Evaluation of Antioxidant Capacity and Synergistic Associations of Quinonemethide Triterpenes and Phenolic Substances from Maytenus ilicifolia (Celastraceae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC-DAD analyses
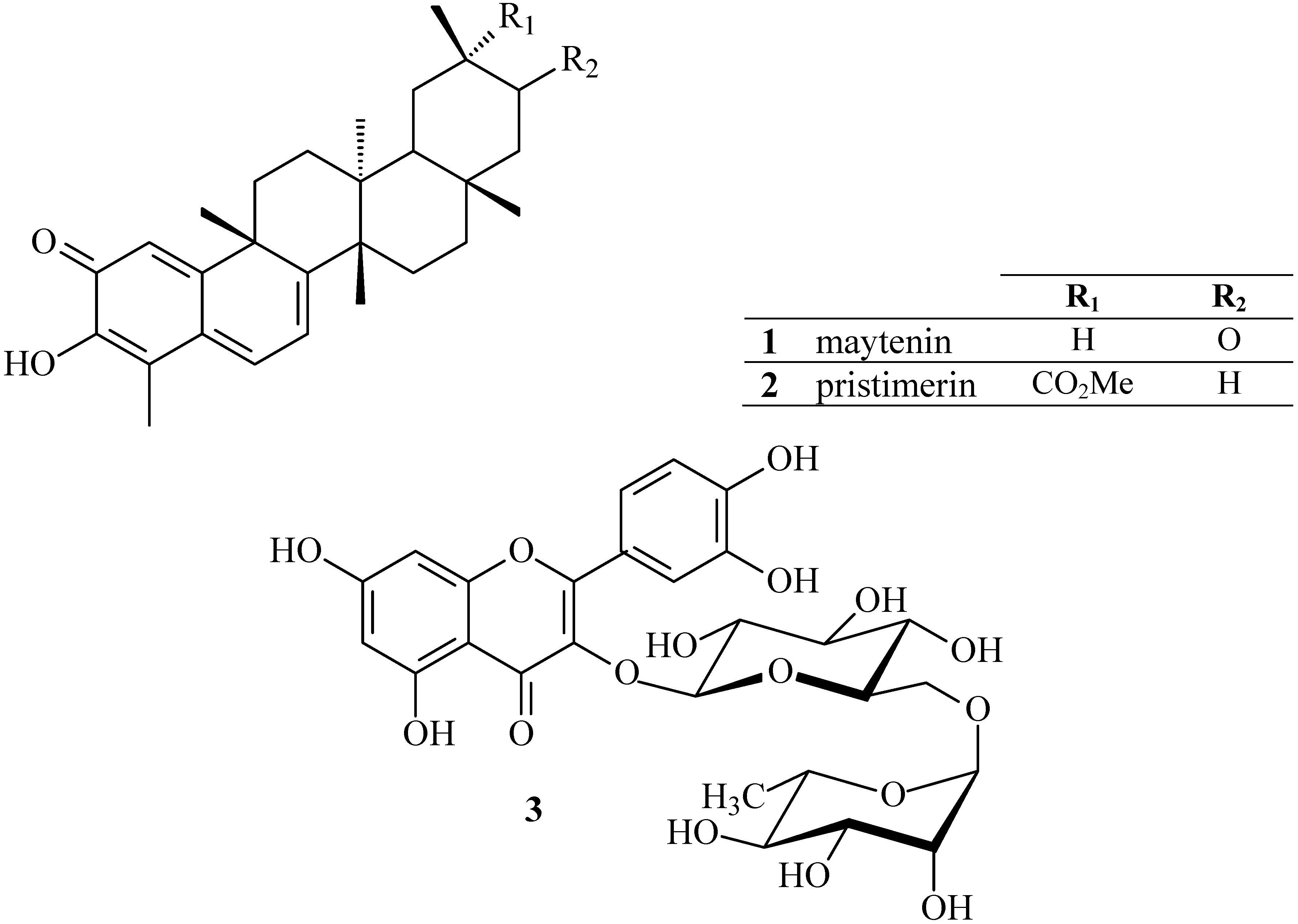
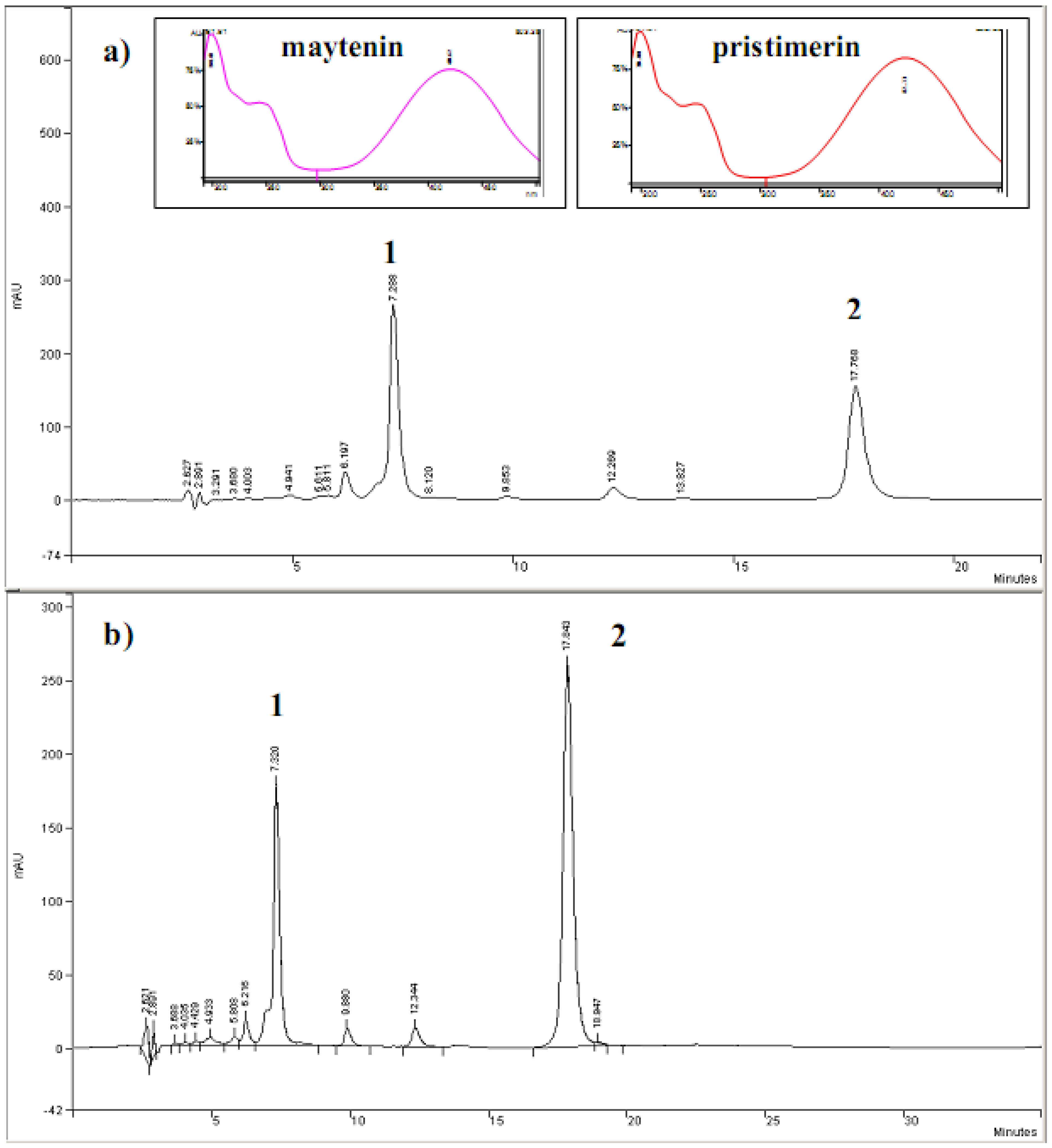
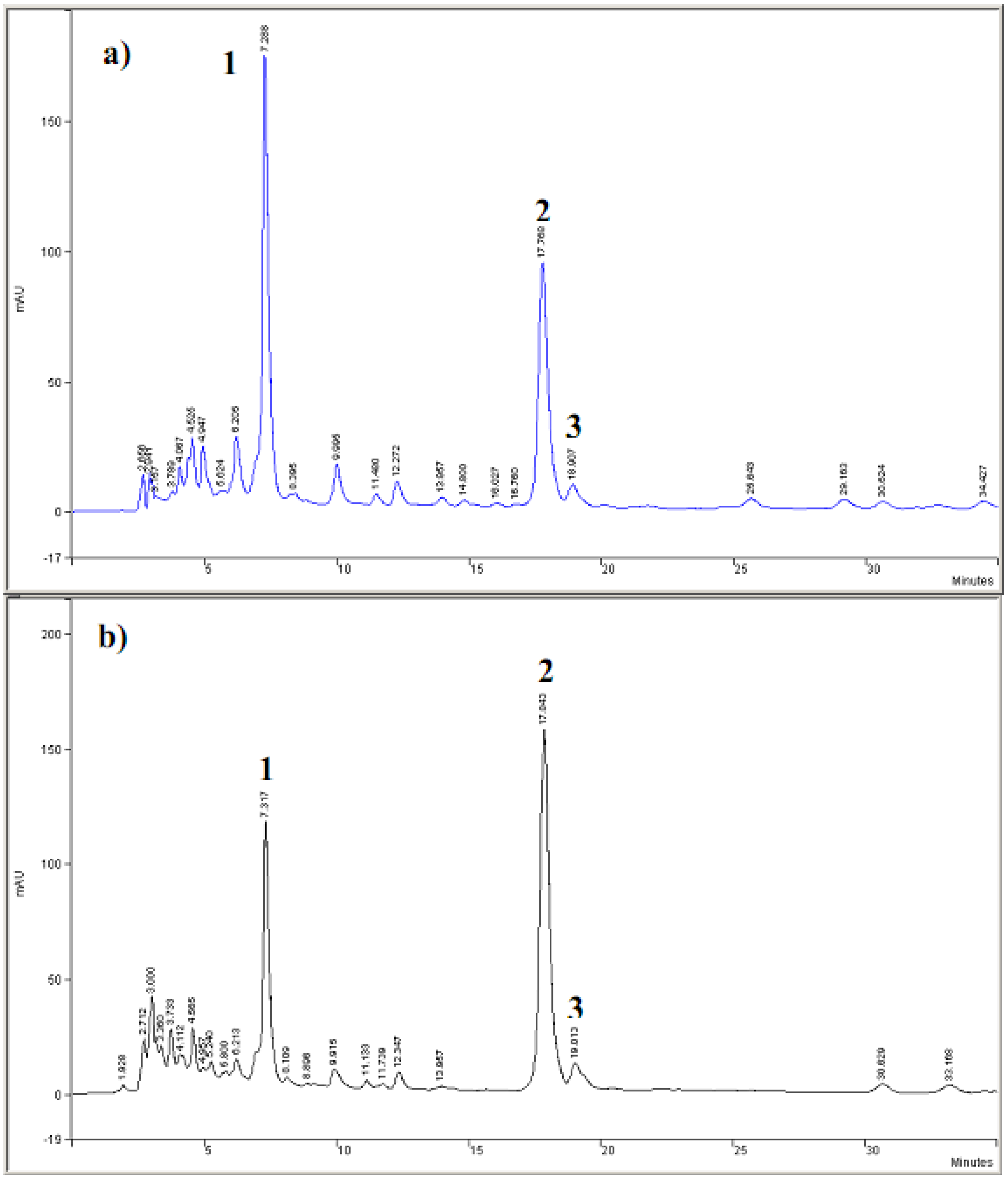
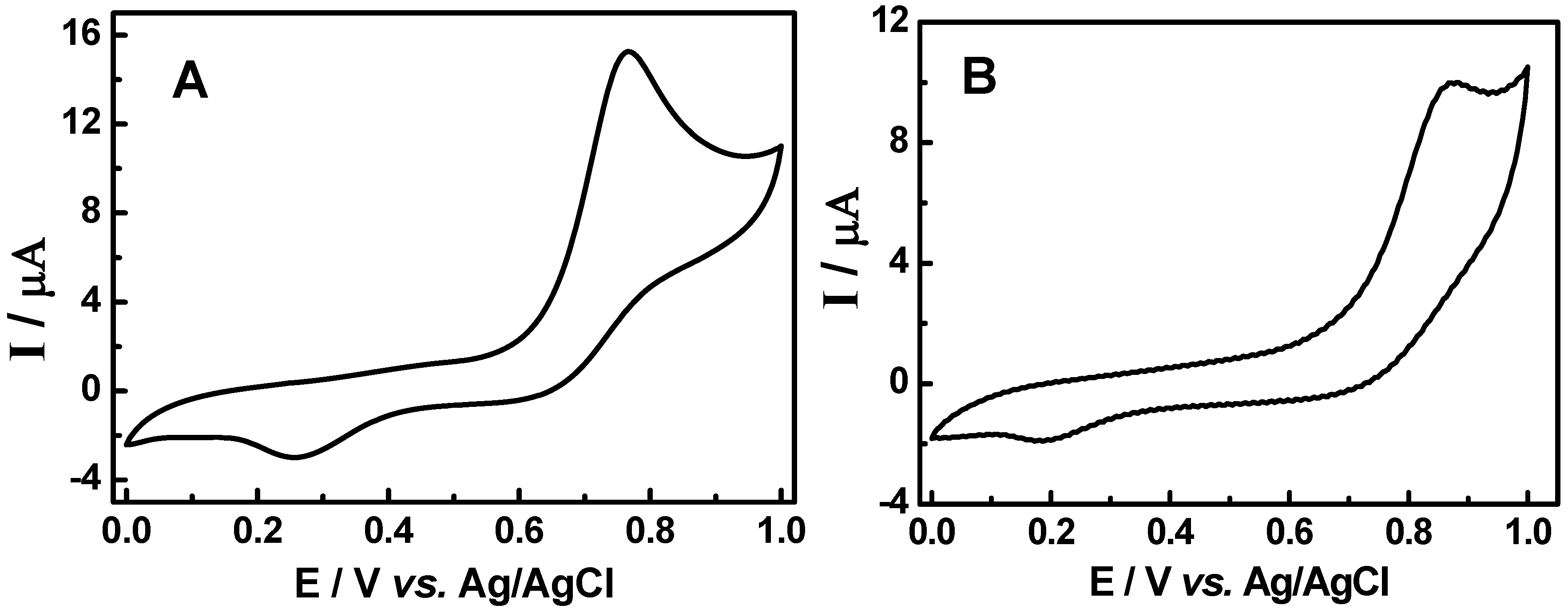
3.2. Cyclic voltammetric behavior of quinonemethide triterpenes and extract of Maytenus ilicifolia
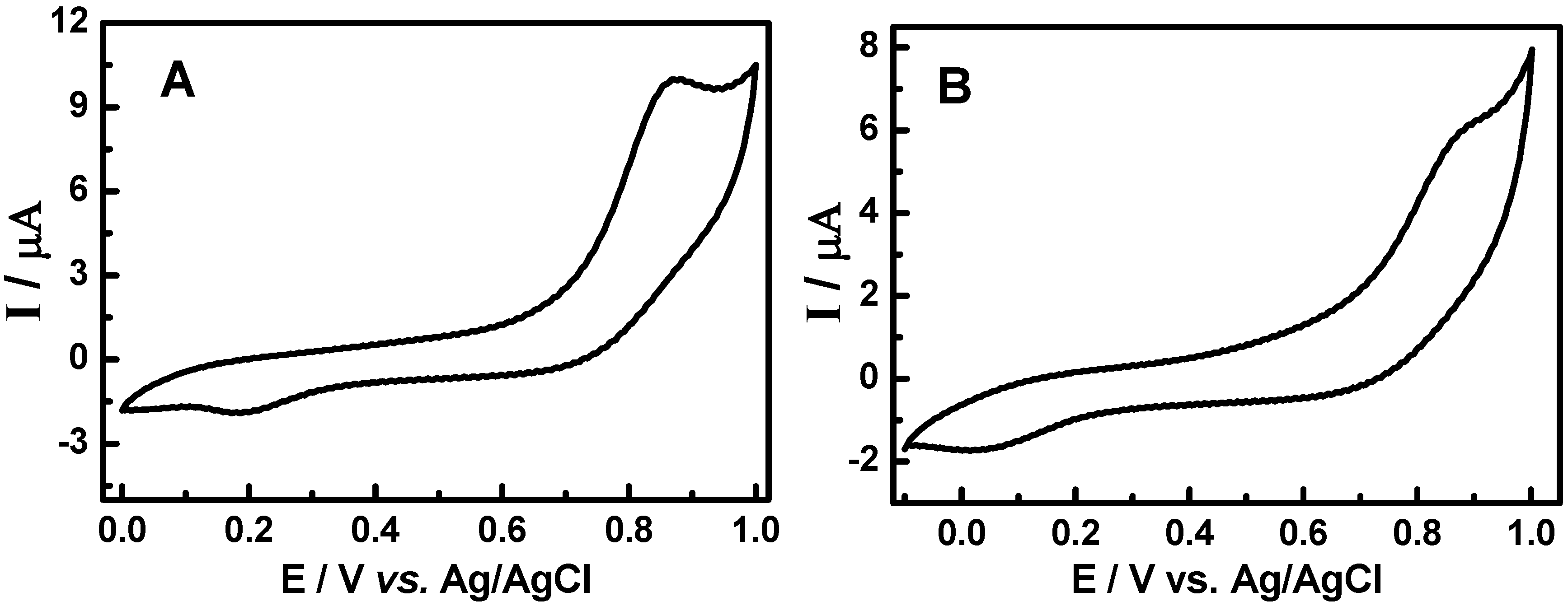

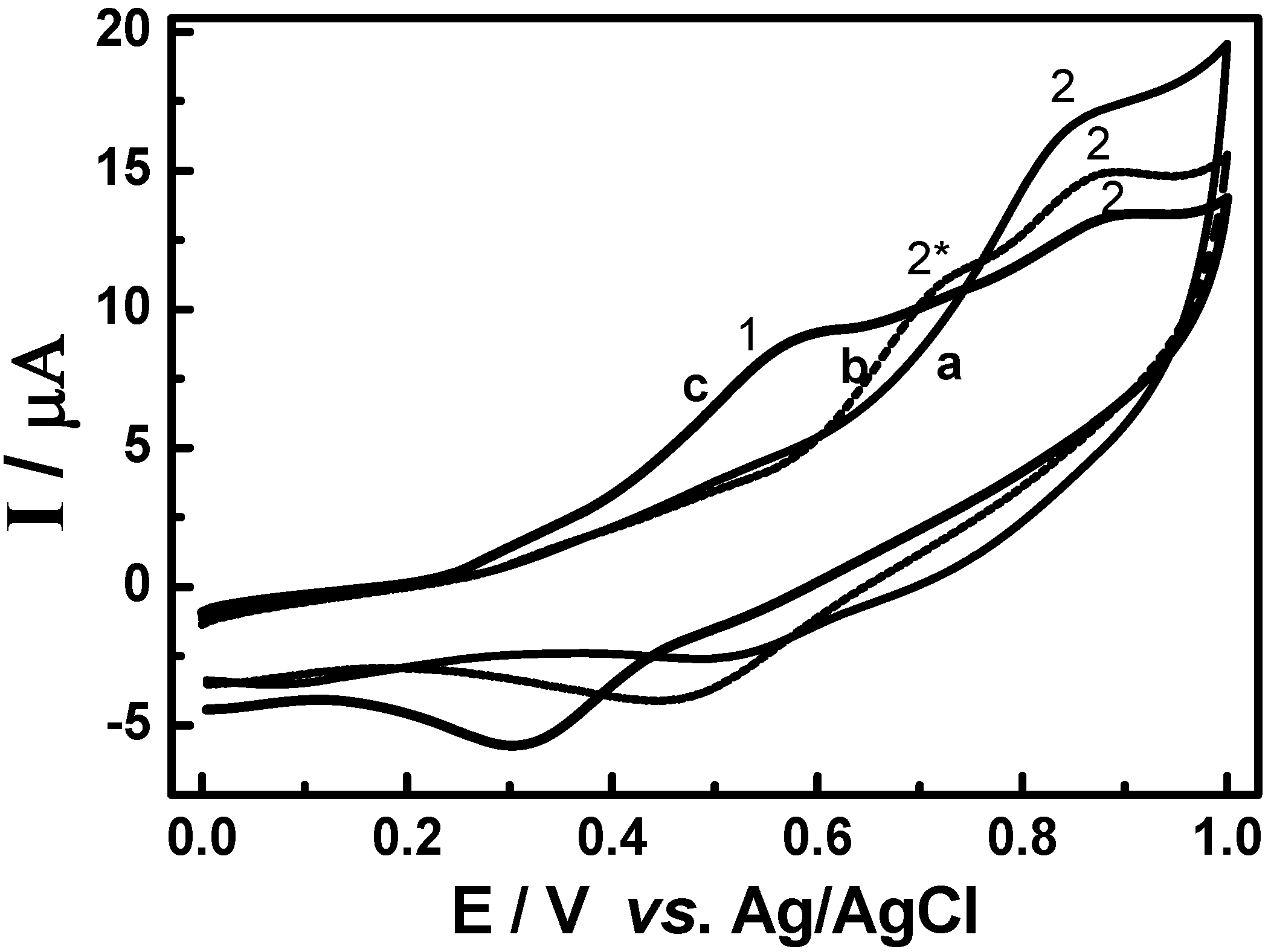
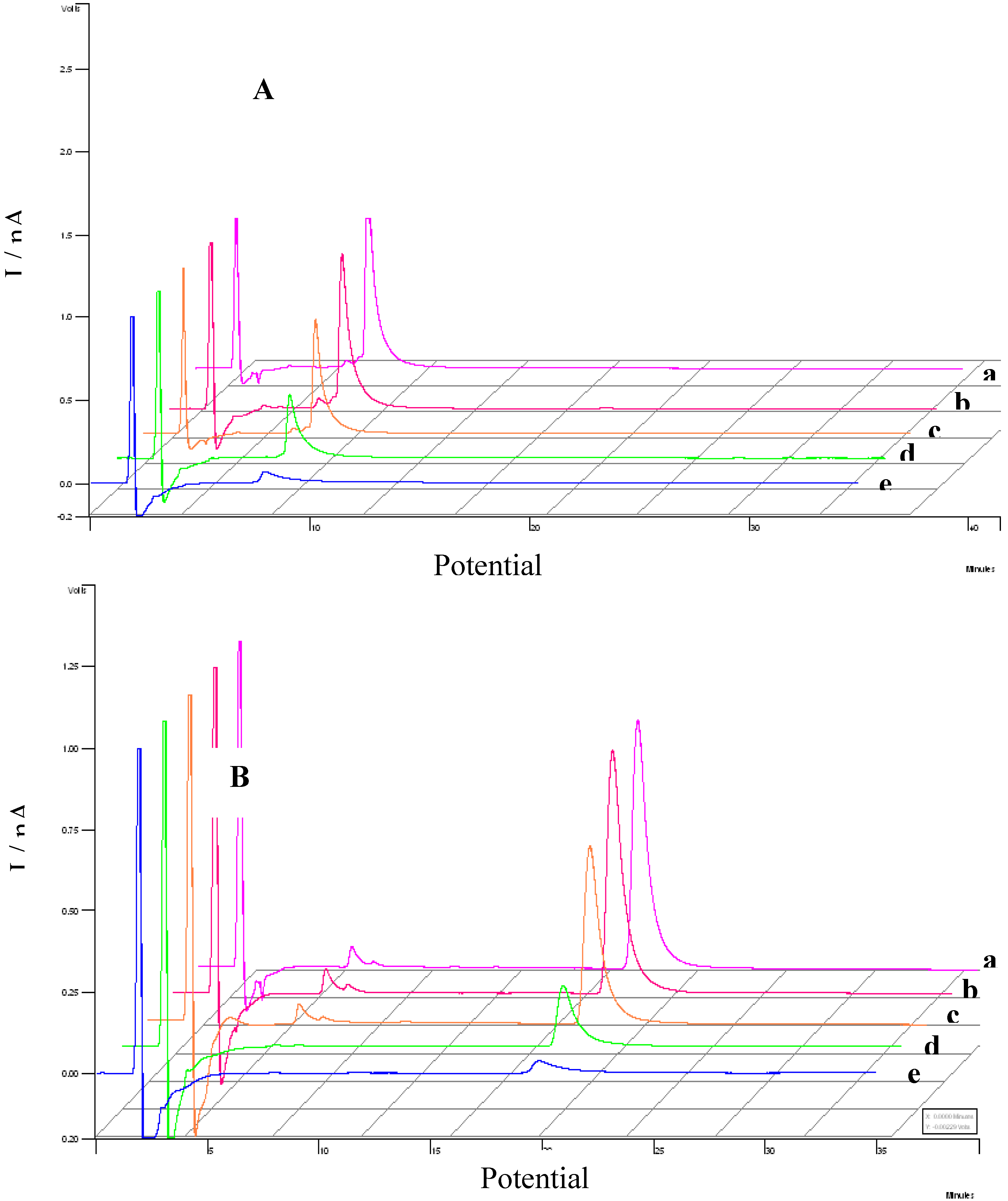
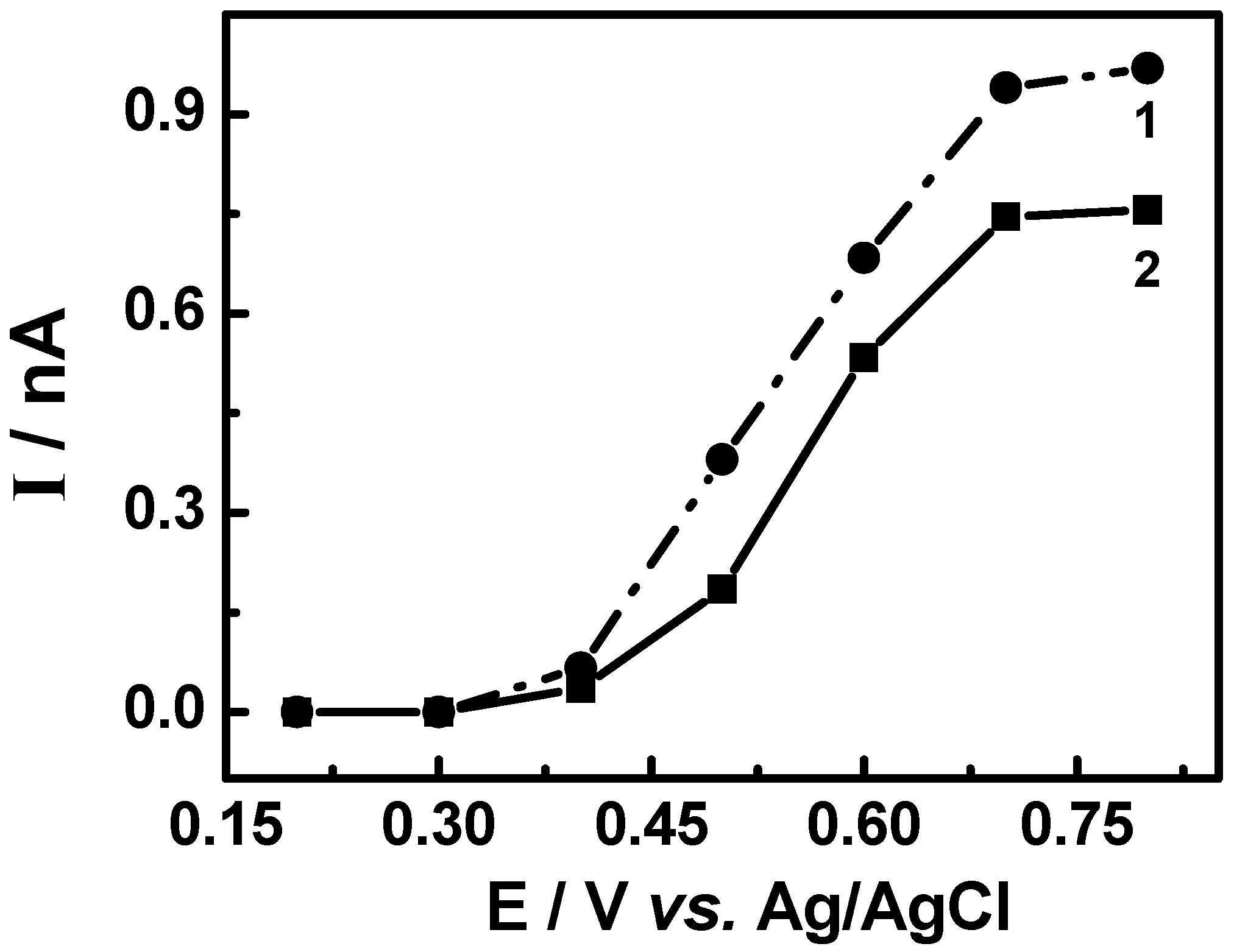
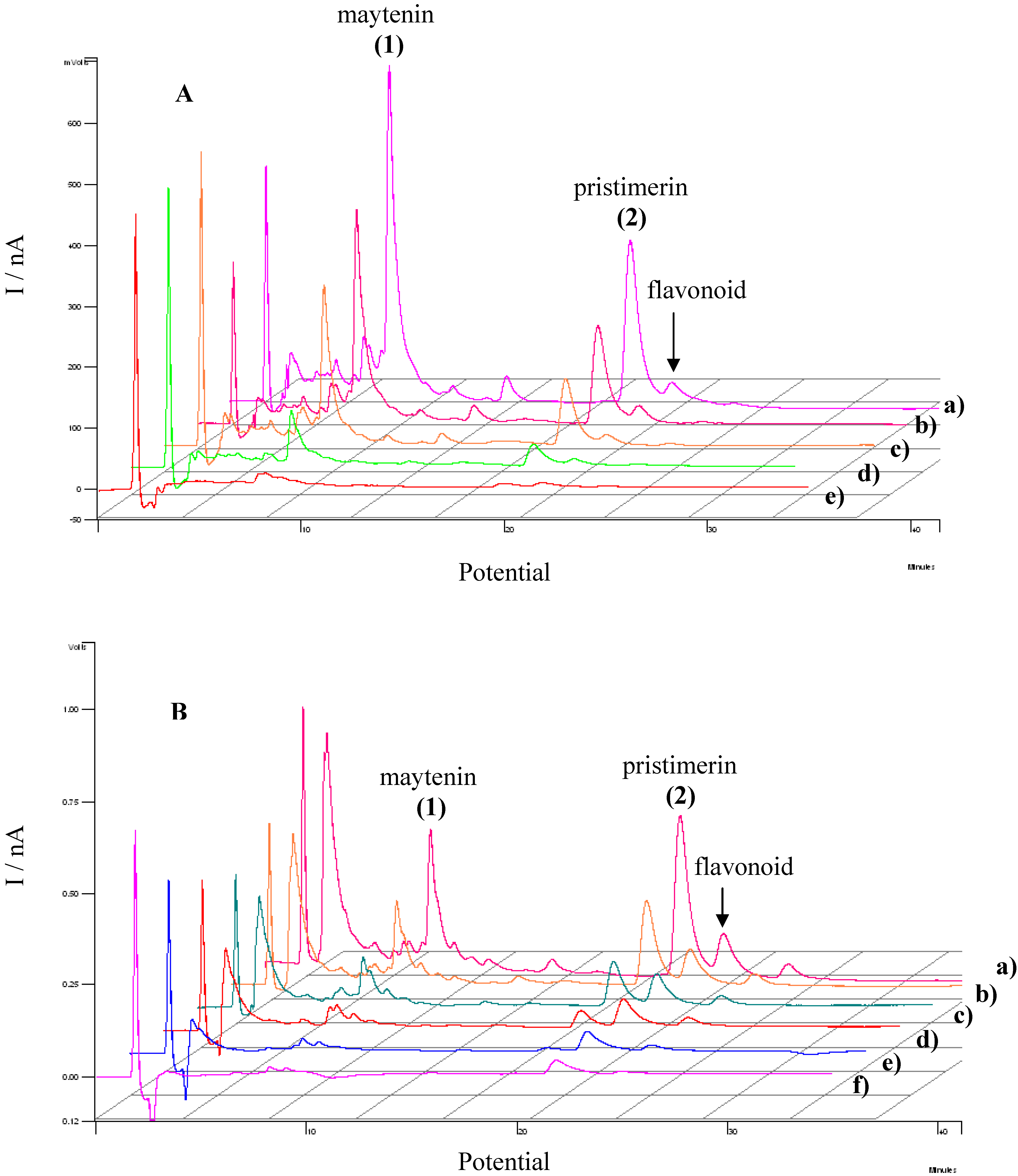
3.3. Evaluation of the extracts E2 and E4 via HPLC with electrochemical detection
3. Experimental
3.1. Instrumental
3.2. Plant material
3.3. Extraction and isolation
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds maytenin and pristimerin are available from the authors.
References
- Jorge, R.M.; Leite, J.P.V.; Oliveira, A.B.; Tagliati, C.A. Evaluation of antinociceptive, anti-inflammatory and antiulcerogenic activities of Maytenus ilicifolia. J. Ethnopharmacol. 2004, 94, 93–100. [Google Scholar] [CrossRef]
- Duarte, M.R.; Debur, M.C. Stem and leaf morphoanatomy of Maytenus ilicifolia. Fitoterapia 2005, 76, 41–49. [Google Scholar] [CrossRef]
- Vilegas, W.; Sanomimiya, M.; Rastrelli, L.; Pizza, C. Isolation and structure elucidation of two new flavonoid glycosides from the infusion of Maytenus aquifolium leaves. Evaluation of the Antiulcer Activity of the Infusion. J. Agric. Food Chem. 1999, 47, 403–406. [Google Scholar] [CrossRef]
- Santos-Oliveira, R.; Coulaud-Cunha, S.; Colaço, W. Review of Maytenus ilicifolia Mart. ex Reissek, Celastraceae. Contribution to the studies of pharmacological properties. Braz. J. Pharmacogn. 2009, 19, 650–659. [Google Scholar]
