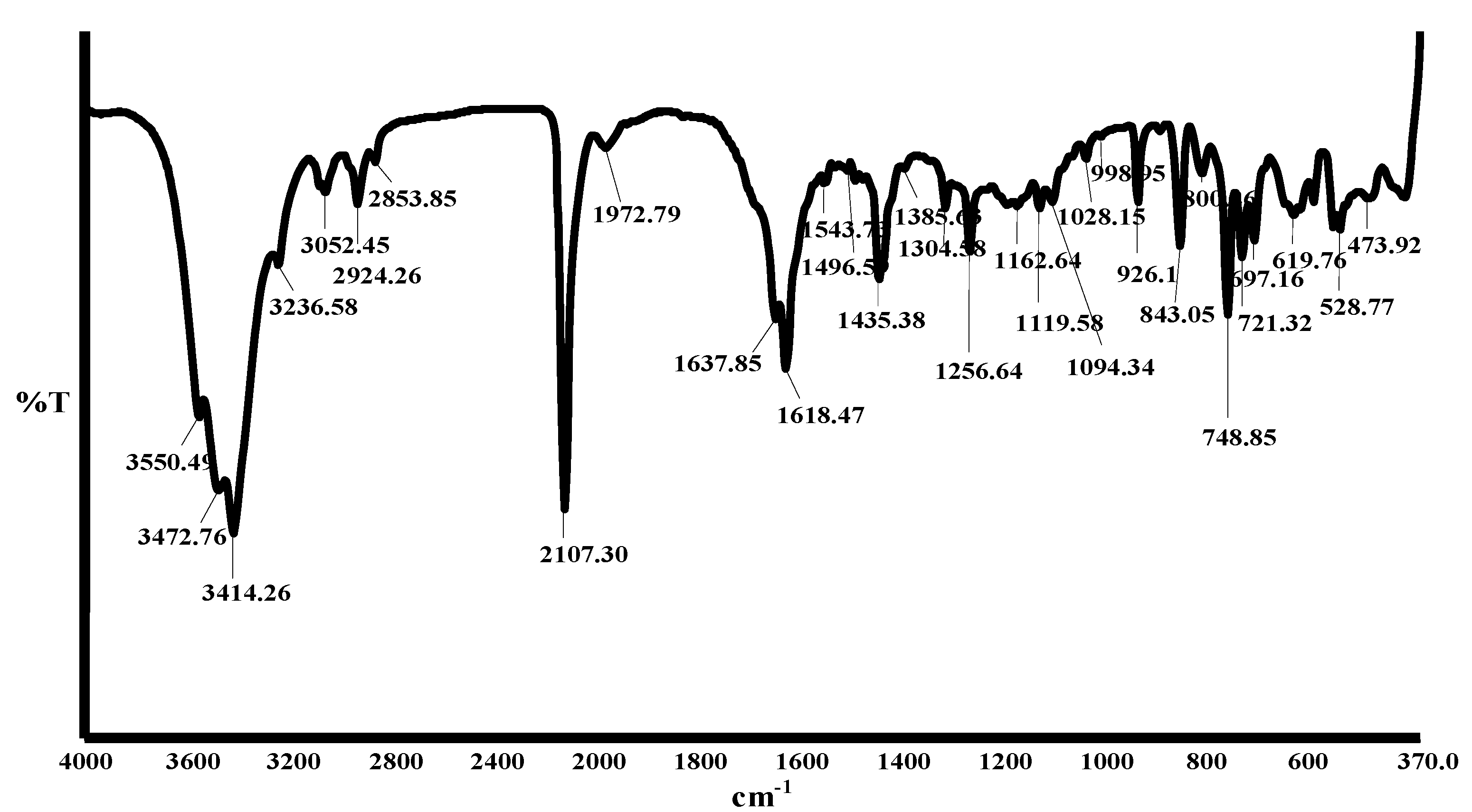
2.2. Infrared spectra
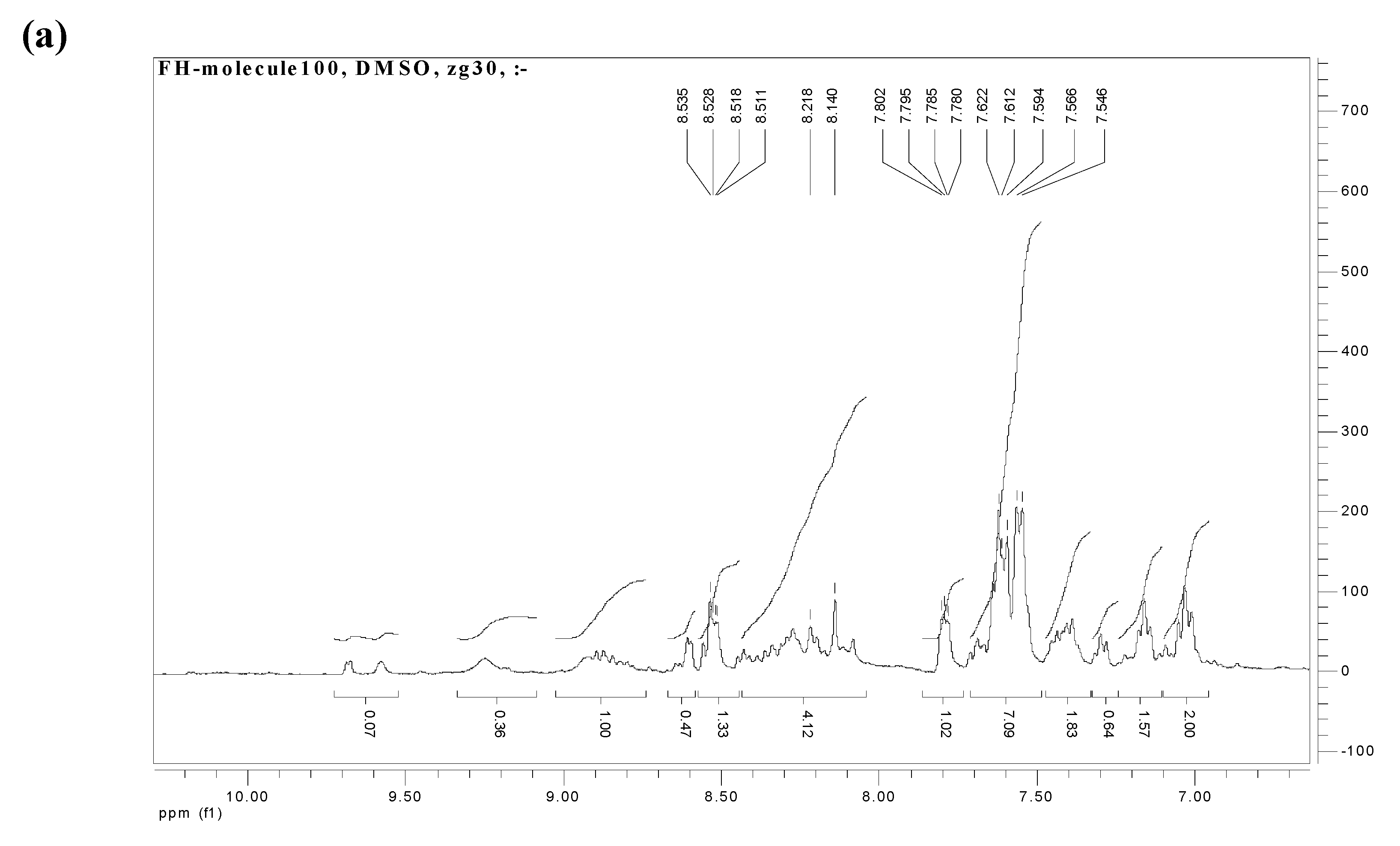
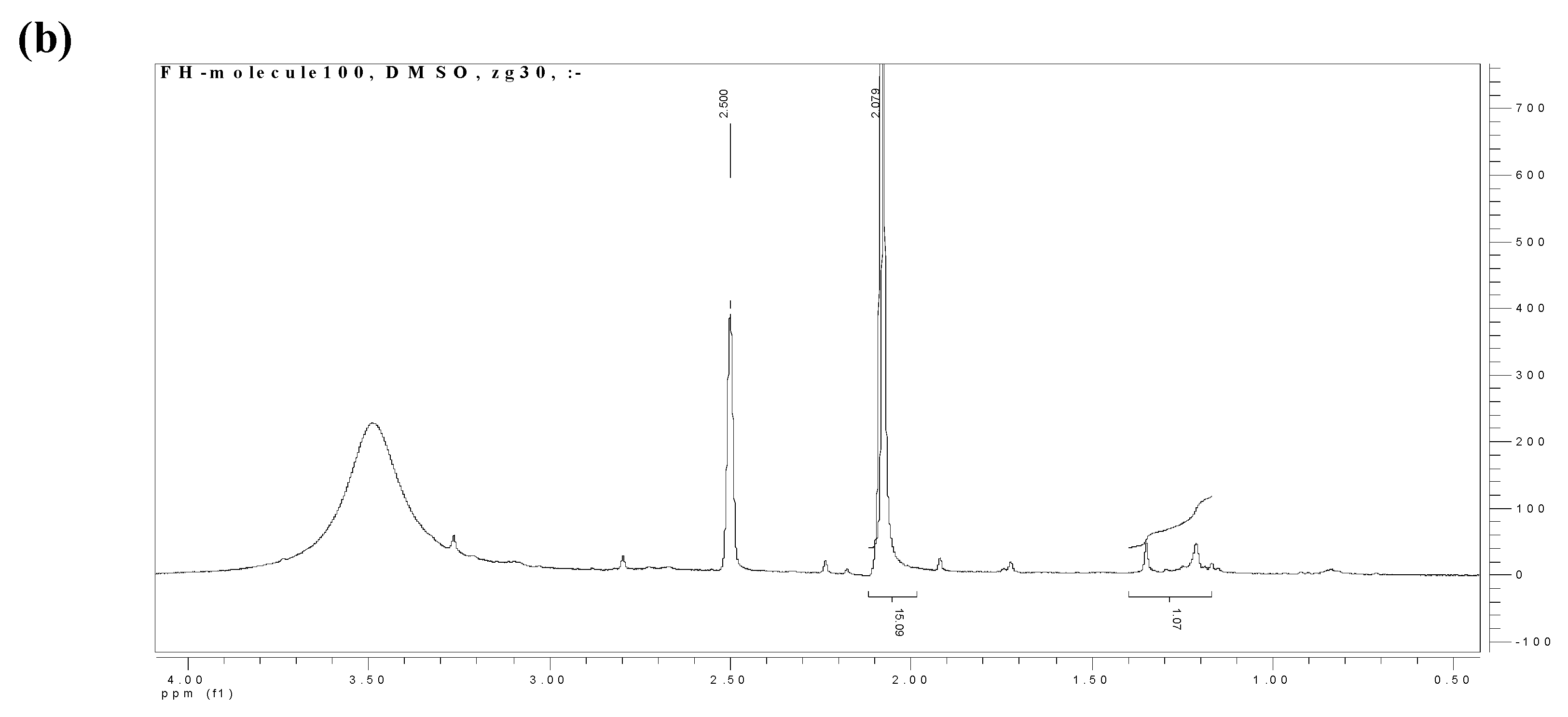
2.3. NMR spectroscopy
