Parabens as Agents for Improving Crocetin Esters’ Shelf-Life in Aqueous Saffron Extracts
Abstract
:1. Introduction
2. Results and Discussion
 440 nm), 291;
440 nm), 291;  257 nm, 94;
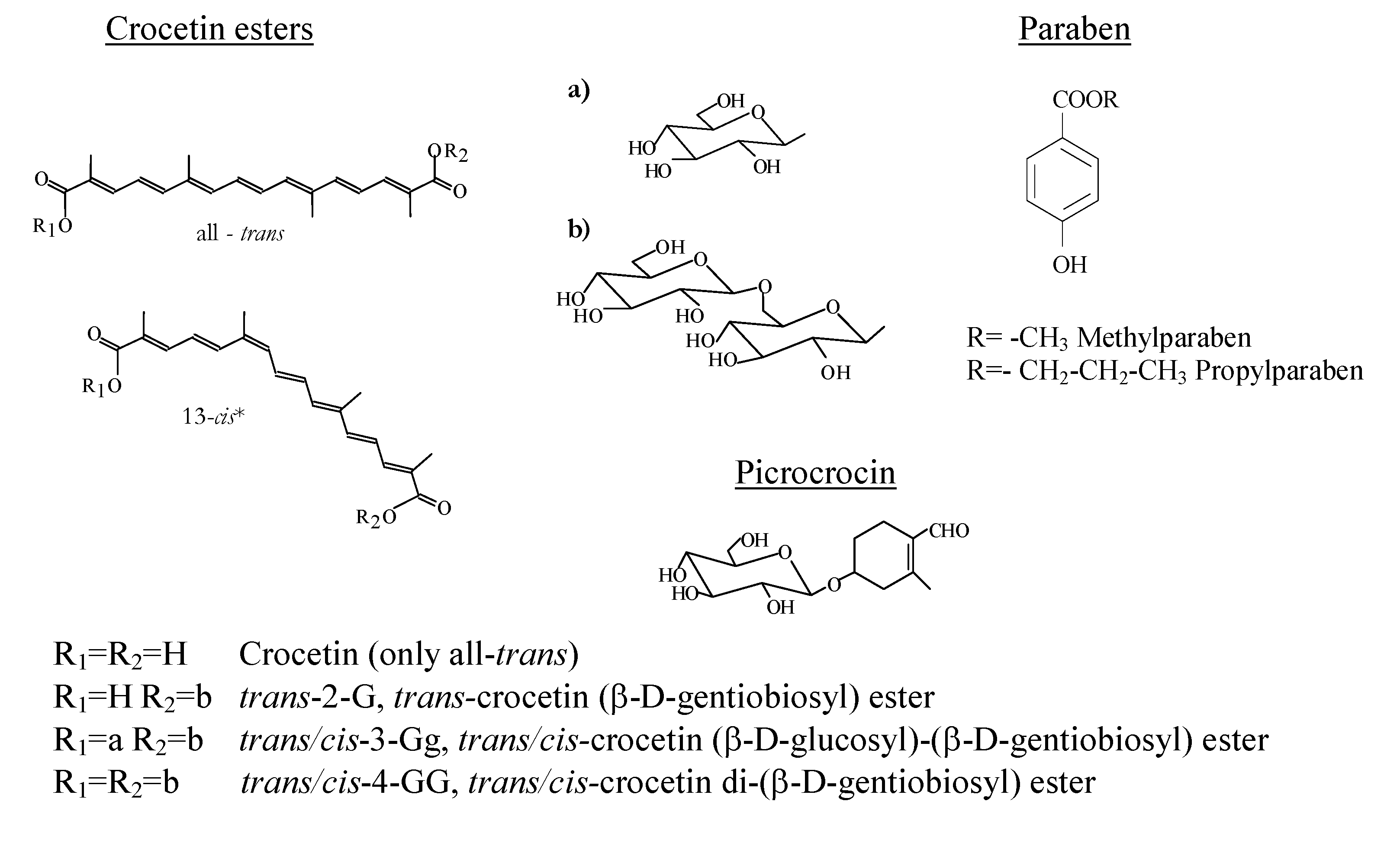
257 nm, 94;  330 nm, 30. Table 1 reports the overall and individual crocetin ester composition of the control sample, expressed as percentage on a dry basis, their retention times and also their kinetic parameters as rate constants (k), determination coefficients (R2) and half-life periods (t1/2). It is noteworthy that all rate constants were negative, as it was degradation, but they were expressed in absolute value. It is necessary to point out that the trans-crocetin di-(β-d-gentiobiosyl) ester (trans-4-GG; Figure 1) represents almost the 60% of the total crocetin ester content present in the aqueous extract and the first two (trans-4-GG and trans-3-Gg) comprise over 80% of the total esters. The half-life period of total crocetin esters was 47 hours, while for trans and cis it was 51 and 39 hours, respectively, indicating a strong effect due to the trans isomer content. The results pointed out that the 3-Gg crocetin esters were more stable than the 4-GG regardless of the isomer (trans or cis) considered.