- Souza, L.M.; Cipriani, T.R.; Serrato, R.V.; Costa, D.E.; Iacomini, M.; Gorin, P.A.J.; Sassaki, G.L. Analysis of flavonol glycoside isomers from leaves of Maytenus ilicifolia by offline and online high performance liquid chromatography–electrospray mass spectrometry. J. Chromatog. A 2008, 1207, 101–109. [Google Scholar] [CrossRef]
- Sannomiya, M.; Vilegas, W.; Ratrelli, L.; Pizza, C. A flavonoid glycoside from Maytenus aquifolium. Phytochemistry 1998, 49, 237–239. [Google Scholar]
- Leite, J.P.V.; Rastrelli, L.; Romussi, G.; Oliveira, A.B.; Vilegas, J.H.Y.; Vilegas, W.; Pizza, C. Isolation and HPLC quantitative analysis of flavonoid glycosides from brazilian beverages (Maytenus ilicifolia and Maytenus aquifolium). J. Agric. Food Chem. 2001, 49, 3796–3801. [Google Scholar]
- Buffa-Filho, W.; Bolzani, V.S.; Furlan, M.; Pereira, A.M.S.; Pereira, S.I.V.; França, S.C. In vitro propagation of Maytenus ilicifolia (Celastraceae) as potential source for antitumoral and antioxidant quinomethide triterpenes production. A rapid quantitative method for their analysis by reverse-phase high-performance liquid chromatography. Arkivoc 2004, 6, 137–146. [Google Scholar]
- Buffa-Filho, W.; Corsino, J.; Bolzani, V.S.; Furlan, M.; Pereira, A.M.S.; França, S.C. Quantitative determination of cytotoxic friedo-nor-oleanane derivatives from five morphological types of Maytenus ilicifolia (Celastraceae) by reverse-phase high-performance liquid chromatography. Phytochemical. Anal. 2002, 13, 75–78. [Google Scholar] [CrossRef]
- Buffa-Filho, W.; Furlan, M.; Pereira, A.M.S.; França, S.C. Indução de metabólito bioativo em cultura de células de Maytenus ilicifolia. Eclet. Quím. 2000, 27, 403–416. [Google Scholar]