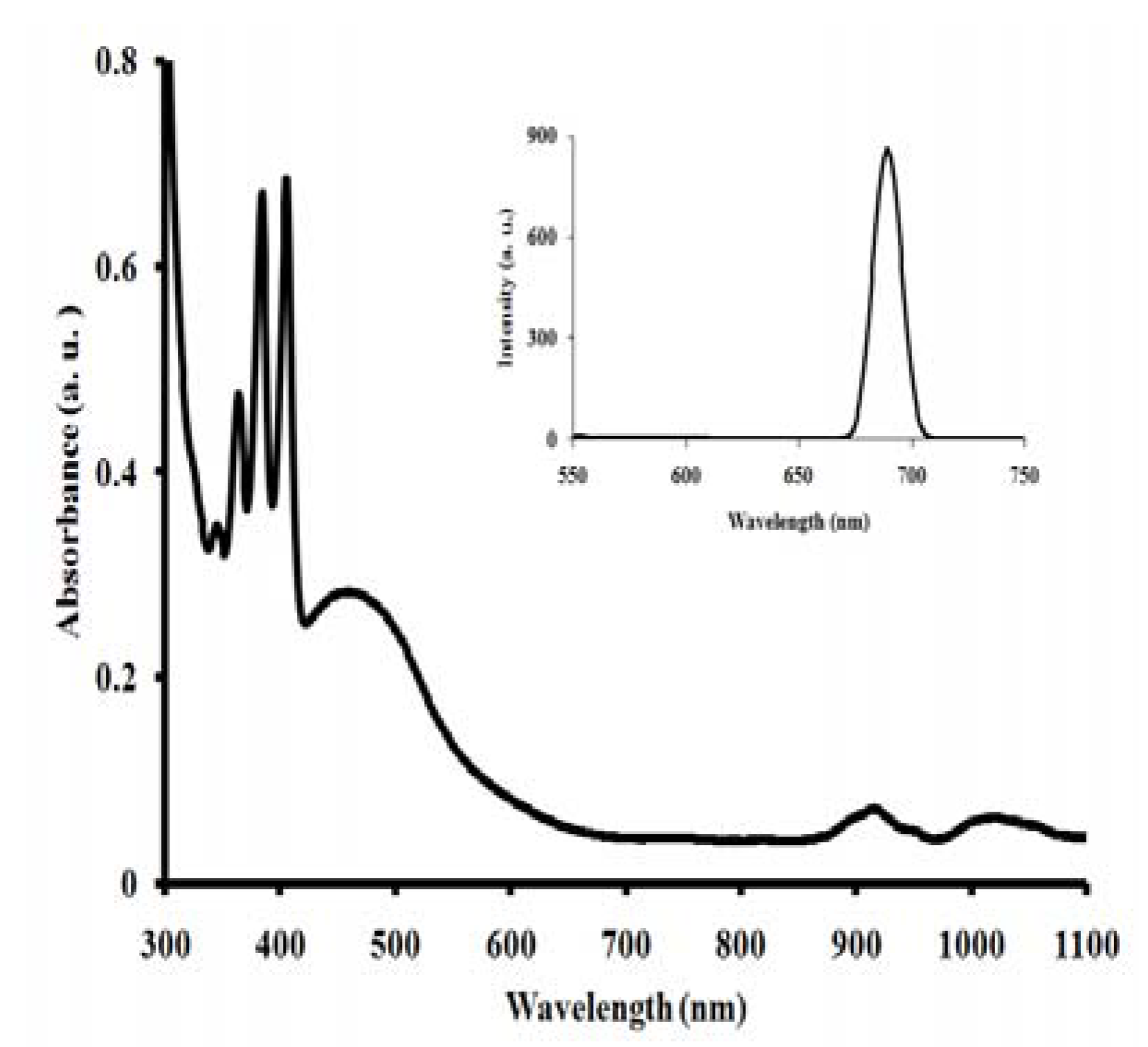
2.4. Electronic and emission spectra
3. Experimental
3.1. Materials and general physical measurements
3.2. Synthesis of ligands
3.2.1. 4-(Dianthracenyl-2,3-dimethylacrylic acid)-7-(anthracenyl-2,3-dimethylacrylic acid)-1,10-phenanthroline (L1)
3.3.2. 4,7-bis(1-Methoxy-1-but-3-yne)-1,10-phenanthroline (2)
3.3. Synthesis of [Ru(II)-L1L2(NCS)2
4. Conclusions
Supplementary Materials
Acknowledgements
- Samples Availability: Samples of the compounds L1, L2 and [RuL1L2(NCS)2 are available from the authors.
