New Triterpene Glucosides from the Roots of Rosa laevigata Michx
Abstract
:Introduction
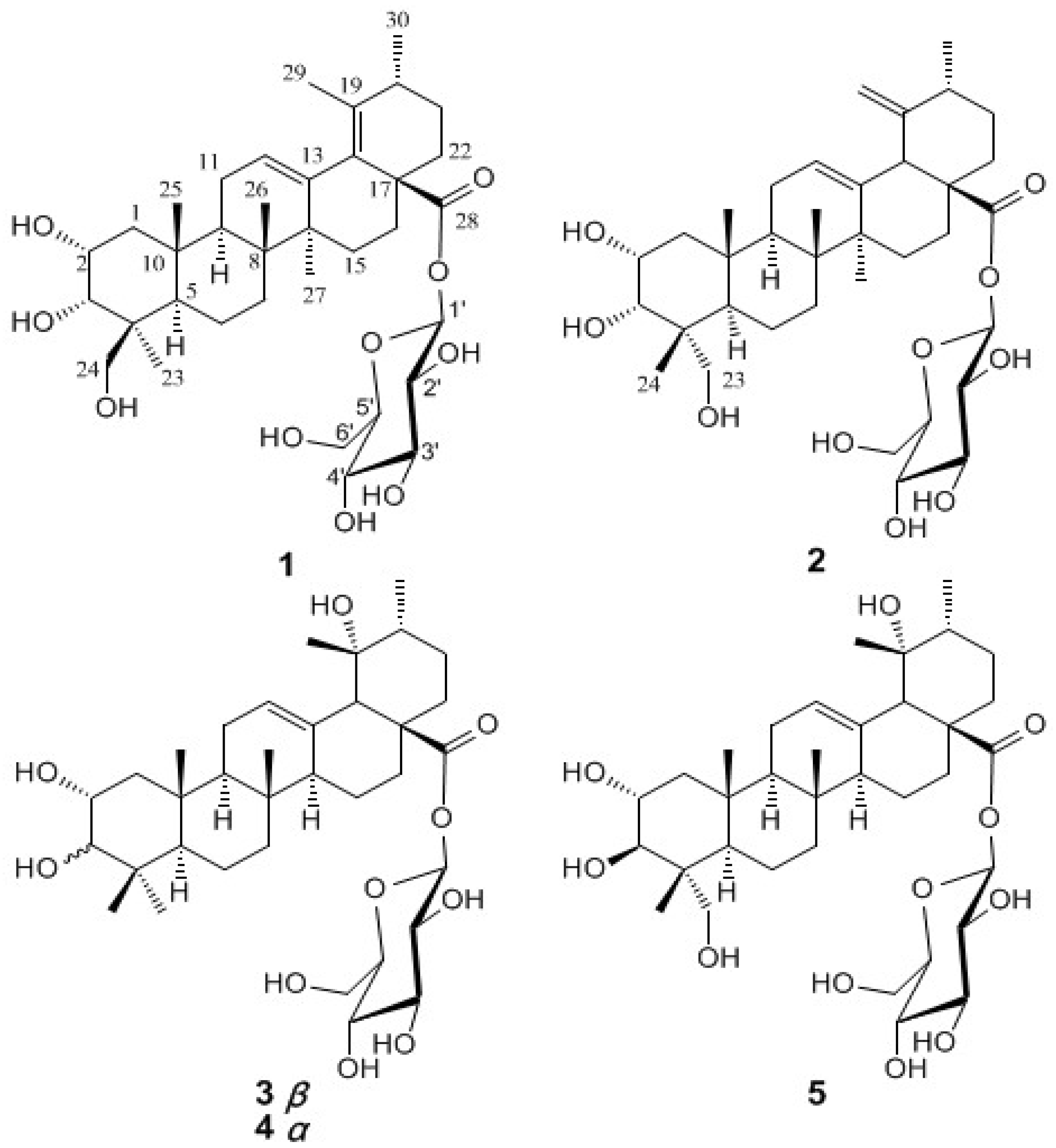
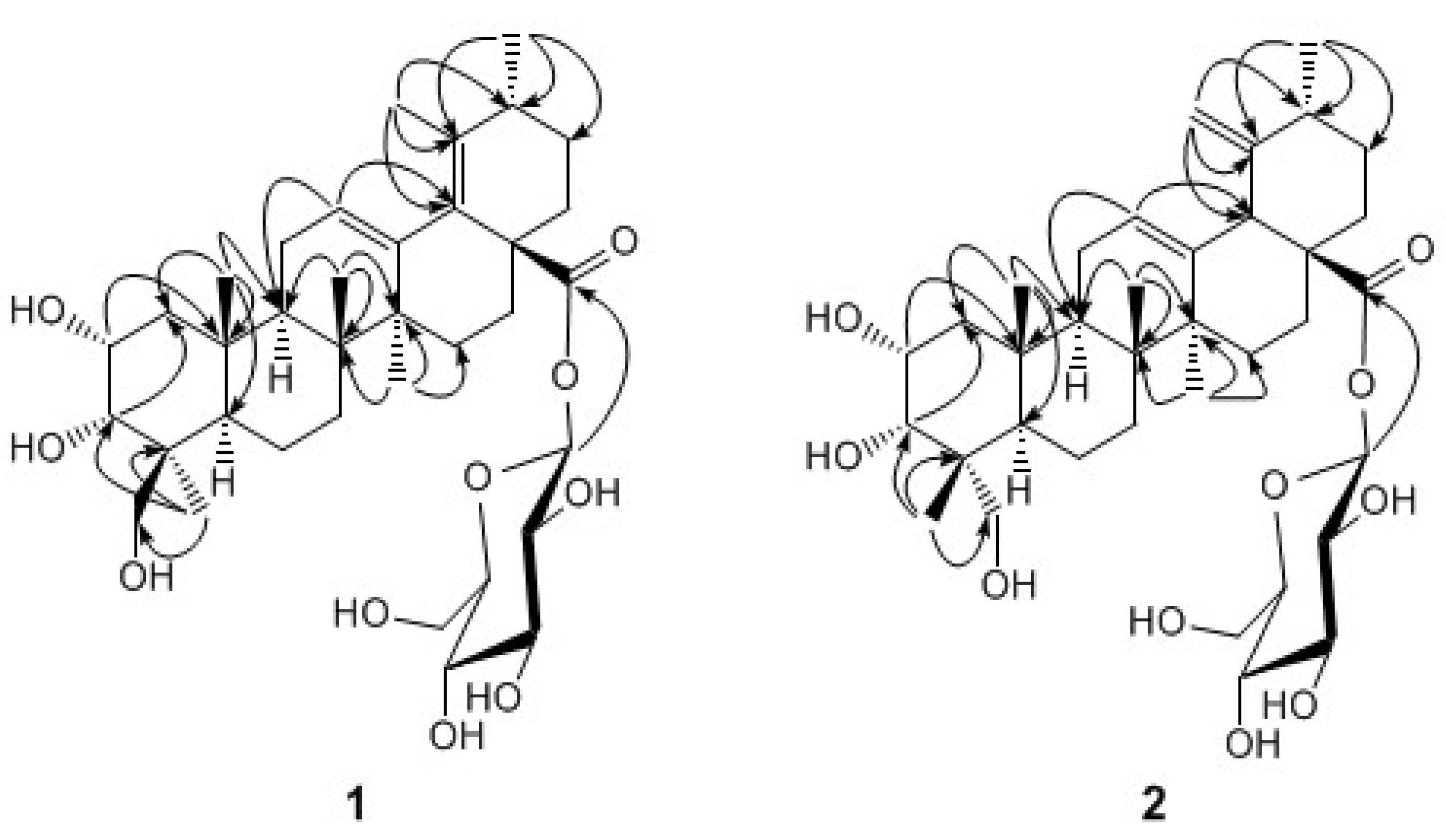
Results and Discussion
| 1 | 2 | ||||
|---|---|---|---|---|---|
| δH (J Hz) | δC | δH (J Hz) | δC | ||
| 1 | 1.84, m; 2.04, m | 43.6 | 1.83, m; 1.93, m | 42.7 | |
| 2 | 4.46, ddd (10.5, 4.3, 3.2) | 66.2 | 4.27, ddd (9.7, 4.1, 2.7) | 66.2 | |
| 3 | 4.60, d (3.2) | 74.1 | 4.14, d (2.7) | 78.9 | |
| 4 | 44.8 | 42.9 | |||
| 5 | 1.78, m | 48.3 | 2.03, m | 43.6 | |
| 6 | 1.43, m; 1.65, m | 18.7 | 1.55, m | 18.3 | |
| 7 | 1.49, m | 35.2 | 1.38, m | 33.1 | |
| 8 | 39.6 | 39.9 | |||
| 9 | 1.85, m | 49.5 | 2.05, m | 49.5 | |
| 10 | 38.4 | 38.4 | |||
| 11 | 2.04, m | 23.9 | 2.05, m | 23.9 | |
| 12 | 5.61, br. s | 126.5 | 5.49, br. s | 128.4 | |