330 nm, 30. Table 1 reports the overall and individual crocetin ester composition of the control sample, expressed as percentage on a dry basis, their retention times and also their kinetic parameters as rate constants (k), determination coefficients (R2) and half-life periods (t1/2). It is noteworthy that all rate constants were negative, as it was degradation, but they were expressed in absolute value. It is necessary to point out that the trans-crocetin di-(β-d-gentiobiosyl) ester (trans-4-GG; Figure 1) represents almost the 60% of the total crocetin ester content present in the aqueous extract and the first two (trans-4-GG and trans-3-Gg) comprise over 80% of the total esters. The half-life period of total crocetin esters was 47 hours, while for trans and cis it was 51 and 39 hours, respectively, indicating a strong effect due to the trans isomer content. The results pointed out that the 3-Gg crocetin esters were more stable than the 4-GG regardless of the isomer (trans or cis) considered. | Compound | Mean contentab±SD (g/100g) | % contenta ± SD | tR (min) | (k±SD)*103 (h-1) | R2 | t1/2 (h) |
|---|---|---|---|---|---|---|
| Total crocetin esters | 31.15±0.05 | 100.00±0.01 | 14.6 | 0.995 | 47 | |
| Total trans | 28.21±0.08 | 93.09±0.22 | 13.7 | 0.995 | 51 | |
| Total cis | 2.94±0.17 | 6.91±0.32 | 17.7 | 0.946 | 39 | |
| Trans-4-GG | 18.72±0.38 | 58.61±1.19 | 10.3 | 29.3 | 0.998 | 24 |
| Trans-3-Gg | 6.75±0.39 | 25.34±1.45 | 10.8 | 11.9 | 0.912 | 58 |
| Trans-2–G | 0.87±0.12 | 4.08±0.56 | 11.5 | 41.8 | 0.972 | 17 |
| Cis-4-GG | 1.97±0.32 | 4.39±0.71 | 12.0 | 18.8 | 0.991 | 37 |
| Cis-3-Gg | 0.82±0.10 | 2.20±0.28 | 12.7 | 11.4 | 0.974 | 61 |
| Crocetin esters | Methylparaben | |||||||||||
| 50 mg/L | 100 mg/L | 150 mg/L | 200 mg/L | |||||||||