- Corsino, J.; Bolzani, V.S.; Pereira, A.M.S.; França, S.C.; Furlan, M. Further sesquiterpene pyridine alkaloids from Maytenus aquifolium. Phytochemistry 1998, 49, 2181–2183. [Google Scholar]
- Corsino, J.; Bolzani, V.S.; Pereira, A.M.S.; França, S.C.; Furlan, M. Bioactive sesquiterpene pyridine alkaloids from Maytenus aquifolium. Phytochemistry 1998, 48, 137–140. [Google Scholar]
- Tiberti, L. A.; Yariwake, J. H.; Ndjoko, K.; Hostettmann, K. Identification of flavonols in leaves of Maytenus ilicifolia and M. aquifolium (Celastraceae) by LC/UV/MS analysis. J. Chromatogr. B 2007, 846, 378–383. [Google Scholar] [CrossRef]
- Vellosa, J.C.R.; Khalil, N.M.; Formenton, V.A.F.; Ximenes, V.F.; Fonseca, L.M.; Furlan, M.; Brunetti, I.L.; Oliveira, O.M.M.F. Antioxidant activity of Maytenus ilicifolia root bark. Fitoterapia 2006, 77, 243–247. [Google Scholar]
- Pessuto, M.B.; Casemiro, I.; Boldieri de Souza, A.; Nicoli, F.M.; Palazzo de Mello, J.C.; Petereit, F.; Luftmann, H. Antioxidant activity of extracts and condensed tannins from leaves of Maytenus ilicifolia Mart. ex Reiss. Quimica Nova 2009, 32, 412–416. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.L. Triterpenoid quinonemethides and related compounds (Celastroloids). Prog. Chem. Org. Nat. Prod. 1996, 67, 1–123. [Google Scholar]
- Nozaki, H.; Suzuki, H.; Hirayama, T.; Kasai, R.; Wu, R.; Lee, K. Antitumour triterpenes of Maytenus diversifolia. Phytochemistry 1986, 25, 479–485. [Google Scholar]
- Chávez, H.; Estévez-Braun, A.; Ravelo, A.G.; González, A.G. New phenolic and quinone-methide triterpenes from Maytenus amazonica. J. Nat. Prod. 1999, 62, 434–436. [Google Scholar] [CrossRef]
