In Vitro Antifungal Activity of the Diterpenoid 7α-Hydroxy-8(17)-labden-15-oic Acid and Its Derivatives against Botrytis cinerea
Abstract
:1. Introduction
2. Results and Discussion
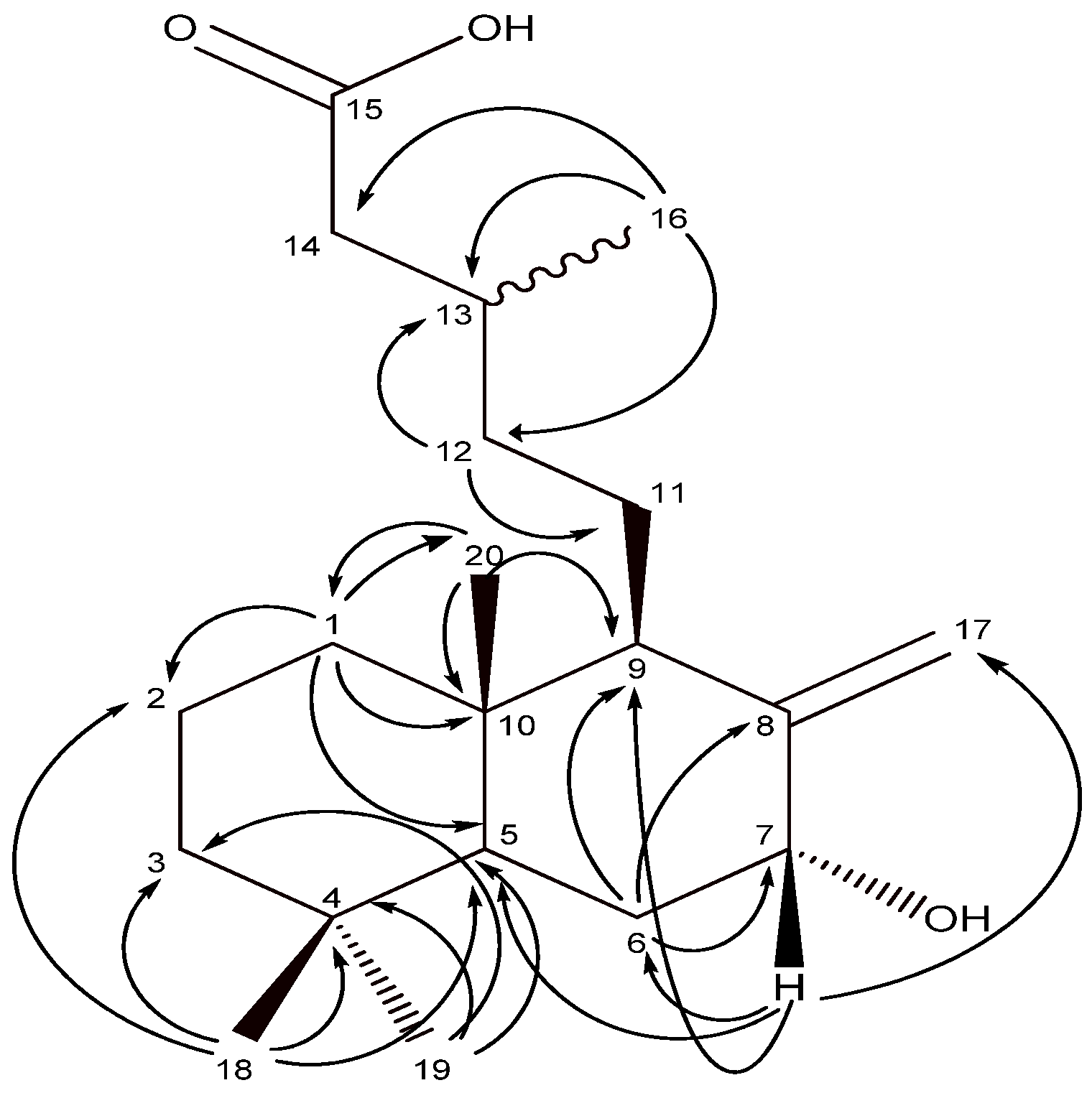
2.1. Isolation of natural diterpenoids and characterization of salvic acid
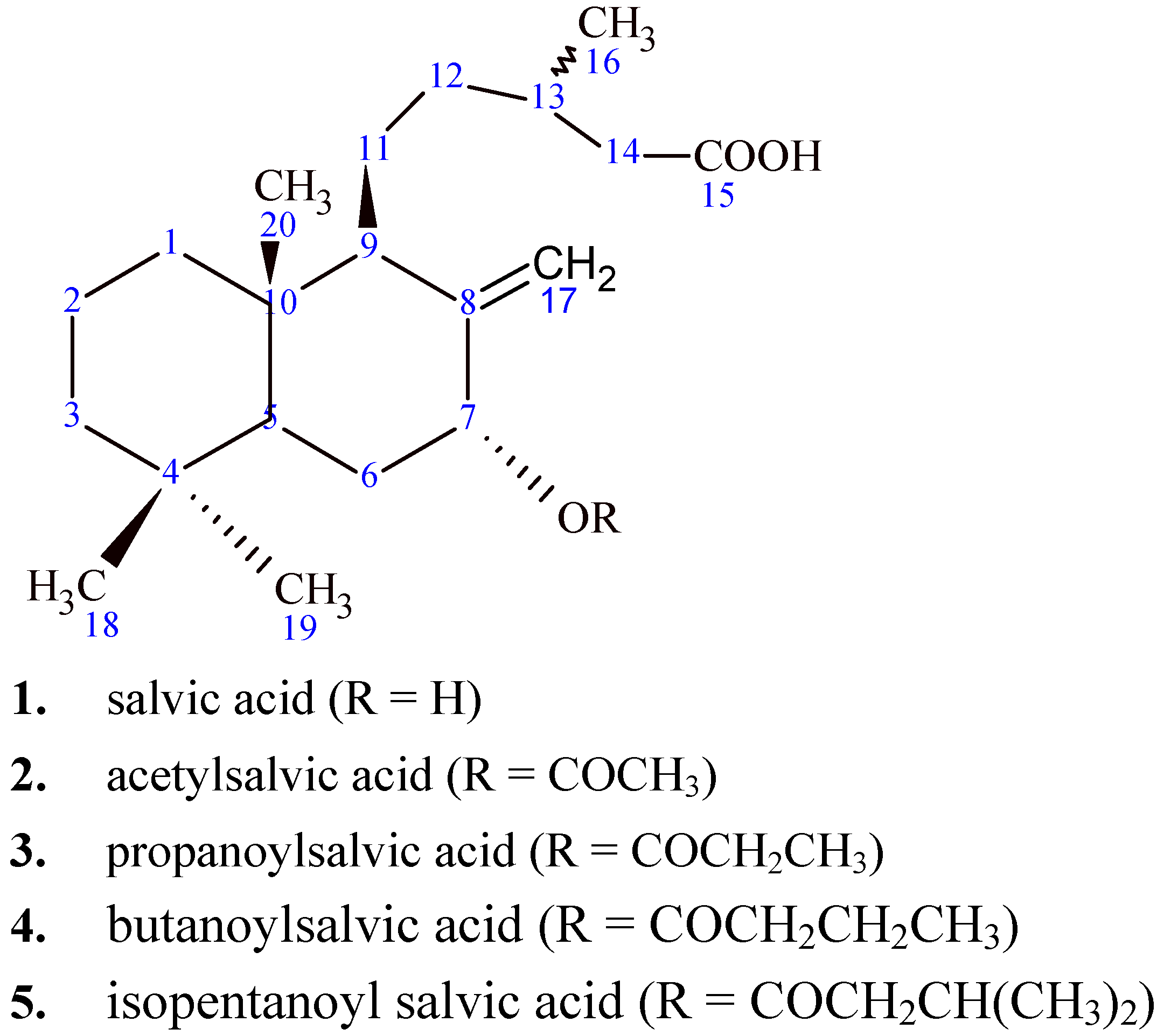
| Carbon | 13C (δ) | 1H (δ) | Carbon | 13C (δ) | 1H (δ) |
|---|---|---|---|---|---|
| 1 | 39.00 | 1.74 (d-t) 1.11 (m) | 11 | 20.80 | 1.28 (m) |
