Alkylation of 2,4-(1H,3H)-Quinazolinediones with Dialkyl Carbonates Under Microwave Irradiations
Abstract
:Introduction

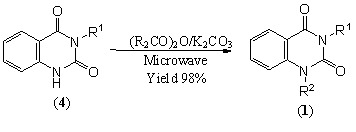
Results and Discussion


| Entries | R1 | R2 | % Yield |
|---|---|---|---|
| 1aa | PhCH2 | CH3 | 94 |
| 1ab | PhCH2CH2 | CH3 | 98 |
| 1ac | 2-CH3OPhCH2CH2 | CH3 | 95 |
| 1ad | 3-CH3OPhCH2CH2 | CH3 | 96 |
| 1ae | 4-CH3OPhCH2CH2 | CH3 | 98 |
| 1af | PhN(CH2)4NCH2CH2CH2 | CH3 | 93 |
| 1ba | PhCH2 | CH3CH2 | 96 |
| 1bb | PhCH2CH2 | CH3CH2 | 96 |
| 1bc | 2-CH3OPhCH2CH2 | CH3CH2 | 95 |
| 1bd | 3-CH3OPhCH2CH2 | CH3CH2 | 94 |
| 1be | 4-CH3OPhCH2CH2 | CH3CH2 | 97 |
| 1bf | PhN(CH2)4NCH2CH2CH2 | CH3CH2 | 92 |
Conclusions
Experimental
General
General Method for Methylation of Quinazoline-2,4-diones 4
The following compounds were prepared in similar fashion:
General Method for Ethylation of 2,4 Quinazoline-2,4-diones 4
The following compounds were prepared in similar fashion:
Acknowledgements
References
- Piers, E.; Grierson, R.J. Alkylation of 1,5-dimethoxy-1,4-cyclohexadiene. A convenient synthesis of 2-alkyl-and-2-alkenyl-1,3-cyclohexanediones. J. Org. Chem. 1977, 42, 3755–3756. [Google Scholar] [CrossRef]
- Merz, A. Phase-transfer-catalyzed Alkylation of Alcoholes by Dimethyl Sulfate in an Aqueous System. Angew. Chem. Int. 2003, 12, 846–847. [Google Scholar] [CrossRef]
- Haniti, M.; Hamid, S.A.; Williams, J.M.J. Ruthenium catalyzed N-alkylation of amines with alcohols. Chem. Commun. 2007, 725–727. [Google Scholar]
- MacPhee, J.A.; Dubois, J.E. Steric effects in synthesis-steric limits to the alkylation of nitriles and carboxylic acids. Tetrahedron 1980, 36, 775–777. [Google Scholar] [CrossRef]
- Goto, S.; Tsuboi, H.; Kanoda, M.; Mukai, K.; Kagara, K. The Process Development of a Novel Aldose Reductase Inhibitor, FK366. Part 1. Improvement of Discovery Process and New Synthesis of 1-Substituted Quinazolinediones. Org. Process Res. Dev. 2003, 7, 700–706. [Google Scholar] [CrossRef]
- Larksarp, C.; Alper, H. Palladium-Catalyzed Cyclocarbonylation of O-Iodoanilines with Heterocumulenes: Regioselective Preparation of 4(3H)-Quinazoline Derivatives. J. Org. Chem. 2000, 65, 2773–2777. [Google Scholar] [CrossRef]
- Hermecz, I.; Kökosi, J. Podanyi, B.; Szasz, G. Synthesis of Indolyl-4(3H)-Quinazolinones. Heterocycles 1994, 37, 903–914. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Rees, C.W. Comprehensive Heterocyclic Chemistry: The Structure, Reactions, Synthesis and Uses of Heterocyclic Compounds, Part 2B; Pergamon Press: New York, USA, 1984; Volume 3. [Google Scholar]
- Pelletier, S.W. Alkaloids: Chemical and Biological Perspectives; John Wiley & Sons Ltd.: New York, USA, 1985; Volume 1. [Google Scholar]
- Scovill, J.; Blank, E.; Konnick, M.; Nenortas, E.; Shapiro, T. Antitrypanosomal Activities of Tryptanthrins. Antimicrob. Agents Chemoter. 2002, 46, 882–883. [Google Scholar] [CrossRef]
- Penn, J.; Mantle, P.G.; Bilton, J.N.; Sheppard, R.N. Glyantrypine, a novel anthranilic acid-containing metabolite of Aspergillus clavatus. J. Chem. Soc. Perkin Trans. 1 1992, 1495–1496. [Google Scholar]
- Wong, S-M.; Musza, L.L.; Kydd, G.C.; Kullnig, R.; Gillum, A.M.; Cooper, R. Fiscalins: new substance P inhibitors produced by the fungus Neosartorya fischeri. J. Antibiot. 1993, 46, 545–553. [Google Scholar] [CrossRef]
- Fujimoto, H.; Negishi, E.; Yamaguchi, K.; Nishi, N.; Yamazaki, M. Isolation of new tremorgenic metabolites from an ascomycete, Corynascus setosus. Chem. Pharm. Bull. 1996, 44, 1843–1848. [Google Scholar] [CrossRef]
- Numata, A.; Takahashi, C.; Matsushita, T.; Miyamoto, T.; Kawai, K.; Usami, Y.; Matsumura, E.; Inoue, M.; Ohishi, H.; Shingu, T. Fumiquinazolines, novel metabolites of a fungus isolated from a saltfish. Tetrahedron Lett. 1992, 33, 1621–1624. [Google Scholar]
- Takahashi, C.; Matsushita, T.; Doi, M.; Minoura, K.; Shingu, T.; Kumeda, Y.; Numata, A. Fumiquinazolines A-G, novel metabolites of a fungus separated from a Pseudolabrus marine fish. J. Chem. Soc. Perkin Trans 1 1995, 2345–2353. [Google Scholar]
