Isolation, Synthesis and Structures of Ginsenoside Derivatives and Their Anti-Tumor Bioactivity
Abstract
:Introduction
Results and Discussion
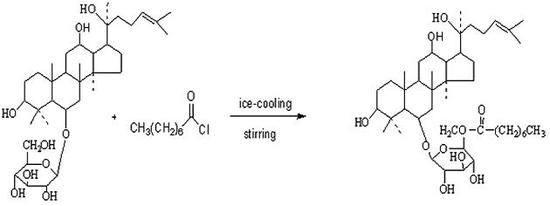
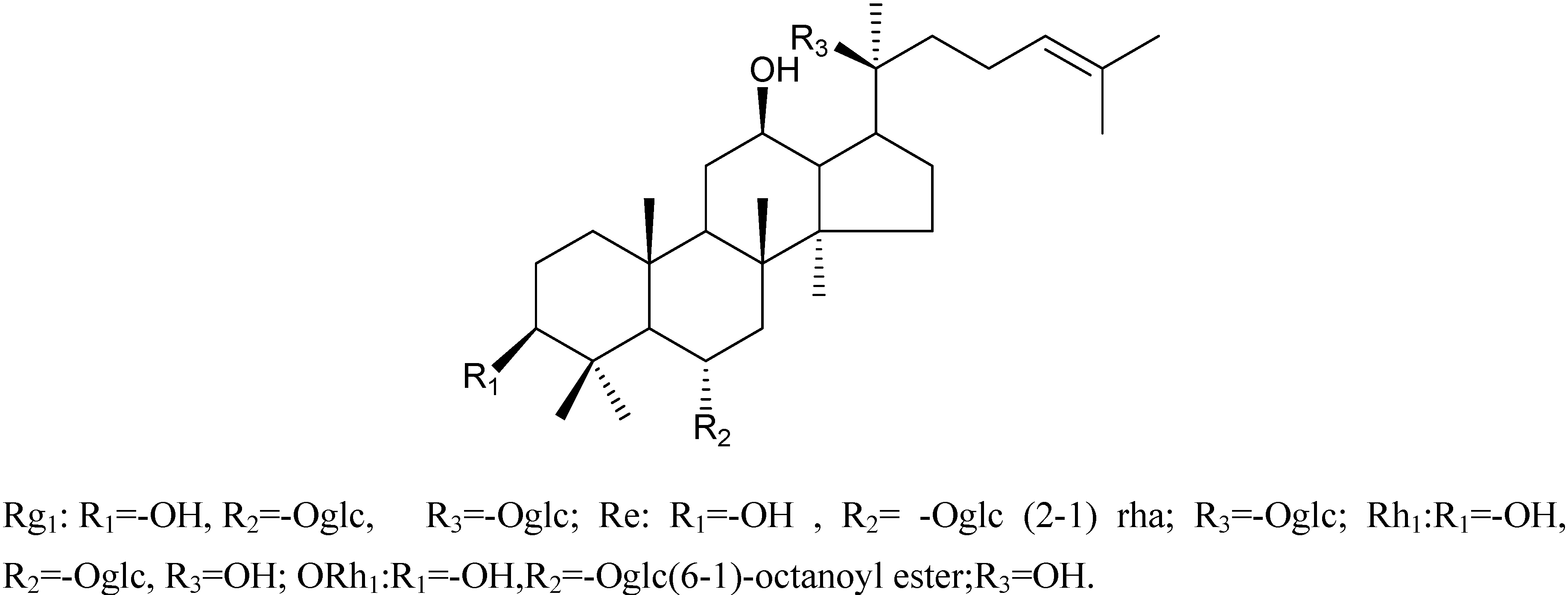
Characterizations of compounds 1-4
| No.(C) | Rh1 | Compound 3 | Compound 4 |
|---|---|---|---|
| 1 | 39.4 | 39.2 | 39.8 |
| 2 | 27.9 | 27.2 | 27.2 |
| 3 | 78.6 | 78.7 | 78.7 |
| 4 | 40.3 | 40.2 | 39.4 |
| 5 | 61.4 | 61.4 | 60.0 |
| 6 | 78.0 | 77.7 | 80.2 |
| 7 | 45.2 | 45.4 | 44.6 |
| 8 | 41.1 | 41.3 | 41.1 |
| 9 | 50.2 | 50.4 | 50.0 |
| 10 | 39.6 | 39.9 | 39.0 |
| 11 | 32.0 | 32.2 | 31.6 |
| 12 | 71.0 | 71.2 | 70.0 |
| 13 | 48.2 | 48.4 | 49.7 |
| 14 | 51.6 | 51.8 | 50.8 |
| 15 | 31.1 | 31.4 | 29.6 |
| 16 | 27.2 | 27.2 | 26.7 |
| 17 | 54.7 | 54.9 | 50.3 |
| 18 | 17.4 | 17.2 | 17.2 |
| 19 | 17.6 | 17.8 | 17.8 |
| 20 | 78.0 | 78.3 | 73.9 |
| 21 | 26.8 | 27.0 | 27.0 |
| 22 | 25.8 | 26.0 | 34.1 |
| 23 | 28.0 | 28.1 | 22.6 |
| 24 | 126.0 | 126.5 | 125.7 |
| 25 | 130.6 | 130.9 | 130.9 |
| 26 | 25.8 | 26.0 | 25.6 |
| 27 | 17.6 | 17.5 | 17.7 |
| 28 | 31.7 | 31.8 | 31.6 |
| 29 | 16.4 | 16.5 | 15.8 |
| 30 | 16.8 | 17.0 | 16.6 |

| No.(C) | Rh1 | Compound 3 | Compound 4 |
|---|---|---|---|
| 1′ | 105.7 | 106.2 | 104.4 |
| 2′ | 75.4 | 75.6 | 74.0 |
| 3 ′ | 80.0 | 80.2 | 80.1 |
| 4′ | 71.8 | 72.0 | 72.0 |
| 5′ | 79.5 | 79.8 | 79.2 |
| 6′ | 63.5 | 63.3 | 64.8 |
| 1′′ | 174.3 | ||
| 2′′ | 34.1 | ||
| 3′′ | 24.9 | ||
| 4′′ | 30.3 | ||
| 5′′ | 29.1 | ||
| 6′′ | 30.9 | ||
| 7′′ | 22.6 | ||
| 8′′ | 14.1 |
Bioactivity evaluation
| Concentration (μM) | Inhibitory rate (%) | IC50(μM) | ||
|---|---|---|---|---|
| Ginsenoside Rh1 | ORh1 | Ginsenoside Rh1 | ORh1 | |
| 0 μM | 0 | 0 | 48.63 | 42.44 |
| 10 μM | 8.97 | 11.44 | ||
| 20 μM | 9.28 | 11.09 | ||
| 40 μM | 54.21 | 62.34 | ||
| 80 μM | 76.54 | 87.16 | ||

Conclusions
Experimental
General
Chemicals and reagents
Extraction and isolation
Enzymatic reaction
Synthesis of Ginsenoside ORh1 (4)

In vitro anti-tumor assays
- Sample Availability: Samples are available from the authors.
References
- Balsiger, B.M.; Murr, M.M.; Poggio, J.L.; Sarr, M.G. Bariatric surgery: Surgery for weight control in patients with morbid obesity. Med. Clin. N. Amer. 2000, 84, 477–489. [Google Scholar] [CrossRef]