| 2 | 19.56 | 1.51 (m) | 12 | 35.62 | 1.04 (m) 1.51 (m) |
| 3 | 42.30 | 1.31 (m) 1.21 (m) | 13 | 31.11 | 1.94 (o(a)) |
| 4 | 33.30 | - | 14 | 41.34 | 2.36 (d-d) 2.15 (d-d) |
| 5 | 47.85 | 1.59 (m) | 15 | 178.46 | - |
| 6 | 31.08 | 1.86 (d-d) | 16 | 20.09 | 0.98 (d) |
| 7 | 74.37 | 4.37 (d(a)) | 17 | 109.99 | 5.03 (t(a)) 4.62 (t(a)) |
| 8 | 149.85 | - | 18 | 33.50 | 0.80 (s) |
| 9 | 51.54 | 2.07 (d-d) | 19 | 21.72 | 0.88 (s) |
| 10 | 40.09 | - | 20 | 13.59 | 0.65 (s) |
2.2. Antifungal activity characterization
| Compounds | ED50a ± SD (μg/mL) |
|---|---|
| 1 | 53.1 ± 4.6 |
| 2 | 60.2 ± 8.4 |
| 3 | 59.5 ± 5.5 |
| 4 | 100.2 ± 4.9 |
| 5 | > 250 |
| Iprodione | 35.0 ± 1.5 |
| Compounds | Volume (Å3) | Log P |
|---|---|---|
| 1 | 974 | 4.56 |
| 2 | 1069 | 4.69 |
| 3 | 1136 | 5.32 |
| 4 | 1190 | 5.72 |
| 5 | 1219 | 6.05 |
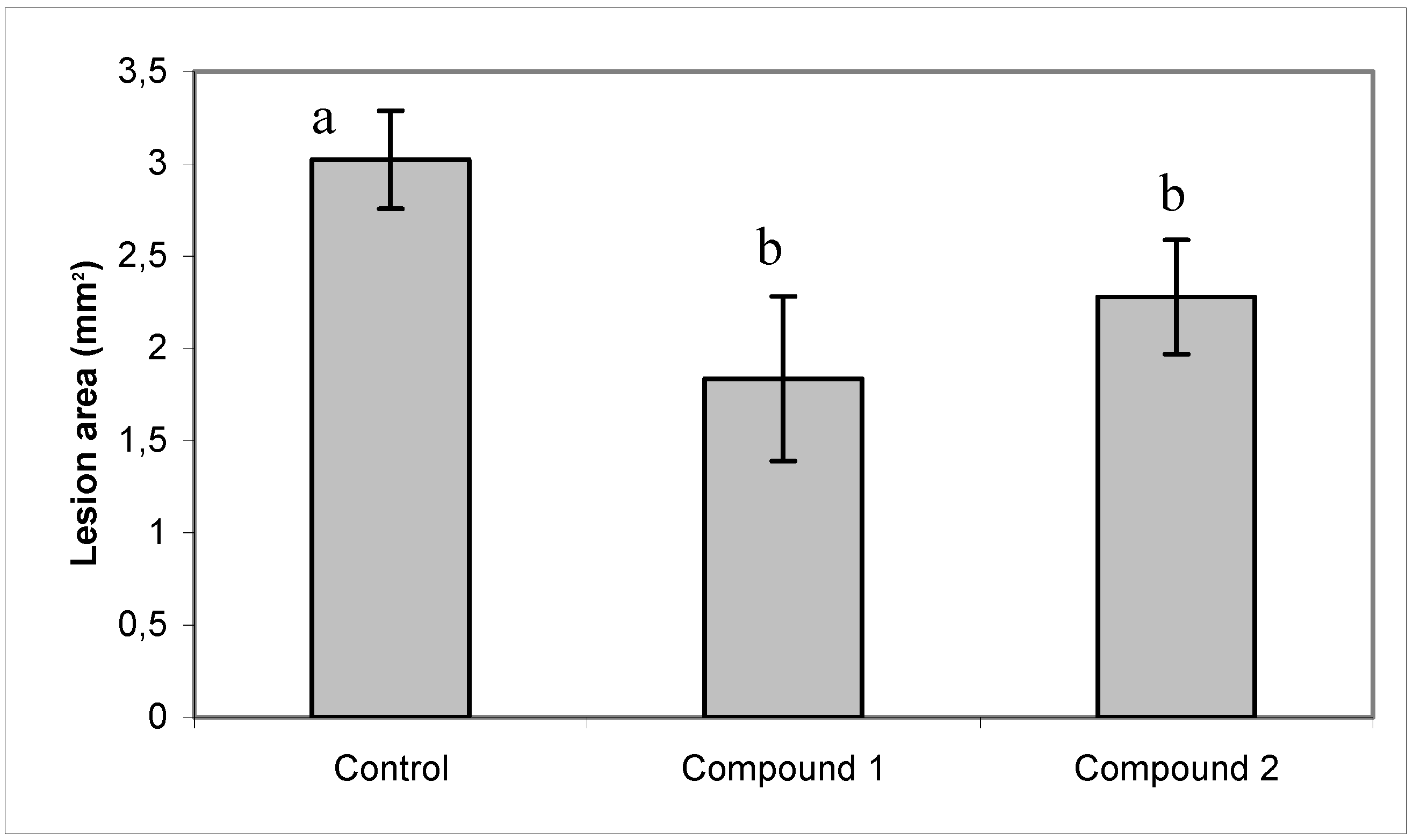
2.3. Effect of compounds 1 and 2 on conidia germination and on ability of B. cinerea to colonize tomato leaves