References
- Halpern, J. Some aspects of the coordination and catalytic chemistry of ruthenium. Pure Appl. Chem. 1987, 59, 173–180. [Google Scholar] [CrossRef]
- Juris, A.; Balzani, V.; Barigelletti, F.; Campagna, S.; Belser, P.; Zelewsky, A.V. Ruthenium(II) polypyridine complexes: photophysics, photochemistry, electrochemistry, and chemiluminescence. Coord. Chem. Rev. 1988, 84, 85–277. [Google Scholar]
- Juris, A.; Campagna, S.; Balzani, V.; Gremaud, G.; Zelewsky, A.V. Absorption spectra, luminescence properties, and electrochemical behaviour of tris-heteroleptic ruthenium(II) polypyridine complexes. Inorg. Chem. 1988, 3652–3655. [Google Scholar]
- Prasanna de Silva, A.; Fox, D.B.; Moody, T.S.; Weir, S.M. Luminescent sensors and photonic switches. Pure Appl. Chem. 2001, 73, 503–511. [Google Scholar] [CrossRef]
- Robertson, N.; McGowan, C.A. A comparison of potential molecular wires as components for molecular electronics. Chem. Soc. Rev. 2003, 32, 96–130. [Google Scholar] [CrossRef]
- Balzani, V.; Bolletta, F.; Ciano, M.; Maestri, M. Electron Transfer Reactions Involving Light. J. Chem. Ed. 1983, 60, 447. [Google Scholar] [CrossRef]
- Juris, A.; Barigelleti, F.; Balzani, V.; Belser, P.; Zelewsky, A.V. New Photosensitizers of the Ruthenium-polypyridine Family for the Water Splitting Reaction. Isr. J. Chem. 1982, 22, 87. [Google Scholar]
- Sun, L.; Hammarstrom, L.; Norrby, T.; Berglund, H.; Davydov, R.; Andersson, M.; Borje, A.; Korall, P.; Philouze, C.; Almgren, M.; Styring, S.; Akermark, B. Intramolecular electron transfer from coordinated manganese(II) to photogenerated ruthenium(III). Chem. Commun. 1997, 607–608. [Google Scholar]
- Jin, Y.; Hua, J.; Wu, W.; Ma, X.; Meng, F. Synthesis, characterization and photovoltaic properties of two novel near-infrared perylene dyes containing benzo[e] indole for dye-sensitized solar cells. Synthet. Metal. 2008, 158, 64–71. [Google Scholar] [CrossRef]
- Tian, F.M.; Wang, P.; Zhu, G.Y. Synthesis and properties of three tris(1,10-phenanthroline) ruthenium(II) derivatives. Chin. Chem. Lett. 1999, 10, 881–884. [Google Scholar]
- Ocakoglu, K.; Zafer, C.; Cetinkaya, B.; Icli, S. Synthesis, characetrization and spectroscopic studies of two new heteroleptic Ru(II) polypyridyl complexes. Dye. Pigment. 2007, 75, 385–394. [Google Scholar] [CrossRef]
- Meyer, T.J. Photochemistry of metal coordination complexes: metal to ligand charge transfer excited state. Pure Appl. Chem. 1986, 58, 1193–1206. [Google Scholar] [CrossRef]
- Bolletta, F.; Maestri, M.; Moggi, L.; Jamieson, M.A.; Serpone, N.; Henry, M.S.; Hoffman, M.Z. Photochemical, photophysical and thermal behaviour of the tris-(1,10-phenanthroline) chromium(II) ion in aqueous solution. Inorg. Chem. 1983, 22, 2502. [Google Scholar] [CrossRef]
- Kalyanasundaram, K. Photochemistry of Polypyridine and Porphyrin Complexes; Academic Press Limited: London, UK, 1992; pp. 87–212. [Google Scholar]