- Niti, S.; Vinay, K.S.; Seo, S.Y. Screening of some medicinal plants foe anti-lipase activity. J. Ethnopharmacol. 2005, 97, 453–456. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Li, T.S.C. Asian and American ginseng: A review. Hort Technol. 1995, 5, 27–34. [Google Scholar]
- Karu, N.; Reifen, R.; Kerem, Z. Weight gain reduction in mice fed panax ginseng saponin, a pancreatic lipase inhibitor. J. Agric. Food Chem. 2007, 55, 2824–2828. [Google Scholar] [CrossRef]
- Park, E.K.; Choo, M.K.; Han, M.J.; Kim, D.H. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int. Arch. Allergy Immunol. 2004, 133, 113–120. [Google Scholar] [CrossRef]
- Ki, S.K.; Noriko, Y.; Kim, H.Y.; Takuya, O.; Yasuo, S.; Takako, Y. Increase in the free radical scavenging activities of American ginseng by heat processing and its safety evaluation. J. Ethnopharmacol. 2007, 113, 225–232. [Google Scholar] [CrossRef]
- Hu, C.; Kitts, D. Free radical scavenging capacity as related to antioxidant activity and ginsenoside composition of Asian and North American ginseng extracts. J. Amer. Oil Chem. Soc. 2001, 78, 249–255. [Google Scholar] [CrossRef]
- Liu, W.C.; Zheng, Y.N.; Masato, S.; Wang, H.M.; Yoshiyuki, K.; Ling, M.S.; Feng, Y.X. Saponins from stems and leaves of Panax quinquefolium prevented high fat diet-induced obesity in mice. Phytomedicine 2008, 15, 1140–1145. [Google Scholar] [CrossRef]
- Lee, Y.N.; Lee, H.Y.; Chung, H.Y.; Kim, S.I.; Lee, S.K.; Park, B.C.; Kim, K.W. In vitro induction of differentiation by ginsenosides in F9 etratocarcinoma cells. Eur. J. Cancer 1996, 32A, 1420–1428. [Google Scholar]
- Kang, J.; Lee, Y.; No, K.; Jung, E.; Sung, J.; Kim, Y.; Nam, S. Ginseng intestinal metabolite-I (GIM-I) reduces doxorubicin toxicity in the mouse testis [J]. Reprod. Toxicol. 2002, 16, 291–298. [Google Scholar] [CrossRef]
- Tawab, M.; Bahr, U.; Karas, M.; Wurglics, M. Degeneration of ginsenosides in humans after oral administration. Drug Metab. Dispos. 2003, 31, 1065–1071. [Google Scholar] [CrossRef]
- Hasegawa, H.; Lee, K.; Nagaoka, T.; Tezuka, Y.; Uchiyama, M.; Kadota, S.; Saiki, I. Pharmacokinetics of ginsenoside deglycosylated by intestinal bacteria and its transformation to biologically active fatty acid esters. Biol. Pharm. Bull. 2000, 23, 298–304. [Google Scholar] [CrossRef]