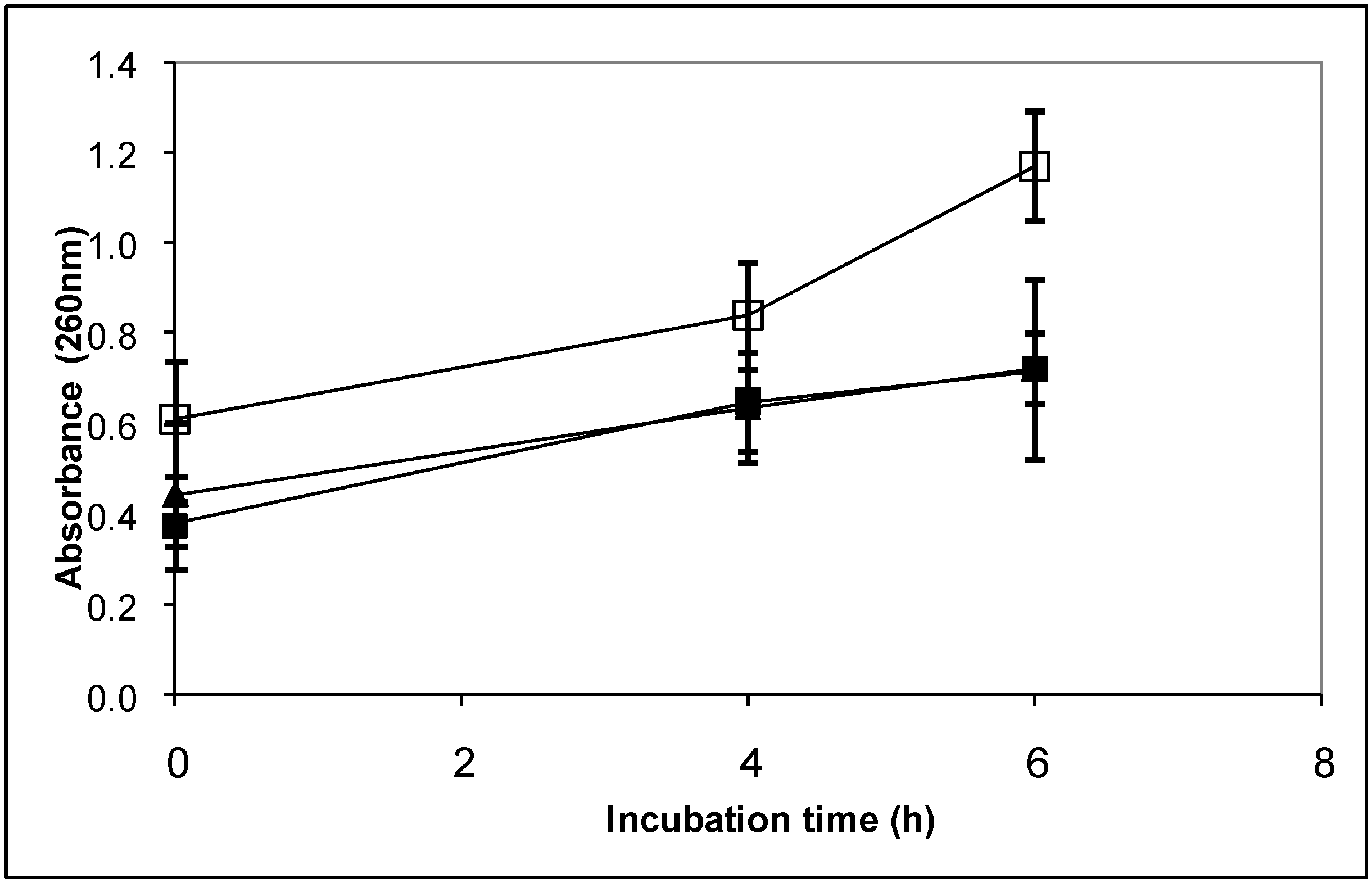
2.4. Mode of action compounds 1 and 2 on B. cinerea
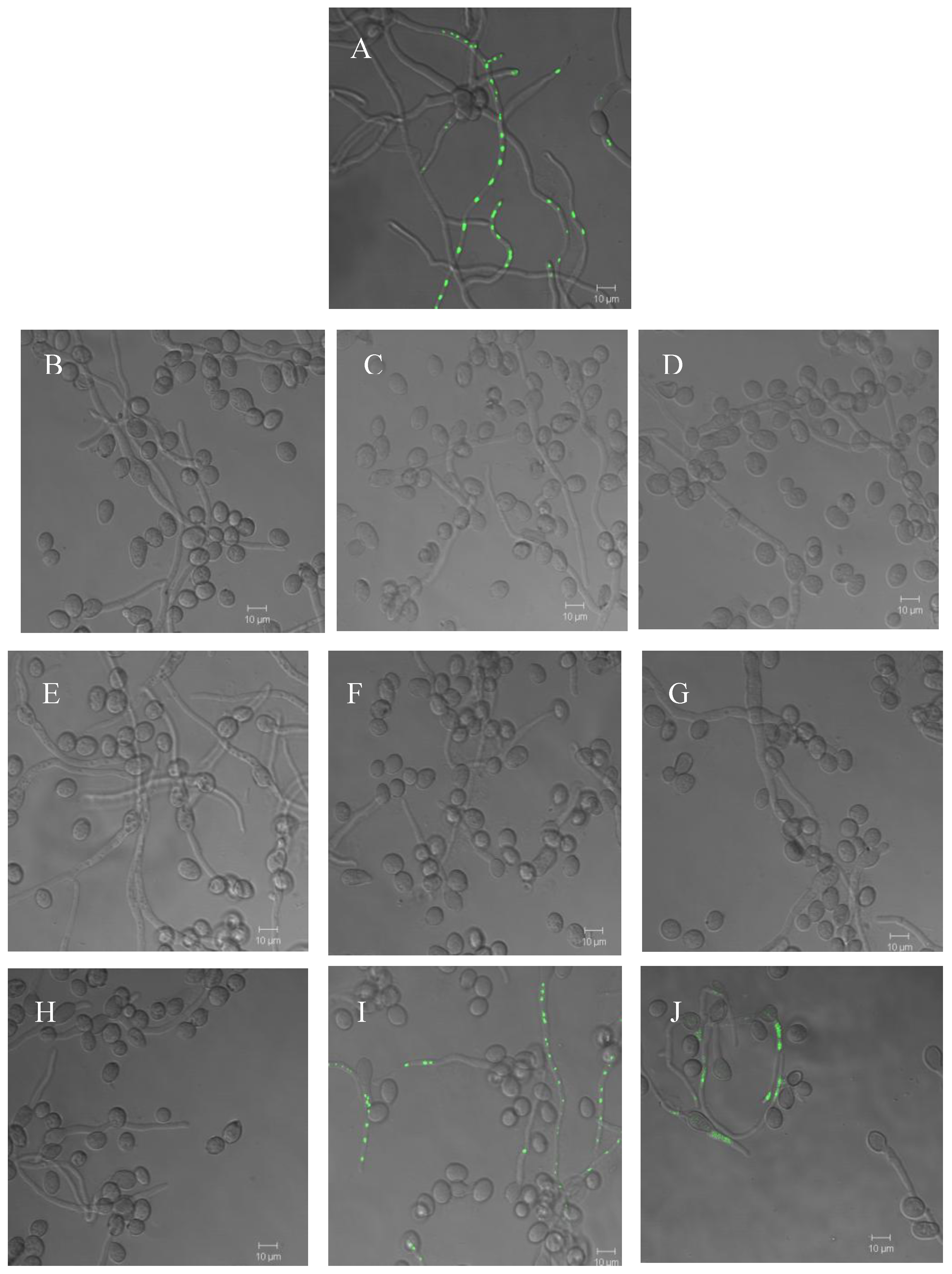
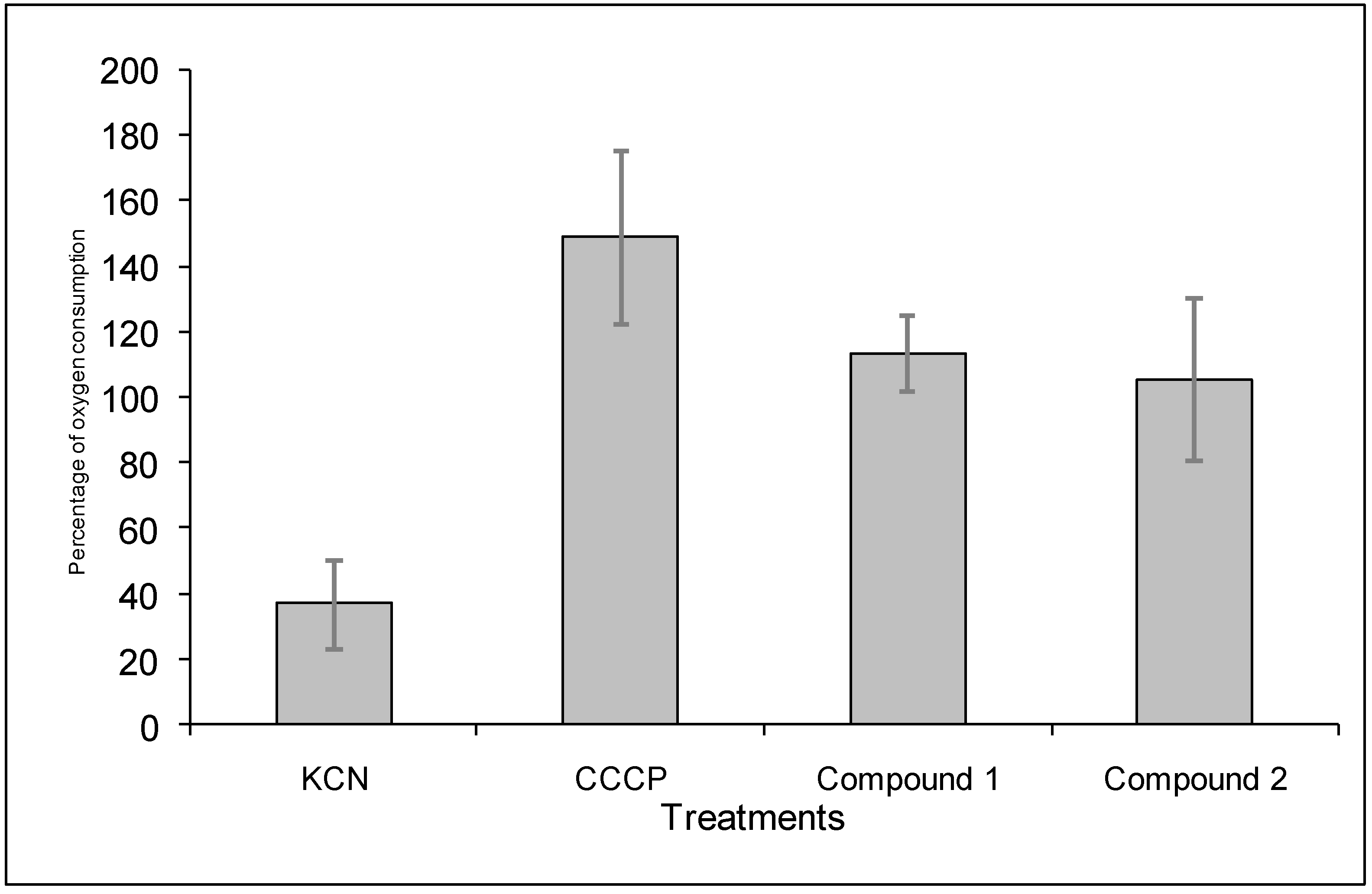
3. Conclusions
4. Experimental
4.1. General
4.2. Chemicals
4.3. Isolation of the natural diterpenoids salvic acid (1) and acetylsalvic acid (2) from E. salvia
4.4. General procedure for the preparation of 7α-acyloxy-8(17)-labden-15-oic acids 3-5
4.5. Fungal Isolate and Culture Condition
4.6. Effect of the compounds on the mycelial growth of B. cinerea in solid media
4.7. Effect on the ability of B. cinerea to colonize tomato leaves.
4.8. Effect on germination of B. cinerea conidia
4.9. Determination of mode of action of compounds 1 and 2
4.9.1. Determination of the effect of the diterpenoids on the membrane integrity of B. cinerea
4.9.2. Determination of the effect of the diterpenoids on the oxygen consumption of B. cinerea conidia
4.10. Determination of log P and Volume
4.11. Statistical analyses
Acknowledgements
References and Notes
- Elad, Y.; Evensen, K. Physiological aspects of resistance to Botrytis cinerea. Phytopathology 1995, 85, 637–643. [Google Scholar]
- Latorre, B.A.; Flores, V.; Sara, A.M.; Roco, A. Dicarboximide-resistant strains of Botrytis cinerea from table grapes in Chile: survey and characterization. Plant. Dis. 1994, 78, 990–994. [Google Scholar] [CrossRef]
