Antioxidant-Prooxidant Properties of a New Organoselenium Compound Library
Abstract
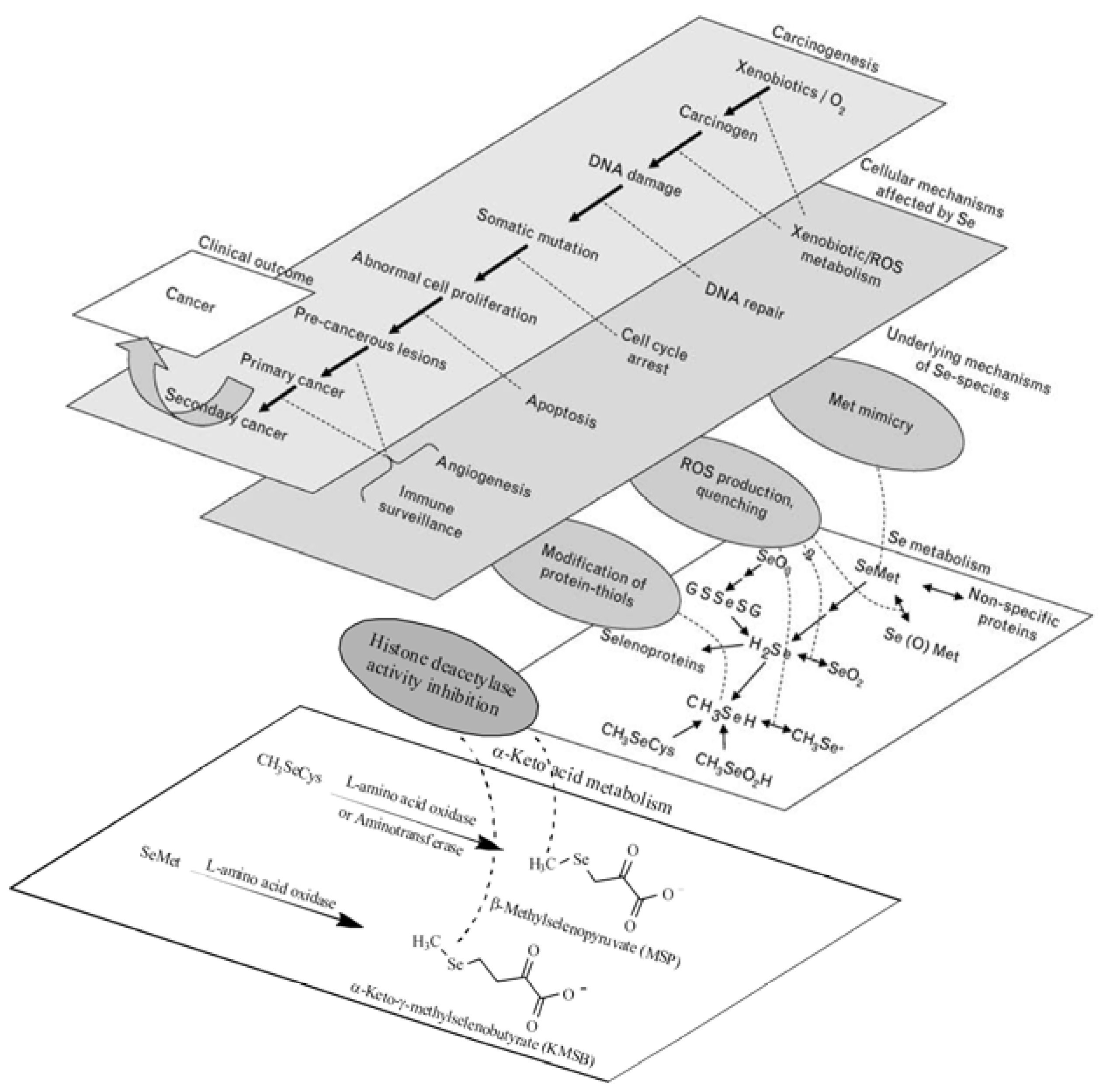
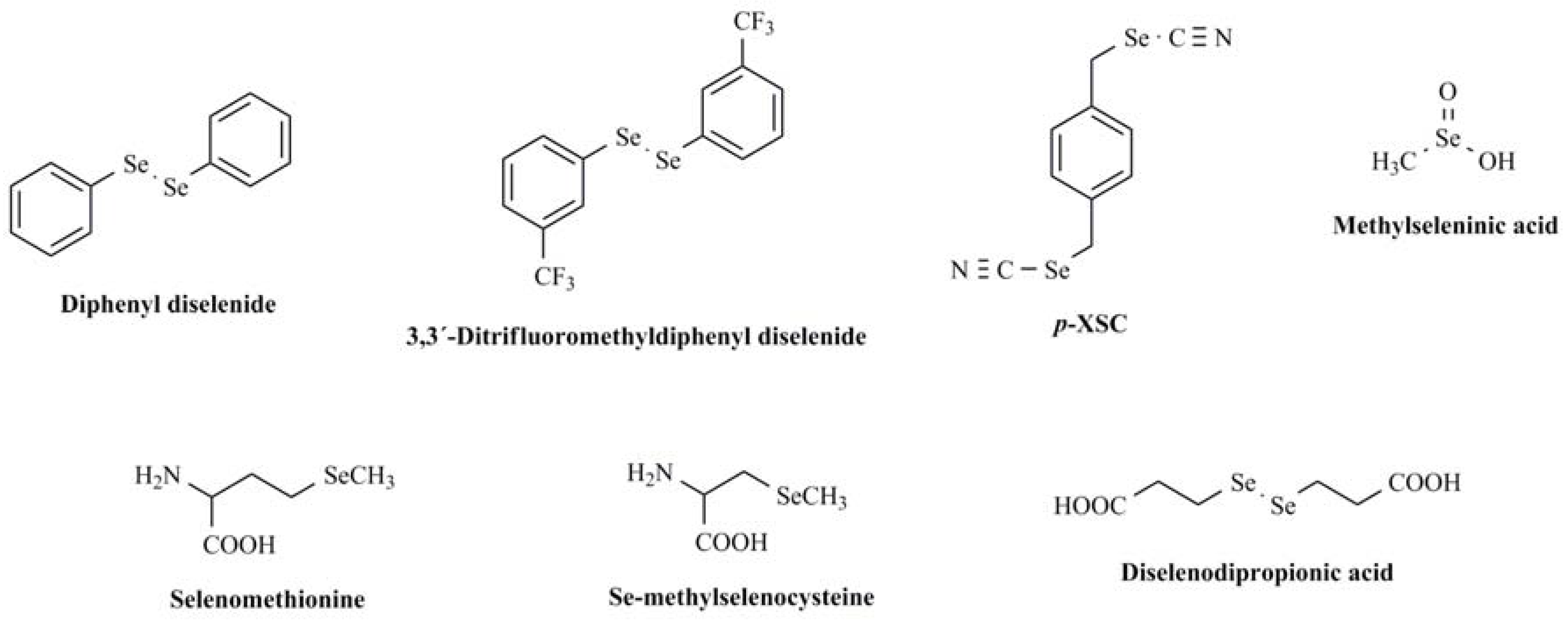
:1. Introduction
2. Results and Discussion
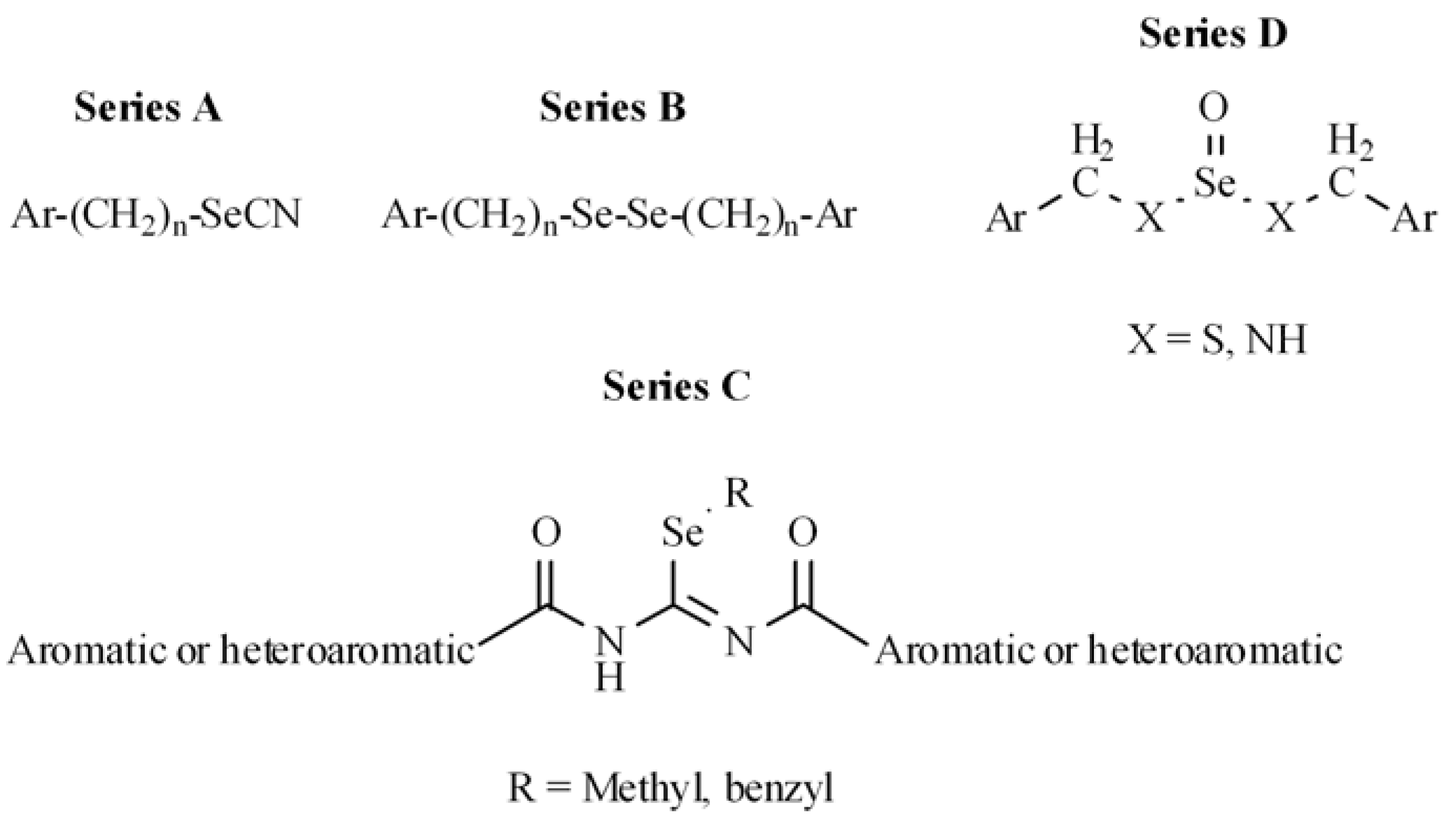
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Superoxide anion scavenging activity
2.2.2. Cytotoxic activity in PC-3 and MCF-7
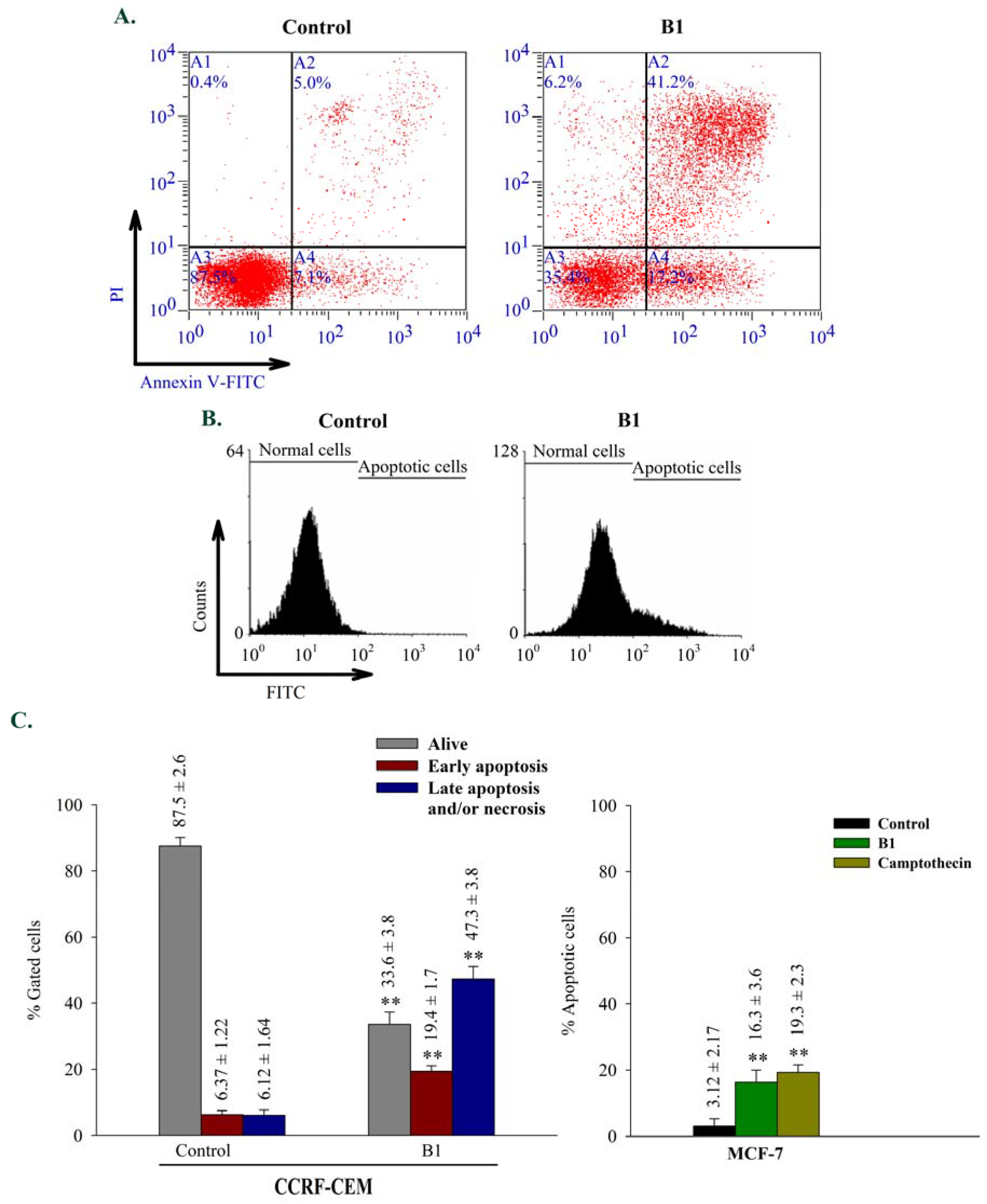
2.2.3. Apoptosis
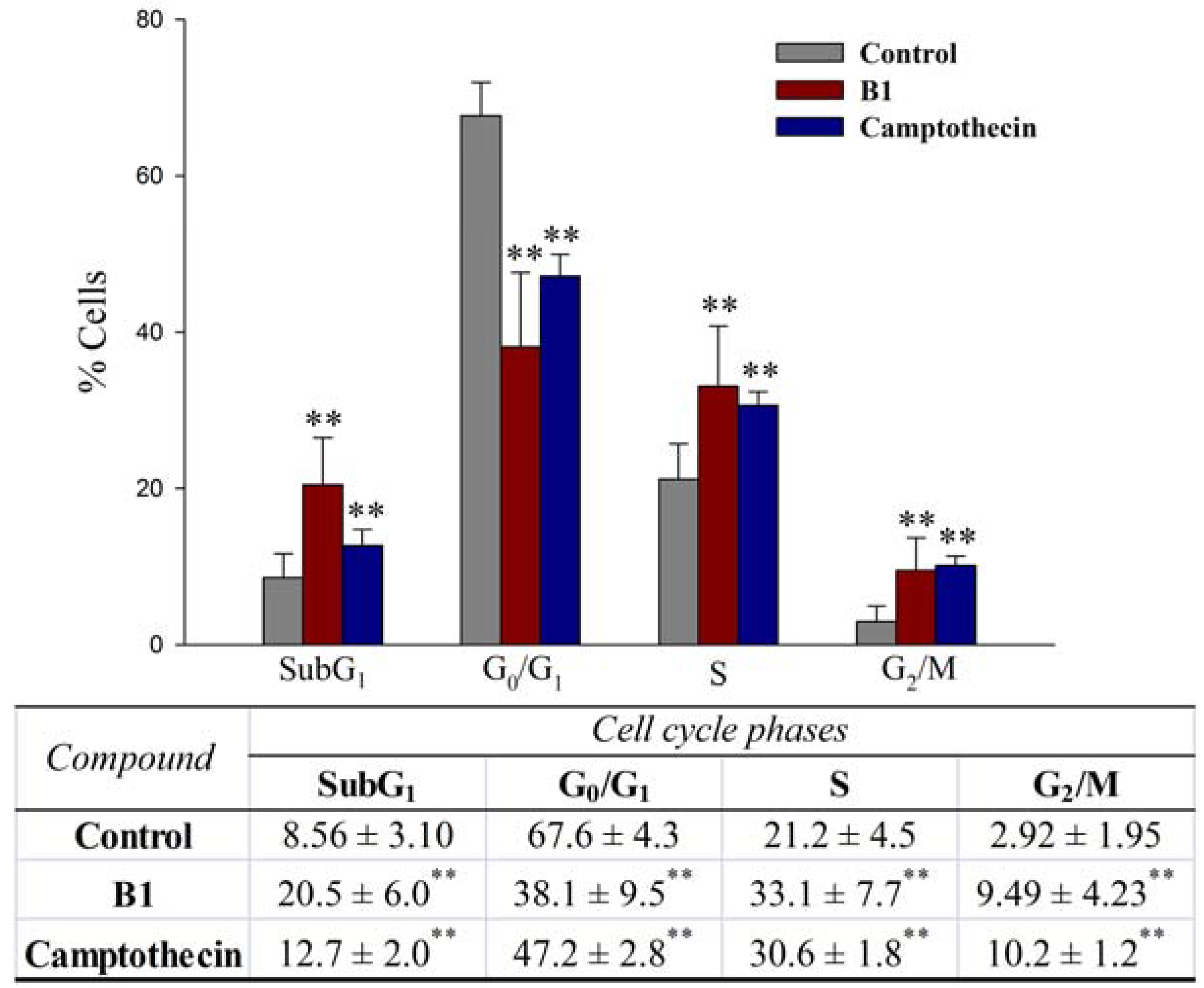
2.2.4. Effects on cell cycle progression
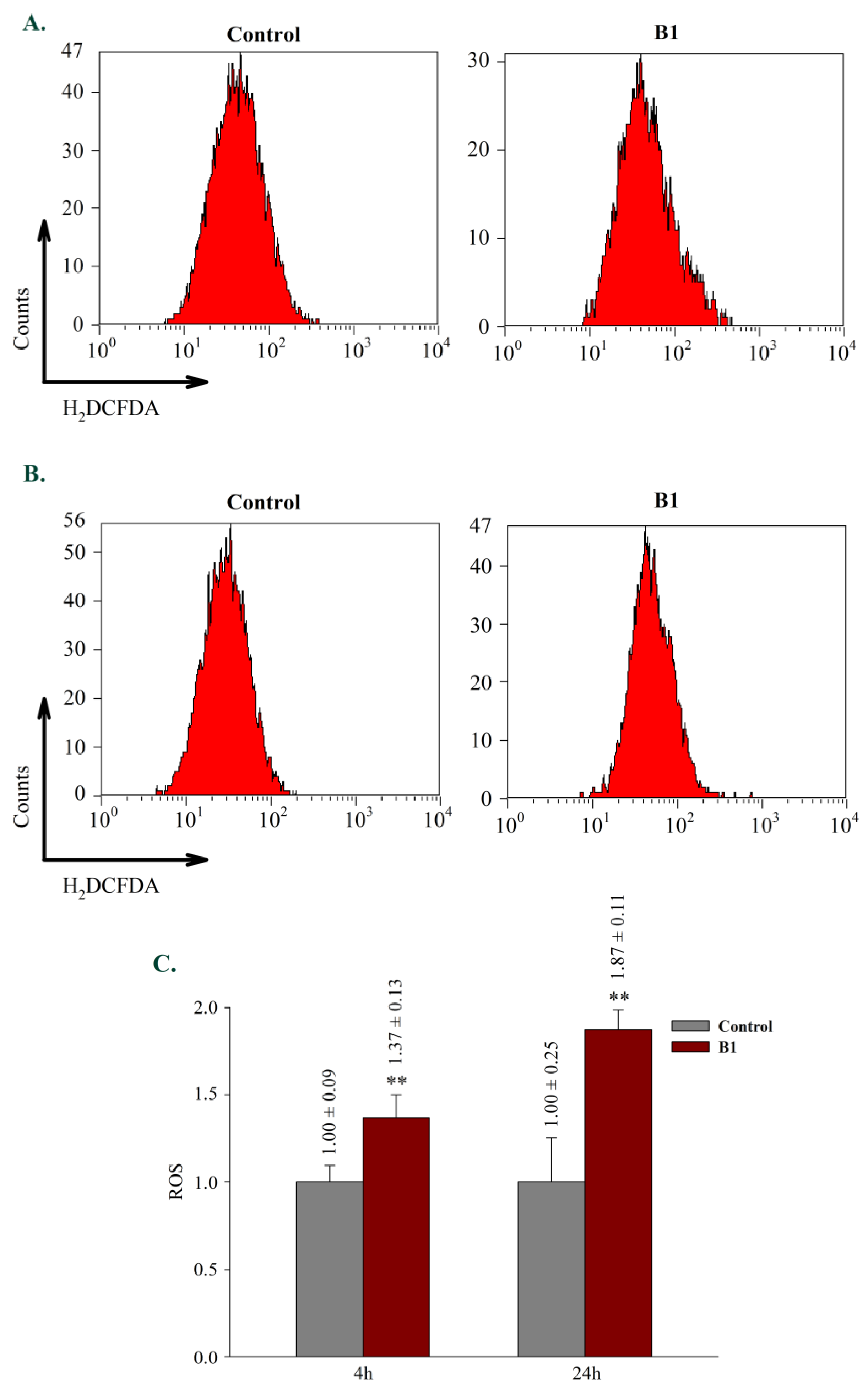
2.2.5. Effects on reactive oxygen species generation (ROS)
3. Experimental
3.1. General
3.2. General Procedure for Preparation of 4-Mercaptomethylbenzonitrile and 4-Nitrophenylmethanethiol
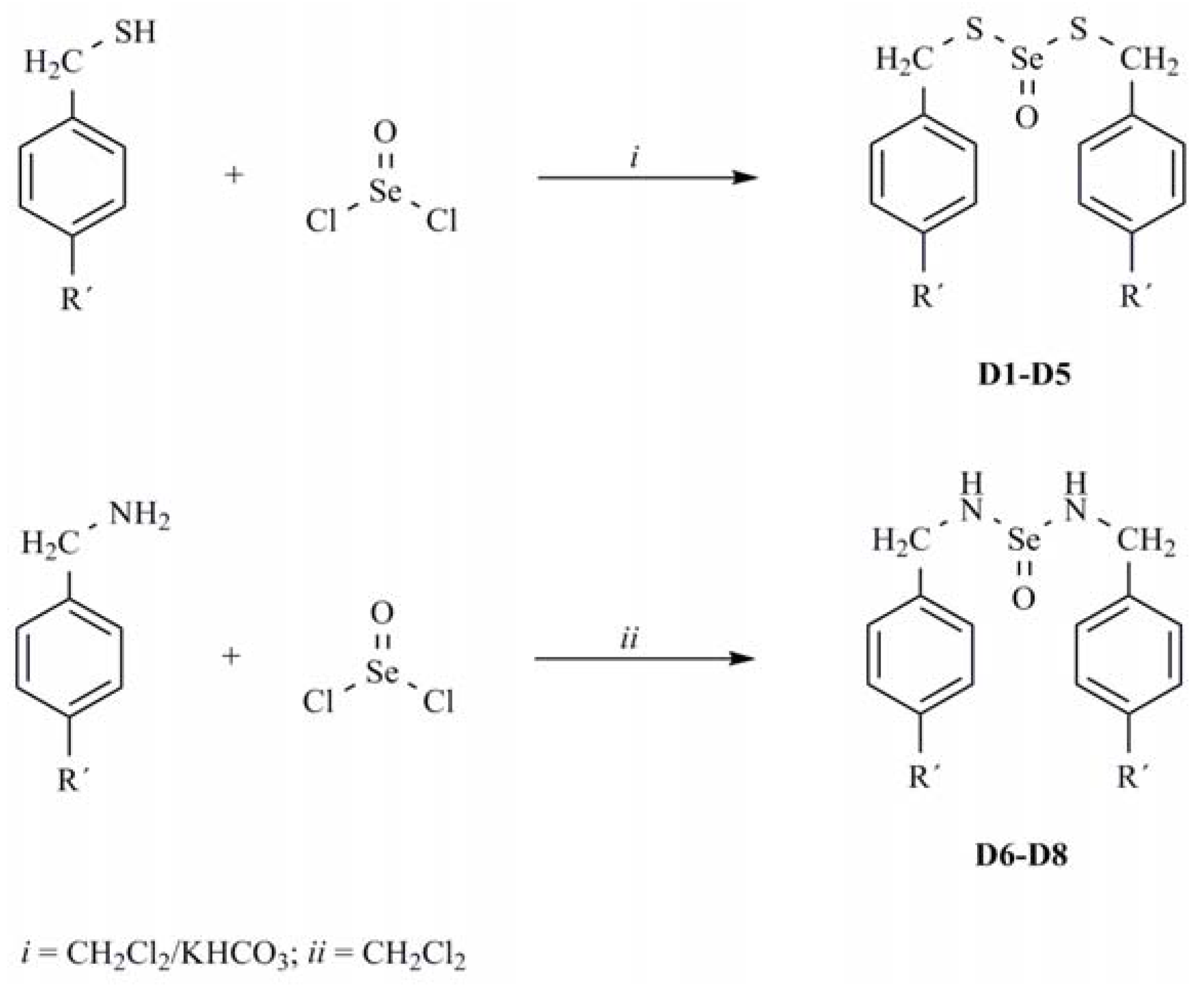
3.3. General Procedure for Preparation of Dithioselenites D1-D5
3.4. General Procedure for Preparation of Selenylureas (D6-D8)
3.5. Biological Activity Assays
3.5.1. Superoxide anion generating activity
3.5.2. Cytotoxic activity in PC-3 and MCF-7
3.5.3. Apoptosis and cell cycle
3.5.4. Measurement of reactive oxygen species
4. Conclusions
Acknowledgements
References and Notes
- Jung, H.J.; Seo, Y.R. Current issues of selenium in cancer chemoprevention. Biofactors 2010, 36, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. Selenium and selenium-antagonistic elements in nutritional cancer prevention. Crit. Rev. Biotechnol. 2009, 29, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; Parsons, J.K.; Bearden, J.D.; Crawford, E.D.; Goodman, G.E.; Claudio, J.; Winquist, E.; Cook, E.D.; Karp, D.D.; Walther, P.; Lieber, M.M.; Kristal, A.R.; Darke, A.K.; Arnold, K.B.; Ganz, P.A.; Santella, R.M.; Albanes, D.; Taylor, P.R.; Probstfield, J.L.; Jagpal, T.J.; Crowley, J.J.; Meyskens, F.L.; Baker, L.H.; Coltman, C.A. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.S.; Algotar, A.M.; Ranger-Moore, J.; Stratton, S.P.; Slate, E.H.; Hsu, C.H.; Thompson, P.A.; Clark, L.C.; Ahmann, F.R. Oral selenium supplementation has no effect on prostate-specific antigen velocity in men undergoing active surveillance for localized prostate cancer. Cancer Prev. Res. 2010, 3, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Micke, O.; Schomburg, L.; Buentzel, J.; Kisters, K.; Muecke, R. Selenium in oncology: from chemistry to clinics. Molecules 2009, 14, 3975–3988. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R. Selenium compounds and selenoproteins in cancer. Chem. Biodivers. 2008, 5, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A.C.; Zoidis, E.; Surai, P.F.; Zervas, G. Selenoproteins and maternal nutrition. Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 2008, 151, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Muecke, R.; Schomburg, L.; Buentzel, J.; Kisters, K.; Micke, O. Selenium or no selenium-that is the question in tumor patients: a new controversy. Integr. Cancer Ther. 2010, 9, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.I.; Combs, G.F. Selenium and anticarcinogenesis: underlying mechanisms. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Hu, H.; Jiang, C.; Schuster, T.; Lü, J. Differential involvement of reactive oxygen species in apoptosis induced by two classes of selenium compounds in human prostate cancer cells. Int. J. Cancer 2007, 120, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Rikiishi, H. Apoptotic cellular events for selenium compounds involved in cancer prevention. J. Bioenerg. Biomembr. 2007, 39, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Nian, H.; Cooper, A.J.; Sinha, R.; Bisson, W.H.; Dashwood, R.H.; Pinto, J.T. α-Keto acid metabolites of naturally-occurring organoselenium compounds as inhibitors of histone deacetylase in human prostate cancer cells. Cancer Prev. Res. 2009, 2, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Spallholz, J.E.; Palace, V.P.; Reid, T.W. Methioninase and selenomethionine but not Se-methylselenocysteine generate methylselenol and superoxide in an in vitro chemiluminescent assay: implications for the nutritional carcinostatic activity of selenoamino acids. Biochem. Pharmacol. 2004, 67, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Boylan, L.M.; Wu, C.K.; Spallholz, J.E. Oxidation of glutathione and superoxide generation by inorganic and organic selenium compounds. Biofactors 2007, 31, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Chaudiere, J.; Courtin, O.; Leclaire, J. Glutathione oxidase activity of selenocystamine: A mechanistic study. Arch. Biochem. Biophys. 1992, 296, 328–336. [Google Scholar] [CrossRef]
- Rosa, R.M.; Moura, D.J.; Romano e Silva, A.C.; Saffi, J.; Pegas-Henriques, J.A. Antioxidant activity of diphenyl diselenide prevents the genotoxicity of several mutagens in Chinese hamster V79 cells. Mutat. Res. 2007, 631, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Brandão, R.; Acker, C.I.; Leite, M.R.; Barbosa, N.B.; Nogueira, C.W. Diphenyl diselenide protects against glycerol-induced renal damage in rats. J. Appl. Toxicol. 2009, 29, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Mishra, B.; Barik, A.; Kumbhare, L.B.; Pandey, R.; Jain, V.K.; Priyadarsini, K.I. 3,3´-diselenodipropionic acid, an efficient peroxyl radical scavenger and GPx mimic, protects erythrocytes (RBCs) from AAPH-induced hemolysis. Chem. Res. Toxicol. 