- Rodríguez, F.M.; López, M.R.; Jiménez, I.A.; Moujir, L.; Ravelo, A.G.; Bazzocchi, I.L. New phenolic triterpenes from Maytenus blepharodes. Semisynthesis of 6-deoxoplepharodol from pristimerine. Tetrahedron 2005, 61, 2513–2519. [Google Scholar]
- Moujir, L.; Gutiérrez-Navarro, A.M.; González, A.G.; Ravelo, A.G.; Luis, J.G. The relationship between structure and antimicrobial activity in quinones from the Celastraceae. Biochem. Syst. Ecol. 1990, 18, 25–28. [Google Scholar] [CrossRef]
- González, A.G.; Alvarenga, N.L.; Ravelo, A.G.; Jamenez, I.A.; Bazzocchi, I.L.; Canela, N.J.; Moujir, L.M. Antibiotic phenol nor-triterpenes from Maytenus canariensis. Phytochemistry 1996, 43, 129–132. [Google Scholar]
- Alvarenga, N.L.; Velázquez, C.A.; Gómez, R.; Canela, N.J.; Bazzocchi, I.L.; Ferro, E.A. A new antibiotic nortriterpene quinone methide from Maytenus catingarum. J. Nat. Prod. 1999, 62, 750–751. [Google Scholar] [CrossRef]
- Chávez, H.; Valdivia, A.; Estévez-Braun, A.; Ravelo, A.G. Structure of new bioactive triterpenes related to 22-β-hydroxy-tingenone. Tetrahedron 1998, 54, 13579–13590. [Google Scholar]
- Jeller, A.H.; Silva, D.H.S.; Lião, L.M.; Bolzani, V.S.; Furlan, M. Antioxidant phenolic and quinonemethide triterpenes from Cheiloclinium cognatum. Phytochemistry 2004, 65, 1977–1982. [Google Scholar]
- Carvalho, P.R.F.; Silva, D.H.S.; Bolzani, V.S.; Furlan, M. Antioxidant quinonemethide triterpenes from Salacia campestris. Chem. Biodivers. 2005, 2, 367–372. [Google Scholar] [CrossRef]
- Cleren, C.; Calingasan, N.Y.; Chen, J.; Beal, M.F. Celastrol protects against MPTP- and 3- nitropropionic acid-induced neurotoxicity. J. Neurochem. 2005, 94, 995–1004. [Google Scholar] [CrossRef]