- Grayer, R.J.; Kokubun, T. Plant-fungal interactions: the search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry 2001, 56, 253–263. [Google Scholar] [CrossRef]
- Salah, M.A.; Bedir, E.; Toyang, N.J.; Khan, I.A.; Harries, M.D.; Wedge, D.E. Antifungal clerodane diterpenes from Macaranga monandra (L) Muell. Et Arg (Euphorbiaceae). J. Agric. Food Chem. 2003, 51, 7607–7610. [Google Scholar]
- Scher, J.M.; Speakman, J.B.; Zapp, J.; Becker, H. Bioactivity guided isolation of antifungal compounds from the liverwort Bazzania trilobata (L.) S.F. Gray. Phytochemistry 2004, 65, 2583–2588. [Google Scholar] [CrossRef]
- Cotoras, M.; Mendoza, L; Folch, C. Characterizacion of the antifungal activity on Botrytis cinerea of the natural diterpenoids kaurenoic acid and 3β-hidroxy-kaurenoic Acid. J. Agric. Food Chem. 2004, 52, 2821–2826. [Google Scholar] [CrossRef]
- Bouchra, C.; Achouri, M.; Hassani, L.M.I.; Hmamouchi, M. Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr. J. Ethnopharmacol. 2003, 89, 165–169. [Google Scholar] [CrossRef]
- Haraguchi, H.; Kuwata, Y.; Inada, K.; Shingu, K.; Miyahara, K.; Nagao, M.; Yagi, A. Antifungal activity from Alpinia galanga and the competition for incorporation of unsaturated fatty acids in cell growth. Planta Med. 1996, 62, 308–313. [Google Scholar] [CrossRef]