- Karwowski, J. P.; Jackson, M.; Rasmussen, R. R.; Humphrey, P. E.; Poddig, J. B.; Kohl, W. L.; Scherr, M.H.; Kadam, S.; McAlpine, J.B. 5-N-Acetylardeemin, a novel heterocyclic compound which reverses multiple drug resistance in tumor cells. J. Antibiot. 1993, 46, 374–379. [Google Scholar] [CrossRef]
- Hochlowski, J.E.; Mullally, M.M.; Spanton, S.G.; Whittern, D.N.; Hill, P.; McAlpine, J.B. 5-N-Acetylardeemin, a novel heterocyclic compound which reverses multiple drug resistance in tumor cells. J. Antibiot. 1993, 46, 380–386. [Google Scholar] [CrossRef]
- Larsen, T.O.; Frydenvang, K.; Frisvad, J.C.; Christophersen, C. UV-Guided Isolation of Alantrypinone, a Novel Penicillium Alkaloid. J. Nat. Prod. 1998, 61, 1154–1157. [Google Scholar] [CrossRef]
- Barrow, C. J.; Sun, H. H. Spiroquinazoline, a Novel Substance P Inhibitor with a New Carbon Skeleton, Isolated from Aspergillus flavipes. J. Nat. Prod. 1994, 57, 471–476. [Google Scholar] [CrossRef]
- Hernández, F.; Buenadicha, F.L.; Avendaño, C.; Söllhuber, M. 1-Alkyl-2,4-dihydro-1H-pyrazino[2,1-b]quinazoline-3,6-diones as glycine templates. Synthesis of Fiscalin B. Tetrahedron Asymmetry 2001, 12, 3387–3398. [Google Scholar]
- Hayao, S.; Havera, H.J.; Strycker, W.G.; Hong, E. Hypotensive, antiadrenergic, and antihistaminic 3-substituded 2-methyl-(or 2-phenyl-)4(3H)-quinazolones. J. Med. Chem. 1969, 12(5), 936–938. [Google Scholar] [CrossRef]
- Shiau, C.Y.; Chern, J.W.; Tien, J.H.; Liu, K C. Reactions of 2-Aminothiobenzamide with Isocyanates: A New Synthesis of 2,3-Dihydroimidazo[1,2-c]quinazolin-5(6H)-one and 3,4-Dihydro-2H-pyrimido[1,2-c]quinazolin-6(7H)-one. J. Heterocyclic Chem. 1989, 26, 595–596. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Shindo, T.; Ishii, K.; Nakamura, H.; Kon, T.; Uno, H. Acrylamide derivatives as antiallergic agents. 2. Synthesis and structure activity relationships of N-[4-[4-(diphenylmethyl)-1-piperazinyl]butyl]-3-(3-pyridyl)acrylamides. J. Med. Chem. 1989, 32, 583–593. [Google Scholar] [CrossRef]
- Hayao, S.; Havera, H.J.; Strycker, W.G.; Leipzig, T.J.; Kulp, R.A.; Hartzler, H.E. New Sedative and Hypotensive 3-Substituted 2,4(1H,3H)-Quinazolinediones. J. Med. Chem. 1965, 8, 807–811. [Google Scholar] [CrossRef]
- Hayao, S. Quinazolinedione derivatives. US Patent 3274194 1965. [Google Scholar]
- Cortez, R.; Rivero, I.A.; Somanathan, R.; Aguirre, G.; Ramirez, F.; Hong, E. Synthesis of Quinazolinedione Using Triphosgene. Synth. Commun. 1991, 21, 285–292. [Google Scholar] [CrossRef]
- Rivero, I.A.; Somanathan, R.; Hellberg, L.H. Synthesis of 3-Dipeptidyl-2,4(1H,3H)-Quinazolinediones as Potential Anti-hypertensive Agents. Synth. Commun. 1998, 28, 2077–2086. [Google Scholar] [CrossRef]
- Dreyer, D.L.; Brenner, R.C. Alkaloids of some Mexican Zanthoxylum species. Phytochemistry 1980, 19, 935–939. [Google Scholar] [CrossRef]
- Rivero, I.A.; Espinoza, K.; Somanathan, R. Synthesis of Quinazoline-2,4-dione Alkaloids and Analogues from Mexican Zanthoxylum Species. Molecules 2004, 9, 609–616. [Google Scholar] [CrossRef]
- Hunig, S.; Quast, H.; Brenninger, W.; Frankenfield, E. Tetramethyl-p-phenylenediamine. Org. Synth. 1973, 5, 1018–1021. [Google Scholar]
- Sample Availability: Available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rivero, I.A.; Guerrero, L.; Espinoza, K.A.; Meza, M.C.; Rodríguez, J.R. Alkylation of 2,4-(1H,3H)-Quinazolinediones with Dialkyl Carbonates Under Microwave Irradiations. Molecules 2009, 14, 1860-1868. https://doi.org/10.3390/molecules14051860
Rivero IA, Guerrero L, Espinoza KA, Meza MC, Rodríguez JR. Alkylation of 2,4-(1H,3H)-Quinazolinediones with Dialkyl Carbonates Under Microwave Irradiations. Molecules. 2009; 14(5):1860-1868. https://doi.org/10.3390/molecules14051860
Chicago/Turabian StyleRivero, Ignacio Alfredo, Leticia Guerrero, Karla Alejandra Espinoza, Martha Cecilia Meza, and Jesús Ramón Rodríguez. 2009. "Alkylation of 2,4-(1H,3H)-Quinazolinediones with Dialkyl Carbonates Under Microwave Irradiations" Molecules 14, no. 5: 1860-1868. https://doi.org/10.3390/molecules14051860