- Polo, A.S.; Itokazu, M.K.; Iha, N.Y.M. Metal complex sensitizers in dye-sensitized solar cells. Coord. Chem. Rev. 2004, 248, 1343–1361. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Moser, J.E.; Baker, R.H.; Comte, P.; Aranyos, V. Stable new sensitizer with improved light harvesting for nanocrystalline dye sensitized solar cells. Advan. Mater. 2004, 16, 1806–1811. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lu, H.C.; Wu, C.G.; Chen, J.G.; Ho, K.C. New ruthenium complexes containing oligoalkylthiophene-substituted 1,10-phenanthroline for nanocrystalline dye sensitized solar cells. Adv. Funct. Mater. 2007, 17, 29–36. [Google Scholar] [CrossRef]
- Jiang, K.J.; Masaki, N.; Xia, J.B.; Noda, S.; Yanagida, S. A novel ruthenium sensitizer with a hydrophobic 2-thiophen-2-yl-vinyl-conjugated bipyridyl ligand for effective dye sensitized TiO2 solar cells. Chem. Commun. 2006, 2460–2462. [Google Scholar]
- Kukrek, A.; Wang, D.; Hou, Y.; Zong, R.; Thummel, R. Photosensitizers containing the 1,8-naphthyridyl moiety and their use in dye-sensitized solar cells. Inorg. Chem. 2006, 45, 10131–10137. [Google Scholar] [CrossRef]
- Klein, C.; Nazeeruddin, M.K.; Liska, P.; Censo, D.D.; Hirata, N.; Palomares, E. Engineering of a novel ruthenium sensitizer and its application in dye sensitized solar cells for conversion of sunlight into electricity. Inorg. Chem. 2005, 44, 178–180. [Google Scholar] [CrossRef]
- Vyas, P.; Bhatt, A.K.; Ramachandraiah, G.; Bedekar, A.V. Environmentally benign chlorination and bromination of aromatic amines, hydrocarbons and naphthols. Tetrahedron Lett. 2003, 44, 4085–4088. [Google Scholar] [CrossRef]
- Yamamoto, T.; Morita, A.; Miyazaki, Y.; Maruyama, T.; Wakayama, H.; Zhou, Z.H.; Nakamura, Y.; Kanbara, T.; Sasaki, S.; Kubota, K. Preparation of π-conjugated poly(thiophene-2,5-diyl), poly(p-phenylene), and related polymers using zerovalent nickel complexes. Linear structure and properties of the π-conjugated polymers. Macromolecules 1992, 25, 1214–1223. [Google Scholar]
- Khatyr, A.; Ziessel, R. Stepwise construction of pyrene bridged polytopic ligands carrying acetylenic tethers. Tetrahedron Lett. 2002, 43, 7431–7434. [Google Scholar] [CrossRef]
- Lipshutz, B.L.; Chung, D.W.; Rich, B. Sonogashira couplings of aryl bromides: room temperature, water only, no copper. Org. Lett. 2008, 10, 3793–3796. [Google Scholar] [CrossRef]
- Venkataraman, D.; Bates, C.G.; Saejueng, P. Methods of 1,3-enyne preparation using copper(I) catalysts. US Patent No. 7,473,797 B2., 6 January 2009. [Google Scholar]
- Evans, I.P.; Spencer, A.; Wilkinson, G. Dichlorotetrakis(dimethyl sulphoxide) ruthenium(II) and its Use as a Source Material for Some New Ruthenium(II) Complexes. J.Chem.Soc. Dalton 1973, 204–208. [Google Scholar]
- Mitsopoulou, C.A.; Veroni, I.; Philippopoulos, A.I.; Falaras, P. Synthesis, characterization and sensitization properties of two novel mono and bis carboxyl-dipyrido-phenazine ruthenium(II) charge transfer complexes. J. Photochem. Photobiol. A: Chem. 2007, 191, 6–12. [Google Scholar] [CrossRef]
- Gunzler, H.; Gremlich, H. IR Spectroscopy - An Introduction; WILEY-VCH Verlag GmbH: Weinheim, Germany, 2002. [Google Scholar]