2007, 20, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Bansal, P.; Kumar, S.J.; Bag, P.P.; Paul, P.; Reddy, N.D.; Kumbhare, L.B.; Jain, V.K.; Chaubey, R.C.; Unnikrishnan, M.K.; Priyadarsini, K.I. In vivo radioprotection studies of 3,3´-diselenodipropionic acid, a selenocystine derivative. Free Radic. Biol. Med. 2010, 48, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Prigol, M.; Hassan, W.; Nogueira, C.W.; Rocha, J.B. Protective effect of binaphthyl diselenide, a synthetic organoselenium compound, on 2-nitropropane-induced hepatotoxicity in rats. Cell Biochem. Funct. 2010, 28, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Machado Mda, S.; Villela, I.V.; Moura, D.J.; Rosa, R.M.; Salvador, M.; Lopes, N.P.; Braga, A.L.; Roesler, R.; Saffi, J.; Henriques, J.A. 3,3-ditrifluoromethyldiphenyl diselenide: a new organoselenium compound with interesting antigenotoxic and antimutagenic activities. Mutat. Res. 2009, 673, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Spallholz, J.E.; Schriver, B.J.; Reid, T.W. Dimethyldiselenide and methylseleninic acid generate superoxide in an in vitro chemiluminescence assay in the presence of glutathione: implications for the anticarcinogenic activity of L-selenomethionine and L-Se-methylselenocysteine. Nutr. Cancer 2001, 40, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The antioxidant role of selenium and seleno-compounds. Biomed. Pharmacother. 2003, 57, 134–144. [Google Scholar] [CrossRef]
- Richie, J.P.; Kleinman, W.; Desai, D.H.; Das, A.; Amin, S.G.; Pinto, J.T.; El-Bayoumy, K. The organoselenium compound 1,4-phenylenebis(methylene) selenocyanate inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induced tumorgenesis and enhances glutathione-related antioxidant levels in A/J mouse lung. Chem. Biol. Interact. 2006, 161, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Hossain, S.U.; Bhattacharya, S. Protective effect of diphenylmethyl selenocyanate against CCl4-induced hepatic injury. J. Appl. Toxicol. 2007, 27, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Drake, E.N. Cancer chemoprevention: Selenium as a prooxidant, not an antioxidant. Med. Hypotheses 2006, 67, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Plano, D.; Sanmartín, C.; Moreno, E.; Prior, C.; Calvo, A.; Palop, J.A. Novel potent organoselenium compounds as cytotoxic agents in prostate cancer cells. Bioorg. Med. Chem. Lett. 2007, 17, 6853–6859. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, C.; Plano, D.; Palop, J.A. Selenium compounds and apoptotic modulation: a new perspective in cancer therapy. Mini Rev. Med. Chem. 2008, 8, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, C.; Plano, D.; Domínguez, E.; Font, M.; Calvo, A.; Prior, C.; Encío, I.; Palop, J.A. Synthesis and pharmacological screening of several aroyl and heteroaroyl selenylacetic acid derivatives as cytotoxic and antiproliferative agents. Molecules 2009, 14, 3313–3338. [Google Scholar] [CrossRef] [PubMed]