- Lei, J.; Li, X.; Gong, X.J.; Zheng, Y.N. Isolation, synthesis and structures of cytotoxic ginsenoside derivatives. Molecules 2007, 12, 2140–2150. [Google Scholar] [CrossRef]
- Keum, Y.S.; Han, S.S.; Chun, K.S.; Park, K.K.; Park, J.H.; Lee, S.K.; Surh, Y.J. Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced cyclooxygenase-2 expression, NF-kappaB activation and tumor promotion. Mutat. Res. 2003, 523–524, 75–85. [Google Scholar] [CrossRef]
- Kim, S.W.; Kwon, H.Y.; Chi, D.W.; Shim, J.H.; Park, J.D.; Lee, Y.H.; Pyo, S.; Rhee, D.K. Reversal of P-glycoprotein-mediated multidrug resistance by ginsenoside Rg (3). Biochem. Pharmacol. 2003, 65, 75–82. [Google Scholar]
- Popovich, D.; Kitts, D. Structure-function relationship exists for ginsenosides in reducing cell proliferation and inducingapoptosis in the human leukemia (THP-1) cell line. Arch. Biochem. Biophys. 2002, 406, 1–8. [Google Scholar] [CrossRef]
- Popovich, D.G.; Kitts, D.D. Ginsenosides can inhibit proliferation and induce apoptosis in cultured leukemia and intestinal cells but effects vary according to the structure of the compounds. FASEB J. 2003, 17, A762. [Google Scholar]
- Park, J.A.; Lee, K.Y.; Oh, Y.J.; Kim, K.W.; Lee, S.K. Activation of caspase-3 protease via a Bcl-2-insensitive pathway during the process of ginsenoside Rh2-induced apoptosis. Cancer Lett. 1997, 121, 73–81. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Han, M.; Hou, J.-G.; Dong, C.-M.; Li, W.; Yu, H.-L.; Zheng, Y.-N.; Chen, L. Isolation, Synthesis and Structures of Ginsenoside Derivatives and Their Anti-Tumor Bioactivity. Molecules 2010, 15, 399-406. https://doi.org/10.3390/molecules15010399
Han M, Hou J-G, Dong C-M, Li W, Yu H-L, Zheng Y-N, Chen L. Isolation, Synthesis and Structures of Ginsenoside Derivatives and Their Anti-Tumor Bioactivity. Molecules. 2010; 15(1):399-406. https://doi.org/10.3390/molecules15010399
Chicago/Turabian StyleHan, Mei, Jin-Gang Hou, Cheng-Ming Dong, Wei Li, Hao-Lun Yu, Yi-Nan Zheng, and Li Chen. 2010. "Isolation, Synthesis and Structures of Ginsenoside Derivatives and Their Anti-Tumor Bioactivity" Molecules 15, no. 1: 399-406. https://doi.org/10.3390/molecules15010399