| (K±SD)*103 (h-1) | R2 | t1/2 (h) | (K±SD)*103 (h-1) | R2 | t1/2 (h) | (K±SD)* 103 (h-1) | R2 | t1/2 (h) | (K±SD)*103 (h-1) | R2 | t1/2 (h) | |
| Total crocetin esters | 17.6 | 0.989 | 39 | 14.8 | 0.966 | 47 | 9.4 | 0.974 | 74 | 8.8 | 0.972 | 79 |
| Total trans | 18.2 | 0.987 | 38 | 15.5 | 0.964 | 45 | 9.2 | 0.977 | 75 | 9.2 | 0.971 | 75 |
| Total cis | 6.8 | 0.931 | 101 | 6.4 | 0.930 | 108 | 4.1 | 0.939 | 169 | 2.6 | 0.840 | 267 |
| Trans-4-GG | 24.3 | 0.996 | 29 | 20.4 | 0.920 | 34 | 9.6 | 0.999 | 72 | 8.2 | 0.940 | 85 |
| Trans-3-Gg | 10.1 | 0.966 | 69 | 10.0 | 0.950 | 69 | 9.7 | 0.903 | 71 | 9.4 | 0.923 | 74 |
| Trans-2-G | 26.4 | 0.961 | 26 | * | * | * | ||||||
| Cis-4-GG | 14.9 | 0.917 | 47 | 9.7 | 0.981 | 71 | 6.6 | 0.938 | 105 | 5.0 | 0.915 | 139 |
| Cis-3-Gg | 9.2 | 0.947 | 75 | 4.6 | 0.983 | 151 | * | * | ||||
| Propylparaben | ||||||||||||
| 50 mg/L | 100 mg/L | 150 mg/L | 200 mg/L | |||||||||
| Total crocetin esters | 14.4 | 0.995 | 48 | 13.1 | 0.983 | 54 | 11.8 | 0.958 | 83 | 7.5 | 0.988 | 92 |
| Total trans | 14.2 | 0.998 | 49 | 12.8 | 0.961 | 52 | 9.6 | 0.971 | 80 | 7.8 | 0.978 | 86 |
| Total cis | 8.0 | 0.940 | 94 | 7.3 | 0.989 | 99 | * | * | ||||
| Trans-4-GG | 14.8 | 0.998 | 47 | 14.3 | 0.990 | 48 | 8.5 | 0.982 | 82 | 7.4 | 0.981 | 94 |
| Trans-3-Gg | 11.7 | 0.980 | 59 | 4.9 | 0.917 | 141 | 4.3 | 0.944 | 161 | 4.3 | 0.971 | 161 |
| Trans-2-G | 16.2 | 0.930 | 43 | 15.2 | 0.916 | 46 | * | * | ||||
| Cis-4-GG | 16.0 | 0.996 | 43 | 11.4 | 0.922 | 61 | 8.4 | 0.999 | 83 | 8.2 | 0.923 | 85 |
| Cis-3-Gg | 9.9 | 0.946 | 70 | 9.0 | 0.938 | 77 | * | * | ||||
2.1. Stability of saffron from different geographical zones
| Country | Greece (9) | Italy (11) | Spain (14) | Iran (12) | ||||||||
| Moisture & volatile content % ± SD | 8.51±0.69 | 8.78±0.43 | 6.59±1.24 | 7.29±0.57 | ||||||||