- Costa, P.M.; Ferreira, P.M.P.; Bolzani, V.S.; Furlan, M.; Santos, V.A.F.F.M.; Corsino, J.; Moraes, M.O.; Costa-Lotufo, L.V.; Montenegro, R.C.; Pessoa, C. Antiproliferative activity of pristimerin isolated from Maytenus ilicifolia (Celastraceae) in human HL-60 cells. Toxicol. In Vitro 2008, 22, 854–863. [Google Scholar] [CrossRef]
- Figueiredo, J.N.; Raz, B.; Séquin, U. Novel quinone methides from Salacia kraussii with in vitro antimalarial activity. J. Nat. Prod. 1998, 61, 718–723. [Google Scholar] [CrossRef]
- Corsino, J.; Carvalho, P.R.F.; Kato, M.J.; Latorre, L.R.; Oliveira, O.M.F.; Araújo, A.R.; Bolzani, V.S.; França, S.C.; Pereira, A.M.S.; Furlan, M. Biosynthesis of friedelane and quinonemethide triterpenoids is compartmentalized in Maytenus aquifolium and Salacia campestris. Phytochemistry 2000, 55, 741–748. [Google Scholar]
- Corsino, J.; Silva, D.H.S.; Zanoni, M.V.B.; Bolzani, V.S.; França, S.C.; Pereira, A.M.S.; Furlan, M. Antioxidant flavan-3-ols and flavonol glycosides from Maytenus aquifolium. Phytother.Res. 2003, 17, 913–916. [Google Scholar]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Kahkonen, M.P.; Hopia, A.I.; Heikki, J.V.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S. Antioxidant activity of plant extracts containing phenolic compounds. J. Agr. Food Chem. 1999, 47, 3954–3962. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar]
- Sun, C.; Chen, K.; Chen, Y.; Chen, Q. Contents and antioxidant capacity of limonin and nomilin in different tissues of citrus fruit of four cultivars during fruit growth and maturation. Food Chem. 2005, 93, 599–605. [Google Scholar] [CrossRef]
- Castellar, M.R.; Obón, J.M.; Fernández-López, J.A.; Cascales, J.A. Assessment of the TEAC method for determining the antioxidant capacity of synthetic red food colorants. Food Res. Int. 2005, 38, 843–845. [Google Scholar] [CrossRef]