- Plano, D.; Lizarraga, E.; Font, M.; Palop, J.A.; Sanmartín, C. Thermal stability and decomposition of sulphur and selenium compounds. J. Therm. Anal. Calorim. 2009, 98, 559–566. [Google Scholar] [CrossRef]
- Plano, D.; Moreno, E.; Font, M.; Encío, I.; Palop, J.A.; Sanmartín, C. Synthesis and in vitro anticancer activities of some selenadiazole derivatives. Arch. Pharm. 2010. [Google Scholar] [CrossRef] [PubMed]
- Plano, D.; Baquedano, Y.; Moreno, D.; Font, M.; Jiménez-Ruiz, A.; Palop, J.A.; Sanmartín, C. Selenocyanates and diselenides: A new class of potent antileishmanial agents. Eur. J. Med. Chem. 2010. submitted. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, E.; Plano, D.; Font, M.; Calvo, A.; Prior, C.; Palop, J.A.; Sanmartín, C. Synthesis and antiproliferative activity of novel symmetrical alkylthio- and alkylseleno- imidocarbamates. Eur. J. Med. Chem. 2010. submitted. [Google Scholar] [CrossRef] [PubMed]
- Friedman, O.M.; Seligman, A.M. Preparation of N-phosphorylated derivates of bis-β-chloroethylamine. J. Am. Chem. Soc. 1954, 76, 655–658. [Google Scholar] [CrossRef]
- Aliphatic sulphur compounds. In Vogel’s Textbook of Practical Organic Chemistry, 50th Edition; Furmiss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. (Eds.) Addison Wesley Longman Limited: Harlow, UK, 1989; p. 787. [Google Scholar]
- Yan, L.; Spallholz, J.E. Generation of reactive oxygen species from the reaction of selenium compounds with thiols and mammary tumor cells. Biochem. Pharmacol. 1993, 45, 429–437. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Inmunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Chen, T.; Wong, Y.S. In vitro antioxidant and antiproliferative activities of selenium-containing phycocyanin from selenium-enriched Spirulina platensis. J. Agric. Food. Chem. 2008, 56, 4352–4358. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.T.; Sinha, R.; Papp, K.; Facompre, N.D.; Desai, D.; El-Bayoumy, K. Differential effects of naturally occurring and synthetic organoselenium compounds on biomarkers in androgen responsive and androgen independent human prostate carcinoma cells. Int. J. Cancer 2007, 120, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, H.; Hawthorn, L.; Ganther, H.E.; Ip, C. Delineation of the molecular basis for selenium-induced growth arrest in human prostate cancer cells by oligonucleotide array. Identification of multidrug resistance-associated protein 1 and glutathione as multidrug resistance mechanisms in human prostate cancer cells: chemosensitization with leukotriene D4 antagonists and buthionine sulfoximine. Cancer Res. 2003, 63, 52–59. [Google Scholar] [PubMed]