| Colouring strength ± SD | 239.30±9.87 | 279.14±12.15 | 260.63±20.39 | 233.11±7.86 | ||||||||
| Crocetin Esters | Mean content (g/100g) | (Content± SD)% | Δ mean content*±SD | Mean content (g/100g) | (Content± SD)% | Δ mean content*±SD | Mean content (g/100g) | (Content± SD)% | Δ mean content*±SD | Mean content (g/100g) | (Content± SD)% | Δ mean content*±SD |
| Total | 25.94b | 100.00±0.01 | 1.08±0.58 | 29.88c | 100.02±0.03 | 1.42±1.09 | 29.31c | 100.00±0.01 | -1.94±0.19 | 24.99a | 100.00±0.01 | 1.55±0.37 |
| Total trans | 20.37a | 83.86a±0.93 | 0.99±0.12 | 26.67c | 91.53c±0.65 | 1.45±1.07 | 22.98b | 85.26a±1.45 | -0.70±0.27 | 20.94a | 88.09b±1.97 | 1.22±0.34 |
| Total cis | 5.57b | 16.14c±0.93 | 0.96±0.34 | 3.21a | 8.41a±0.65 | 0.73±0.41 | 6.33b | 14.74c±1.45 | 1.52±0.72 | 4.05a | 11.91b±1.97 | 1.60±0.46 |
| Trans-4-GG | 11.77a | 44.79a±2.57 | 2.29±0.59 | 17.79c | 58.05d±1.44 | 2.56±0.62 | 14.35b | 50.15c±3.07 | 1.92±0.61 | 12.03a | 47.01b±1.38 | 4.21±0.60 |
| Trans-3-Gg | 5.75a | 26.25ab±0.84 | -0.92±0.12 | 6.63a | 25.92a±1.39 | 0.90±0.79 | 6.55a | 27.45b±2.29 | -1.13±0.32 | 6.37a | 29.84c±1.54 | -1.27±0.25 |
| Trans-2-G | 1.37c | 7.78c±0.95 | -2.61±0.41 | 0.50a | 2.46a±0.53 | -2.21±1.65 | 0.55a | 2.87a±0.88 | -2.32±0.59 | 1.01b | 5.89b±1.77 | -4.50±0.31 |
| Cis-4-GG | 3.44b | 9.33b±0.48 | 1.97±0.43 | 2.06a | 5.74a±0.75 | 1.41±1.27 | 4.51c | 9.63b±3.37 | 1.32±0.21 | 2.57ab | 7.16a±1.25 | 2.83±0.49 |
| Cis-3-Gg | 1.93b | 6.26c±0.94 | -0.75±0.49 | 0.89a | 2.08a±0.28 | 2.14±0.78 | 1.46a | 4.35b±1.46 | -1.26±0.50 | 1.16ab | 3.78b±0.72 | -0.91±0.45 |
3. Experimental
3.1. Samples
3.2. Chemicals and reagents
3.3. Procedure and Instrumentation