- Haraguchi, H.; Oike, S.; Muroi, H.; Kubo, I. Mode of antibacterial action of totarol, a diterpene from Podocarpus nagi. Planta Med. 1996, 62, 122–125. [Google Scholar] [CrossRef]
- Tapia, L.; Torres, J.; Mendoza, L.; Urzúa, A.; Ferreira, J.; Pavani, M.; Wilkens, M. Effect of 13-epi-Sclareol on the Bacterial Respiratory Chain. Planta Med. 2004, 70, 1058–1063. [Google Scholar] [CrossRef]
- Urzúa, A.; Caroli, M.; Vasquez, L.; Mendoza, L.; Wilkens, M.; Tojo, E. Antimicrobial study of the resinous exudate and of diterpenoids isolated from Eupatorium salvia (Asteraceae). J. Ethnopharmacol. 1998, 2, 251–254. [Google Scholar]
- Hoeneisen, M.; Sammes, P.; Silva, M.; Watson, W. A new diterpenic acid and other constituents from Eupatorium salvia. Rev. Latinoamer Quim. 1979, 10, 37–40. [Google Scholar]
- Calabuig, M.T.; Cortés, M.; Francisco, C.G.; Hernández, R.; Suárez, E. Labdane diterpenes from Cistus symphytifolius. Phytochemistry 1981, 20, 2255–2258. [Google Scholar] [CrossRef]
- Arnoldi, A.; Merlini, L. Lipophilicity-antifungal activity relationships for some isoflavonoid phytoalexins. J .Agric. Food Chem. 1990, 38, 834–838. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, X.; Yang, H. Development of novel pesticides based on phytoalexins: Part 2. Quantitative structure-activity relationships of 2-heteroaryl-4-chromanone derivatives. Pest Manag. Sci. 2002, 58, 1063–1067. [Google Scholar] [CrossRef]