- Van Brussel, J.P.; Oomen, M.A.; Vossebeld, P.J.; Wiemer, E.A.; Sonneveld, P.; Mickisch, G.H. Identification of multidrug resistance-associated protein 1 and glutathione as multidrug resistance mechanisms in human prostate cancer cells: chemosensitization with leukotriene D4 antagonists and buthionine sulfoximine. BJU Int. 2004, 93, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Kang, D.H.; Min, J.; Lee, C.S.; Ha, E.M.; Lee, E.S.; Kwon, Y.; Na, Y. Synthesis and pharmacological evaluation of new methyloxiranylmethoxyxanthone analogues. Eur. J. Med. Chem. 2010, 45, 4221–4228. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Wu, M.; Botnen, J.H. Methylselenol, a selenium metabolite, induces cell cycle arrest in G1 phase and apoptosis via the extracellular-regulated kinase 1/2 pathway and other cancer signaling genes. J. Nutr. 2009, 139, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lee, H.J.; Chai, Y.; Hu, H.; Wang, L.; Zhang, Y.; Jiang, C.; Lü, J. Persistent P21Cip1 induction mediates G1 cell cycle arrest by methylseleninic acid in DU145 prostate cancer cells. Curr. Cancer Drug Target. 2010, 10, 307–318. [Google Scholar] [CrossRef]
- Pinto, J.T.; Lee, J.I.; Sinha, R.; Macewan, M.E.; Cooper, A.J. Chemopreventive mechanisms of α-keto acid metabolites of naturally occurring organoselenium compounds. Amino Acids 2010. [Google Scholar] [CrossRef] [PubMed]
- Philchenkov, A.; Zavelevich, M.; Khranovskaya, N.; Surai, P. Comparative analysis of apoptosis induction by selenium compounds in human lymphoblastic leukemia MT-4 cells. Exp. Oncol. 2007, 29, 257–261. [Google Scholar] [PubMed]
- Naithani, R. Organoselenium compounds in cancer chemoprevention. Mini Rev. Med. Chem. 2008, 8, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Fimognari, C.; Nüsse, M.; Cesari, R.; Iori, R.; Cantelli-Forti, G.; Hrelia, P. Growth inhibition, cell-cycle arrest and apoptosis in human T-cell leukemia by the isothiocyanate sulforaphane. Carcinogenesis 2002, 23, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.R.; Aithal, K.; Rao, B.N.; Udupa, N.; Rao, B.S. Cytotoxic, genotoxic and oxidative stress induced by 1,4-naphthoquinone in B16F1 melanoma tumor cells. Toxicol. in Vitro 2009, 23, 242–250. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, L.F.; Losada, A.; Alcaide, V.; Álvarez, A.M.; Cuadrado, A.; González, L.; Nakayama, K.; Nakayama, K.I.; Fernández-Sousa, J.M.; Munoz, A.; Sánchez-Puelles, J.M. Aplidin (TM) induces the mitochondrial apoptotic pathway via oxidative stress-mediated JNK and p38 activation and protein kinase C delta. Oncogene 2002, 21, 7533–7544. [Google Scholar] [CrossRef]