 is the colouring strength, Ai is the percentage peak area of the crocetin ester i at 440 nm, and Єt,c is the molecular coefficient absorbance value (89000 for trans-crocetin esters and 63350 for cis-crocetin esters) [23].
is the colouring strength, Ai is the percentage peak area of the crocetin ester i at 440 nm, and Єt,c is the molecular coefficient absorbance value (89000 for trans-crocetin esters and 63350 for cis-crocetin esters) [23].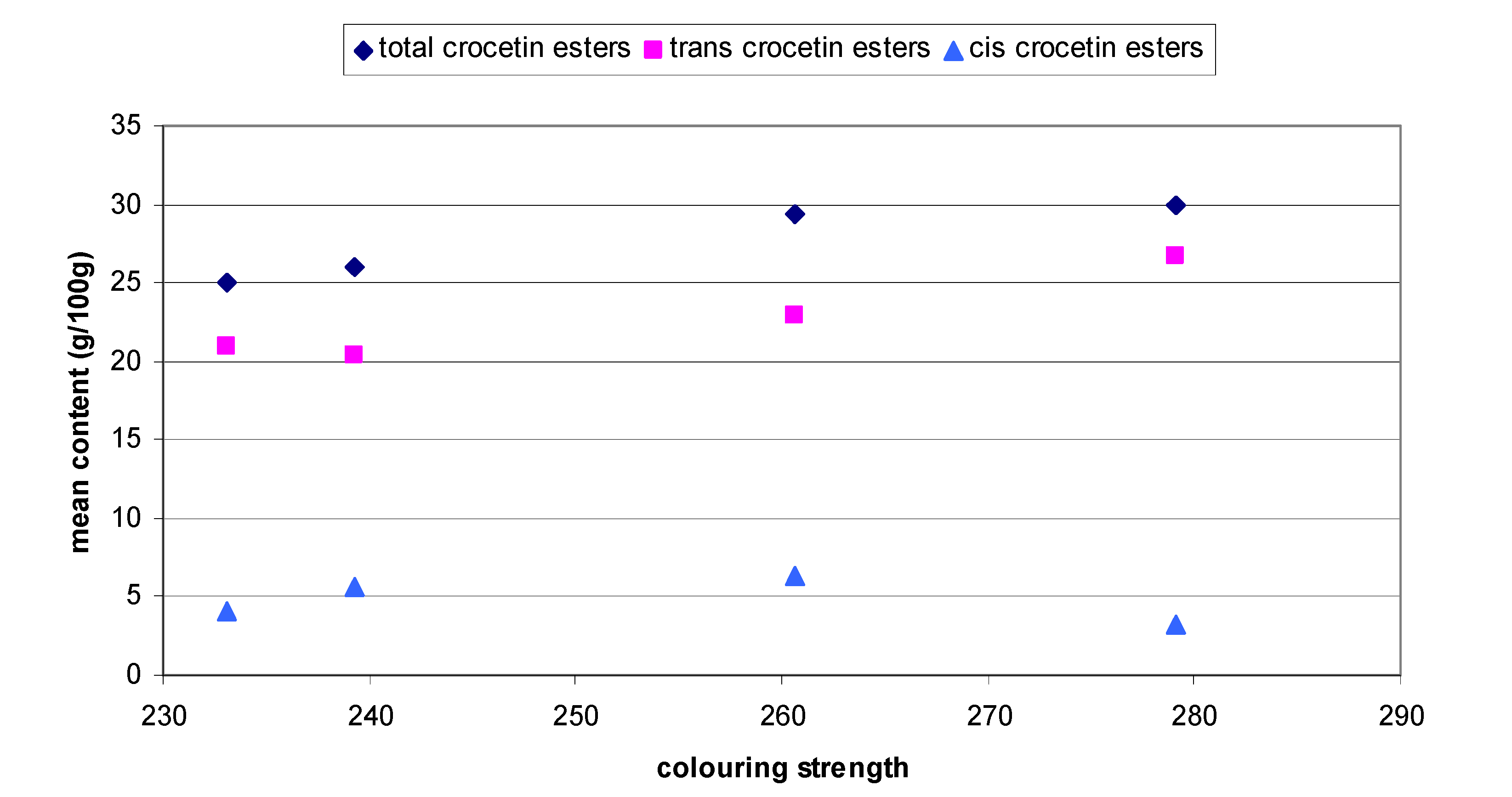
3.4. Geographical sample differentiation according to the stability
 at 257 nm,
at 257 nm,  at 330 nm and
at 330 nm and  at 440 nm values were calculated according to ISO 3632 (2003) [21]. Every sample was measured in triplicate. The kinetic parameters of each reaction-reaction order, rate constants (k), and half-life periods (t1/2) were obtained using the integral method [24]. This method uses a trial-and-error procedure to find reaction order. If the order assumed is correct, the appropriate plot of the concentration-time data[concentration against time (zero-order), ln concentration against time (first-order), and concentration-1 against time (second-order)] should be linear. The result showing the best correlation coefficient (R2) was selected.
at 440 nm values were calculated according to ISO 3632 (2003) [21]. Every sample was measured in triplicate. The kinetic parameters of each reaction-reaction order, rate constants (k), and half-life periods (t1/2) were obtained using the integral method [24]. This method uses a trial-and-error procedure to find reaction order. If the order assumed is correct, the appropriate plot of the concentration-time data[concentration against time (zero-order), ln concentration against time (first-order), and concentration-1 against time (second-order)] should be linear. The result showing the best correlation coefficient (R2) was selected. 