- Zou, X.J.; Jin, G.Y.; Zhang, Z.X. Synthesis, fungicidal activity, and QSAR of pyridazinonethiadiazole. J. Agric. Food Chem. 2002, 50, 1451–1454. [Google Scholar] [CrossRef]
- Voda, K.; Boh, B.; Vrtacnik, M. A quantitative structure–antifungal activity relationship study of oxygenated aromatic essential oil compounds using data structuring and PLS regression analysis. J. Mol. Model. 2004, 10, 76–84. [Google Scholar] [CrossRef]
- Niewiadomy, A.; Matysiak, J.; Fekner, Z.; Czeczco, R. Synthesis, antifungal activity and SAR of N-substituted and N, N-disubstituted 2,4-dihydroxythiobenzamide. J. Pestic. Sci. 2006, 31, 14–22. [Google Scholar] [CrossRef]
- Lunde, C.S.; Kubo, I. Effect of Polygodial on the Mitochondrial ATPase of Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2000, 44, 1943–1953. [Google Scholar] [CrossRef]
- Tamura, H.; Mizutani, A.; Yukioka, H.; Miki, N.; Ohba, H.; Masuko, M. Effect of the methoxyiminoacetamide fungicide, SSF129, on respiratory activity in Botrytis cinerea. Pestic. Sci. 1999, 55, 681–686. [Google Scholar] [CrossRef]
- Muñoz, G.; Hinrichsen, P.; Brygoo, Y.; Giraud, T. Genetic characterisation of Botrytis cinerea populations in Chile. Mycol. Res. 2002, 106, 594–601. [Google Scholar] [CrossRef]