- Cão, G.H.; Prior, R.L. Measurement of oxygen radical absorbance capacity in biological samples. Methods Enzymol. 1999, 299, 50–62. [Google Scholar]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant capacity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Capecka, E.; Mareczek, A.; Leja, M. Antioxidant activity of fresh and dry herbs of some Lamiaceae species. Food Chem. 2005, 93, 223–226. [Google Scholar] [CrossRef]
- Jin-wei, L.; Shao-dong, D.; Xiao-lin, D. Comparison of antioxidant capacities of extracts from five cultivars of Chinese jujube. Process Biochem. 2005, 40, 3607–3613. [Google Scholar] [CrossRef]
- Vellosa, J.C.R.; Santos, V.A.F.F.M.; Barbosa, V.F.; Khalil, N.M.; Furlan, M.; Brunetti, I.L.; Oliveira, O.M.M.F. Profile of Maytenus aquifolium action over free radicals and reactive oxygen species. Braz. J. Pharm. Sci. 2007, 43, 447. [Google Scholar]
- Chevion, S.; Robers, M.A.; Chevion, M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radical Bio. Med. 2000, 28, 860–870. [Google Scholar] [CrossRef]
- Chevion, S.; Chevion, M.; Chock, P.B.; Beecher, G.R. The antioxidant capacity of edible plants: extraction protocol and direct evaluation by cyclic voltammetry. J. Med. Food 1999, 2, 1–11. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free radicals in biology and medicine, 3rd ed.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Santos, D.P.; Bergamini, M.F.; Santos, V.A.F.F.M.; Furlan, M.; Zanoni, M.V.B. Preconcentration of Rutin at a Poly Glutamic Acid Modified Electrode and its Determination by Square Wave Voltammetry. Anal. Lett. 2007, 40, 3430–3442. [Google Scholar] [CrossRef]
- Kotani, A.; Miyaguchi, Y.; Tomita, E.; Takamura, K.; Kusu, F. Determination of organic acids by high-performance liquid chromatography with electrochemical detection during wine brewing. J. Agric. Food Chem. 2004, 52, 1440–1444. [Google Scholar] [CrossRef]