3.5. Statistical analysis
4. Conclusions
Acknowledgements
References and Notes
- Carmona, M.; Zalacain, A.; Sánchez, A.M.; Novella, J.L.; Alonso, G.L. Crocetin Esters, Picrocrocin and its Related Compounds Present in Crocus sativus Stigmas and Gardenia jasminoides Fruits. Tentative Identification of Seven New Compounds by LC-ESI-MS. J. Agric. Food Chem. 2006, 54, 973–979. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain Aramburu, A.; Pardo, J.E.; López, E.; Alvarruiz, A.; Alonso Díaz-Marta, G.L. Influence of Different Drying and Aging Conditions on Saffron Constituents. J. Agric. Food Chem. 2005, 53, 3974–3979. [Google Scholar] [CrossRef]
- Alonso, G.L.; Varón, R.; Gómez, R.; Navarro, F.; Salinas, M.R. Auto-Oxidation in Saffron at 40ºC and 75% Relative Humidity. J. Food Sci. 1990, 55, 595–596. [Google Scholar] [CrossRef]
- Alonso, G.L.; Sánchez, M.A.; Salinas, M.R.; Esteban-Infantes, F.J. Inhibición de la autooxidación de las substancias responsables de las características del azafrán. In Proceeding of the II Congreso Internacional de Química de la ANQUE, Spain, 1992; pp. 67–73.
- Alonso, G.L.; Varón, R.; Salinas, M.R.; Navarro, F. Autooxidation of crocin and picrocrocin in saffron under different storage conditions. Boll.Chim. Farm. 1993, 132, 116–120. [Google Scholar]
- Alonso, G.L.; Sánchez, M.A.; Salinas, M.R.; Esteban-Infantes, F.J. Cinética de la pérdida de poder colorante en extractos acuosos de azafrán a distintas temperaturas. In Presented at the IX Congreso Nacional de Química, Spain, 1993.
- Tsimidou, M.; Tsatsaroni, E. Stability of Saffron Pigments in Aqueous Extracts. J. Food Sci. 1993, 58, 1073–1075. [Google Scholar] [CrossRef]
- Orfanou, O.; Tsimidou, M. Influence of Selected Additives on the Stability of Saffron Pigments in Aqueous Extracts. In Food flavours: Generation, Analysis and Process Influence; Charalambous, G., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1995; pp. 881–894. [Google Scholar]
- Sánchez, A.M.; Carmona, M.; Ordoudi, S.; Tsimidou, M.; Alonso, G.L. Kinetics of Individual Crocetin Ester Degradation in Aqueous Extracts of Saffron (Crocus sativusL.) upon Thermal Treatment in the Dark. J. Agric. Food Chem. 2008, 56, 1627–1637. [Google Scholar] [CrossRef]
- Carmona, M.; Sánchez, A.M.; Ferreres, F.; Zalacain, A.; Tomás-Barberán, F.; Alonso, G.L. Identification of the flavonoid fraction in saffron spice by LC/DAD/MS/MS: Comparative Study of Samples from Different Geographical Origins. Food Chem. 2007, 100, 445–450. [Google Scholar] [CrossRef]
- Castellar, M.R. Biotransformación de la picrocrocin con β-glucosidasa inmovilizada. Doctoral thesis, Universidad de Murcia, Murcia, Spain, 1992; p. 19. [Google Scholar]
- Soni, M.G.; Carabin, I.G.; Burdock, G.A. Safety Assessment of Esters of p-Hydroxybenzoic Acid (parabens). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef]
- García-Jiménez, J.F.; Valencia, M.C.; Capitán-Vallvey, L.F. Simultaneous Determination of Antioxidants, Preservatives and Sweetener Additives in Food and Cosmetics by Flow Injection Analysis Coupled to a Monolithic Column. Anal. Chim. Acta. 2007, 594, 226–233. [Google Scholar] [CrossRef]