- Puntel, R.L.; Roos, D.H.; Folmer, V.; Nogueira, C.W.; Galina, A.; Aschner, M.; Rocha, J.B. Mitochondrial dysfunction induced by different organochalchogens is mediated by thiol oxidation and is not dependent of the classical mitochondrial permeability transition pore opening. Tox. Sci. 2010, 117, 133–143. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds A1-A13, B1-B13, C1-C25 and D1-D8 are available from the authors. |

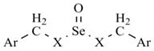
| Ref. | Ar | X | Yield (%) | M.p. (°C) | Recryst. Solvent | Molecular formula | CHN (%) Calcd/Found |
|---|---|---|---|---|---|---|---|
| D1 | p-chlorophenyl | S | 9 | 126–128 | Methanol | C14H14Cl2OS2Se | C, 40.77/40.54 H, 3.39/3.48 N, 0.00/0.00 |
| D2 | p-methoxyphenyl | S | 14 | 96–98 | Methanol | C16H18O3S2Se | C, 47.88/48.10 H, 4.49/4.76 N, 0.00/0.00 |
| D3 | p-trifluoromethylphenyl | S | 11 | 131–133 | Methanol | C16H12F6OS2Se | C, 40.25/40.51 H, 2.52/2.68 N, 0.00/0.00 |
| D4 | p-cyanophenyl | S | 18 | 170–172 | Methanol | C16H12N2OS2Se | C, 49.10/49.37 H, 3.07/3.35 N, 0.00/0.00 |
| D5 | p-nitrophenyl | S | 7 | 124–126 | Methanol | C14H12N2O5S2Se | C, 38.98/39.10 H, 2.78/2.60 N, 0.00/0.00 |
| D6 | p-chlorophenyl | NH | 22 | 245–247 | Ethanol | C14H14Cl2N2OSe | C, 44.68/44.49 H, 3.72/3.40 N, 7.44/7.56 |
| D7 | p-methoxyphenyl | NH | 39 | 176–178 | Ethanol | C16H20N2O3Se | C, 52.32/52.40 H, 5.45/5.40 N, 7.62/7.37 |
| D8 | p-trifluoromethylphenyl | NH | 8 | 159–161 | Ethanol | C16H14F6N2OSe | C, 43.34/43.49 H, 3.16/3.10 N, 6.32/6.30 |
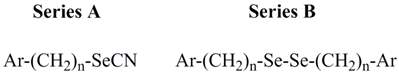
| Compound | Ar | n | CLUa | PC-3 cell line | MCF-7 cell line |
|---|---|---|---|---|---|
| IC50 (μM) | IC50 (μM) | ||||
| A1 | 4-aminophenyl | 0 | 0.242 | 10.0 | >10 |
| A2 | 4-(N,N-dimethylamino)phenyl | 0 | 1.095 | 2.0 | >10 |
| A3 | 4-amino-3-carboxyphenyl | 0 | 0.075 | NEb | >10 |
| A4 | 4-acetamido-3-carboxyphenyl | 0 | 0.301 | > 10 | >10 |
| A5 | 4-bromophenyl | 1 | 0.372 | > 10 | >10 |
| A6 | phenyl | 1 | 0.814 | NE | >10 |
| A7 | 4-nitrophenyl | 1 | 0.091 | 8.9 | >10 |
| A8 | 4-trifluoromethylphenyl | 1 | 0.798 | > 10 | >10 |
| A9 | 4-methylthiophenyl | 1 | 0.810 | NE | NE |
| A10 | 4-methylphenyl | 1 | 1.490 | NE | NE |
| A11 | 4-cyanophenyl | 1 | 0.580 | > 10 | NE |
| A12 | naphthyl | 1 | 0.141 | 6.0 | 9.4 |
| A13 | 4-nitrophenyl | 2 | 0.232 | NE | NE |