- Mendoza, L.; Araya-Maturana, R.; Cardona, W.; Delgado-Castro, T.; García, C.; Lagos, C.; Cotoras, M. In vitro sensitivity of Botrytis cinerea to anthraquinone and anthrahydroquinone derivates. J. Agric. Food Chem. 2005, 53, 10080–10084. [Google Scholar]
- Thevissen, K.; Terras, F.R.G.; Broekaert, W.F. Permeabilization of fungal membranes by plant defensins inhibit fungal growth. Appl. Environ. Microbiol. 1999, 65, 5451–5458. [Google Scholar]
- Samples Availability: Samples of the compounds 1 and 2 are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mendoza, L.; Espinoza, P.; Urzua, A.; Vivanco, M.; Cotoras, M. In Vitro Antifungal Activity of the Diterpenoid 7α-Hydroxy-8(17)-labden-15-oic Acid and Its Derivatives against Botrytis cinerea. Molecules 2009, 14, 1966-1979. https://doi.org/10.3390/molecules14061966
Mendoza L, Espinoza P, Urzua A, Vivanco M, Cotoras M. In Vitro Antifungal Activity of the Diterpenoid 7α-Hydroxy-8(17)-labden-15-oic Acid and Its Derivatives against Botrytis cinerea. Molecules. 2009; 14(6):1966-1979. https://doi.org/10.3390/molecules14061966
Chicago/Turabian StyleMendoza, Leonora, Pamela Espinoza, Alejandro Urzua, Marcela Vivanco, and Milena Cotoras. 2009. "In Vitro Antifungal Activity of the Diterpenoid 7α-Hydroxy-8(17)-labden-15-oic Acid and Its Derivatives against Botrytis cinerea" Molecules 14, no. 6: 1966-1979. https://doi.org/10.3390/molecules14061966