- Ali, M.S.; Chaudhary, R.S.; Takieddin, M.A. Simultaneous Determination of Metronidazole Benzoate, Methylparaben, and Propylparaben by High-Performance Liquid Chromatograph. Drug Develop. Ind. Pharm. 1999, 25, 1143–1147. [Google Scholar] [CrossRef]
- Mahuzier, P.E.; Altria, K.D.; Clark, B.J. Selective and Quantitative Analysis of 4-Hydroxybenzoate Preservatives by Microemulsion Electrokinetic Chromatography. J. Chromatogr. 1927, 924, 465–470. [Google Scholar] [CrossRef]
- Wang, S.P.; Chang, C.L. Determination of Parabens in Cosmetic Products by Supercritical Fluid Extraction And Capillary Zone Electrophoresis. Anal. Chim. Acta 1998, 377, 85–93. [Google Scholar] [CrossRef]
- Kreuz, D.M.; Howard, A.L.; Ip, D. Determination of Indinavir, Potassium Sorbate, Methylparaben, and Propylparaben in Aqueous Pediatric Suspensions. J. Pharm. Biomed.Anal. 1999, 19, 725–735. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chou, S.S.; Sheu, F.; Shyu, Y.T. Simultaneous Determination of Sweeteners and Preservatives in Preserved Fruits by Micellar Electrokinetic Capillary Chromatography. J. Chromatogr. Sci. 2000, 38, 345–352. [Google Scholar] [CrossRef]
- Labat, L.; Kummer, E.; Dallet, P.; Dubost, J.P. Comparison of High-Performance Liquid Chromatography and Capillary Zone Electrophoresis for the Determination of Parabens in a Cosmetic Product. J. Pharm. Biomed. Anal. 2000, 23, 763–769. [Google Scholar] [CrossRef]
- Skaria, C.V.; Gaisford, S.; O’Neill, M.A.A.; Buckton, G.; Beezer, A.E. Stability Assessment of Pharmaceuticals by Isothermal Calorimetry: two Component Systems. Int. J. Pharm. 2005, 292, 127–135. [Google Scholar] [CrossRef]
- ISO 3632-1,2 Technical Specification. Saffron (Crocus sativus L.). Part 1 (Specification) and Part 2 (Test methods).; International Organisation for Standarization: Geneva, Switzerland, 2003.
- Morteza, P.H.; Reza, F.M.; Nasrin, S.; Ehsan, N.; Ali, R.S.; Amini, M. Deterioration of Parabens in Preserved Magnesium Hydroxide Oral Suspensions. J. Appl. Sci. 2007, 7, 3322–3325. [Google Scholar] [CrossRef]
- Speranza, G.; Dadà, G.; Manitto, P.; Monti, D.; Gramatica, P. 13-cis-crocin: a New Crocinoid of Saffron. Gazz.Chim. Ital. 1984, 114, 189–192. [Google Scholar]
- Fogler, H.S. Elements of Chemical Reaction Engineering; Amundson, Ed.; Prentice-Hall: Englewood Cliffs, NJ, 1992. [Google Scholar]
- Sample Availability: Not available.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Maggi, L.; Carmona, M.; Zalacain, A.; Tomé, M.M.; Murcia, M.A.; Alonso, G.L. Parabens as Agents for Improving Crocetin Esters’ Shelf-Life in Aqueous Saffron Extracts. Molecules 2009, 14, 1160-1170. https://doi.org/10.3390/molecules14031160
Maggi L, Carmona M, Zalacain A, Tomé MM, Murcia MA, Alonso GL. Parabens as Agents for Improving Crocetin Esters’ Shelf-Life in Aqueous Saffron Extracts. Molecules. 2009; 14(3):1160-1170. https://doi.org/10.3390/molecules14031160
Chicago/Turabian StyleMaggi, Luana, Manuel Carmona, Amaya Zalacain, Magdalena Martínez Tomé, María Antonia Murcia, and Gonzalo Luis Alonso. 2009. "Parabens as Agents for Improving Crocetin Esters’ Shelf-Life in Aqueous Saffron Extracts" Molecules 14, no. 3: 1160-1170. https://doi.org/10.3390/molecules14031160