| B1 | 4-aminophenyl | 0 | 3.928 | 1.7 | 4.3 |
| B2 | 4-(N,N-dimethylamino) phenyl | 0 | 0.281 | > 10 | >10 |
| B3 | 4-amino-3-carboxyphenyl | 0 | 0.129 | NE | >10 |
| B4 | 4-acetamido-3-carboxyphenyl | 0 | 0.085 | NE | NE |
| B5 | 4-bromophenyl | 1 | 0.315 | NE | NE |
| B6 | phenyl | 1 | 1.543 | NE | NE |
| B7 | 4-nitrophenyl | 1 | 0.141 | NE | NE |
| B8 | 4-trifluoromethylphenyl | 1 | 0.735 | > 10 | >10 |
| B9 | 4-methylthiophenyl | 1 | 1.089 | > 10 | >10 |
| B10 | 4-methylphenyl | 1 | 0.570 | NE | NE |
| B11 | 4-cyanophenyl | 1 | 0.119 | NE | NE |
| B12 | naphthyl | 1 | 0.101 | NE | NE |
| B13 | 4-nitrophenyl | 2 | 0.176 | 5.0 | >10 |
| Control | 0.103 | - | - | ||
| Diselenodipropionic acid | 2.526 | - | - | ||
| MSAc | - | 8.4 [40] | - | ||
| Etoposide | - | 13.6 ± 2.2 [41] | 17.5 ± 1.2 [42] |
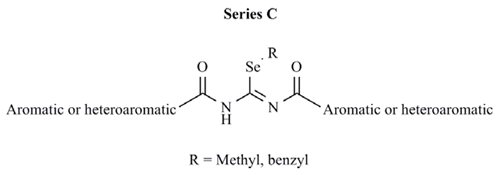
| Comp. | Aromatic or heteroaromatic | R | CLUa | PC-3 cell line | MCF-7 cell line |
|---|---|---|---|---|---|
| IC50 (μM) | IC50 (μM) | ||||
| C1 | 3-pyridyl | methyl | 0.110 | >10 | 6.2 |
| C2 | 4-methylphenyl | methyl | 0.157 | 1.8 | 3.0 |
| C3 | 4-cyanophenyl | methyl | 0.085 | >10 | 0.1 |
| C4 | 3,5-dimethoxyphenyl | methyl | 0.040 | 6.5 | 2.8 |
| C5 | 2-chloro-3-pyridyl | methyl | 0.221 | 9.1 | 1.3 |
| C6 | 2-furyl | methyl | 2353 | 7.4 | >10 |
| C7 | 2-thienyl | methyl | 0.165 | 6.1 | >10 |
| C8 | 3-chloro-2-thienyl | methyl | 0.517 | 6.3 | 8.3 |
| C9 | 5-nitro-3-thienyl | methyl | 2760 | 1.2 | 6.0 |
| C10 | 2-isoxazolyl | methyl | 0.408 | 7.6 | 0.6 |
| C11 | 2-benzothienyl | methyl | NDb | > 10 | >10 |
| C12 | 3,4-methylenedioxybenzyl | methyl | 0.043 | 8.1 | >10 |
| C13 | 3-quinolinyl | methyl | 0.100 | 7.6 | >10 |
| C14 | 2-phenyl-4-quinolinyl | methyl | 0.054 | 7.7 | NE |
| C15 | 9-acridinyl | methyl | 0.185 | > 10 | NE |
| C16 | 2-furyl | benzyl | 508 | > 10 | >10 |
| C17 | 2-thienyl | benzyl | 0.073 | NEc | NE |
| C18 | 3-chloro-2-thienyl | benzyl | 0.052 | NE | >10 |
| C19 | 5-nitro-3-thienyl | benzyl | 0.063 | > 10 | >10 |
| C20 | 2-isoxazolyl | benzyl | 0.216 | NE | >10 |
| C21 | 2-benzothienyl | benzyl | 0.076 | > 10 | >10 |
| C22 | 3,4-methylenedioxybenzyl | benzyl | 0.046 | NE | NE |
| C23 | 3-quinolinyl | benzyl | 0.051 | > 10 | NE |
| C24 | 2-phenyl-4-quinolinyl | benzyl | 0.061 | > 10 | >10 |
| C25 | 9-acridinyl | benzyl | 0.051 | > 10 | NE |
| Control | 0.060 | - | - | ||
| Diselenodipropionic acid | 2.526 | - | - | ||
| MSAd | - | 8.4 [40] | - | ||
| Etoposide | - | 13.6 ± 2.2 [41] | 17.5 ± 1.2 [42] |
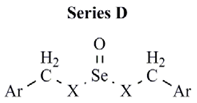
| Comp. | Ar | X | CLUa | PC-3 cell line | MCF-7 cell line |
|---|---|---|---|---|---|