- Skrinjar, M.; Kolar, M.H.; Jelsek, N.; Hras, A.R.; Bezjak, M.; Knez, Z. Application of HPLC with electrochemical detection for the determination of low levels of antioxidants. J. Food Compos. Anal. 2007, 20, 539–545. [Google Scholar] [CrossRef]
- Compton, R.G.; Banks, C.E. Understanding Voltammetry; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2007. [Google Scholar]
- Castro-Gamboa, I.; Cardoso, C.L.; Silva, D.H.S.; Cavalheiro, A.J.; Furlan, M.; Bolzani, V.S. HPLC-ElCD: An useful tool for the pursuit of novel analytical strategies for the detection of antioxidant secondary metabolites. J. Braz. Chem. Soc. 2003, 14, 771–776. [Google Scholar] [CrossRef]
- Rückert, U.; Likussar, W.; Michelitsch, A. Simultaneous determination of total hypericin and hyperforin in St. John’s Wort extracts by HPLC with electrochemical detection. Phytochem. Analysis 2007, 18, 204–208. [Google Scholar]
- Esparza, I.; Salinas, Í.; Santamaría, C.; García-Mina, J.M.; Fernández, J.M. Electrochemical and theoretical complexation studies for Zn and Cu with individual polyphenols. Anal. Chim. Acta 2005, 543, 267–274. [Google Scholar] [CrossRef]
- Ghica, M.E.; Brett, A.M.O. Electrochemical oxidation of rutin. Electroanal. 2005, 17, 313–318. [Google Scholar]
- Pietta, P. Flavonoids in medicinal plant; Marcel Dekker: New York, NY, USA, 1998; p. 61. [Google Scholar]
- Baizer, M.M.; Lund, H. Organic Electrochemistry; Harper and Row: New York, NY, USA, 1972; p. 300. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1980; p. 833. [Google Scholar]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzimol. 1990, 186, 343–355. [Google Scholar]