- Chen, C.Y.; Wu, S.J.; Wu, C.G.; Chen, J.G.; Ho, K.C. A ruthenium complex with super high light-harvesting capacity for dye-sensitized solar cells. Angew. Chem. 2006, 118, 5954–5957. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wu, S.J.; Li, J.Y.; Wu, C.G.; Chen, J.G.; Ho, K.C. A new route to enhance the light-harvesting capability of ruthenium complexes for dye-sensitized solar cells. Advan. Mater. 2007, 19, 3888–3891. [Google Scholar] [CrossRef]
- Brown, D.W.; Floyd, A.J.; Sainsbury, M. Organic Spectroscopy; John Wiley & Sons: New York, NY, USA, 1988; pp. 24–53. [Google Scholar]
- Cooper, J.W. Spectroscopic Techniques for Organic Chemists; John Wiley & Sons: New York, NY, USA, 1980; pp. 53–117. [Google Scholar]
- Nazeeruddin, M.K.; Zakeeruddin, S.M.; Humphry-Baker, R.; Gorelsky, S.I.; Lever, A.B.P.; Gratzel, M. Synthesis, spectroscopic and a ZINDO study of cis- and trans- (X2) Bis(4,4'-dicarboxylic acid-2,2'-bipyridine)-Ruthenium(II) complexes (X = Cl-, H2O, NCS-). Coord. Chem. Rev. 2000, 208, 213–226. [Google Scholar]
- Wu, S.J.; Chen, C.Y.; Chen, J.G.; Li, J.Y.; Tung, Y.L.; Ho, K.C.; Wu, C.G. An efficiency light-harvesting ruthenium dye for solar cell application. Dye. Pigment. 2009, 84, 1–7. [Google Scholar]
- Li, X.; Gui, J.; Yang, H.; Wu, W.; Li, F.; Tian, H.; Huang, C. A new carbozole-based phenanthrenyl ruthenium complex as sensitizer for a dye-sensitized solar cell. Inorg. Chim. Acta 2008, 361, 2835–2840. [Google Scholar] [CrossRef]
- Qu, J.Q.; Zhang, J.Y.; Grimsdale, A.C.; Mullen, K. Dendronized Perylene Diimide Emitters: Synthesis, Luminescence and Electron and Energy Transfer Studies. Macromolecules 2004, 37, 8297–8306. [Google Scholar] [CrossRef]
- Xu, B.; Holdcroft, S. Molecular Control of Luminescence from Poly-(3-hexylthiophenes). Macromolecules 1993, 26, 4457–4460. [Google Scholar] [CrossRef]
- Sumby, C.J.; Steel, P.J. Mono- and dinuclear ruthenium complexes of bridging ligands incorporating two di-2-pyridylamine motifs: Synthesis, spectroscopy and electrochemistry. Polyhedron 2007, 26, 5370–5381. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Adeloye, A.O.; Ajibade, P.A. Synthesis and Characterization of a Ru(II) Complex with Functionalized Phenanthroline Ligands Having Single-Double Linked Anthracenyl and 1-Methoxy-1-buten-3-yne Moieties. Molecules 2010, 15, 7570-7581. https://doi.org/10.3390/molecules15117570
Adeloye AO, Ajibade PA. Synthesis and Characterization of a Ru(II) Complex with Functionalized Phenanthroline Ligands Having Single-Double Linked Anthracenyl and 1-Methoxy-1-buten-3-yne Moieties. Molecules. 2010; 15(11):7570-7581. https://doi.org/10.3390/molecules15117570
Chicago/Turabian StyleAdeloye, Adewale O., and Peter A. Ajibade. 2010. "Synthesis and Characterization of a Ru(II) Complex with Functionalized Phenanthroline Ligands Having Single-Double Linked Anthracenyl and 1-Methoxy-1-buten-3-yne Moieties" Molecules 15, no. 11: 7570-7581. https://doi.org/10.3390/molecules15117570