| IC50 (μM) | IC50 (μM) | ||||
| D1 | p-chlorophenyl | S | 0.160 | NEb | NE |
| D2 | p-methoxyphenyl | S | 890 | NE | NE |
| D3 | p-trifluoromethylphenyl | S | 1451 | NE | >10 |
| D4 | p-cyanophenyl | S | 701 | NE | NE |
| D5 | p-nitrophenyl | S | 4743 | NE | NE |
| D6 | p-chlorophenyl | NH | 0.072 | 3.9 | 8.4 |
| D7 | p-methoxyphenyl | NH | 0.562 | NE | >10 |
| D8 | p-trifluoromethylphenyl | NH | 1973 | NE | NE |
| Control | 0.137 | - | - | ||
| Diselenodipropionic acid | 2.526 | - | - | ||
| MSAc | - | 8.4 [40] | - | ||
| Etoposide | - | 13.6 ± 2.2 [41] | 17.5 ± 1.2 [42] |
| Ref. | IR (KBr; υ/cm−1) | 1H NMR (400 MHz, DMSO-d6, δ/ppm, J in Hz) | MS 70 eV; s; (% abundance) |
|---|---|---|---|
| D1 | 3030 | 4.75 (d, 4H, 2 -S-CH2-), 7.32 (d, 4H, H3 + H5 + H3’ + H5’), 7.46 (d, 4H, H2 + H6 + H2’ + H6’). | 125 (100) |
| D2 | 3003 | 3.73 (s, 6H, 2OCH3), 4.84 (s, 4H, 2 -S-CH2-), 6.87 (d, 4H, H3 + H5 + H3’ + H5’), 7.17 (d, 4H, H2 + H6 + H2’ + H6’). | 90 (100) |
| D3 | 3008 | 5.04 (d, 4H, 2 -S-CH2-), 7.62 (d, 4H, H3 + H5 + H3’ + H5’ ), 7.73 (d, 4H, H2 + H6 + H2’ + H6’). | 159 (100) |
| D4 | 3061 | 5.03 (d, 4H, 2 -S-CH2-), 7.59 (d, 4H, H3 + H5 + H3’ + H5’ ), 7.85 (d, 4H, H2 + H6 + H2 + H6’). | 116 (100) |
| D5 | 3112 | 4.98 (d, 4H, 2 -S-CH2-), 7.75 (d, 4H, H3 + H5 + H3’ + H5’ ), 8.16 (d, 4H, H2 + H6 + H2’ + H6’). | 136 (100) |
| D6 | 3324 | 4.21 (d, 4H, 2 –NH-CH2-), 6.54 (t, 2H, 2-NH-CH2-), 7.28 (d, 4H, H3 + H5 + H3’ + H5’ ), 7.36 (d, 4H, H2 + H6 + H2’ + H6’). | 140 (100) |
| D7 | 3351-3321 | 3.73 (s, 6H, 2OCH3), 4.14 (d, 4H, 2 –NH-CH2-), 6.28 (t, 2H, 2-NH-CH2-), 6.87 (d, 4H, H3 + H5 + H3’ + H5’ ), 7.17 (d, 4H, H2 + H6 + H2’ + H6’). | 136 (100) |
| D8 | 3335 | 4.32 (d, 4H, 2 –NH-CH2-), 6.69 (t, 2H, 2-NH-CH2-), 7.46 (d, 4H, H3 + H5 + H3’ + H5’ ), 7.68 (d, 4H, H2 + H6 + H2’ + H6’). | 174 (100) |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Plano, D.; Baquedano, Y.; Ibáñez, E.; Jiménez, I.; Palop, J.A.; Spallholz, J.E.; Sanmartín, C. Antioxidant-Prooxidant Properties of a New Organoselenium Compound Library. Molecules 2010, 15, 7292-7312. https://doi.org/10.3390/molecules15107292
Plano D, Baquedano Y, Ibáñez E, Jiménez I, Palop JA, Spallholz JE, Sanmartín C. Antioxidant-Prooxidant Properties of a New Organoselenium Compound Library. Molecules. 2010; 15(10):7292-7312. https://doi.org/10.3390/molecules15107292
Chicago/Turabian StylePlano, Daniel, Ylenia Baquedano, Elena Ibáñez, Iosu Jiménez, Juan Antonio Palop, Julian E. Spallholz, and Carmen Sanmartín. 2010. "Antioxidant-Prooxidant Properties of a New Organoselenium Compound Library" Molecules 15, no. 10: 7292-7312. https://doi.org/10.3390/molecules15107292