- Moure, A.; Cruz, J.M.; Franco, D.; Dominguez, J.M.; Sineiro, J.; Dominguez, H.; Nuñez, M.J.; Parajó, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Ferancová, A.; Heilerová, L.; Korgová, E.; Silhár, S.; Stepánek, I.; Labuda, J. Anti/pro-oxidative properties of selected standard chemicals and tea extracts investigated by DNA-based electrochemical biosensor. Eur. Food Res. Technol. 2004, 219, 416–420. [Google Scholar] [CrossRef]
- van Acker, S.A.B.E.; van den Berg, D.J.; Tromp, M.N.J.L.; Griffioen, D.H.; van Bennekom, W.P.; van der Vijgh, W.J.F.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radical Biol. Med. 1996, 20, 331–342. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aparecida de Freitas Formenton Macedo dos Santos, V.; Pereira dos Santos, D.; Castro-Gamboa, I.; Zanoni, M.V.B.; Furlan, M. Evaluation of Antioxidant Capacity and Synergistic Associations of Quinonemethide Triterpenes and Phenolic Substances from Maytenus ilicifolia (Celastraceae). Molecules 2010, 15, 6956-6973. https://doi.org/10.3390/molecules15106956
Aparecida de Freitas Formenton Macedo dos Santos V, Pereira dos Santos D, Castro-Gamboa I, Zanoni MVB, Furlan M. Evaluation of Antioxidant Capacity and Synergistic Associations of Quinonemethide Triterpenes and Phenolic Substances from Maytenus ilicifolia (Celastraceae). Molecules. 2010; 15(10):6956-6973. https://doi.org/10.3390/molecules15106956
Chicago/Turabian StyleAparecida de Freitas Formenton Macedo dos Santos, Vânia, Daniela Pereira dos Santos, Ian Castro-Gamboa, Maria Valnice Boldrin Zanoni, and Maysa Furlan. 2010. "Evaluation of Antioxidant Capacity and Synergistic Associations of Quinonemethide Triterpenes and Phenolic Substances from Maytenus ilicifolia (Celastraceae)" Molecules 15, no. 10: 6956-6973. https://doi.org/10.3390/molecules15106956