| 13 | 138.6 | 137.6 | |||
| 14 | 45.1 | 41.9 | |||
| 15 | 1.23, m; 2.41, m | 28.9 | 1.10, m; 2.38, m | 29.0 | |
| 16 | 1.62, m, 2.57, m | 35.5 | 1.75, m; 1.86, m | 25.7 | |
| 17 | 50.3 | 49.8 | |||
| 18 | 135.2 | 3.76, s | 52.2 | ||
| 19 | 133.7 | 153.3 | |||
| 20 | 2.03, m | 34.5 | 1.83, m | 37.5 | |
| 21 | 1.23, m; 2.04, m | 26.7 | 1.22, m; 1.37,m | 30.7 | |
| 22 | 1.67, m; 2.17, m | 30.9 | 1.79, m; 1.94, m | 37.1 | |
| 23 | 0.98, s | 65.1 | 3.73, d (10.2); 3.88,d (10.2) | 71.2 | |
| 24 | 3.80, m; 4.12, m | 21.9 | 0.85, s | 17.7 | |
| 25 | 1.05, s | 17.8 | 1.02, s | 17.2 | |
| 26 | 1.14, s | 18.4 | 1.14, s | 17.4 | |
| 27 | 1.68, s | 23.8 | 1.12, s | 26.2 | |
| 28 | 174.8 | 176.1 | |||
| 29 | 1.71, s | 19.5 | 4.95, br. s; 5.10, br. s | 110.4 | |
| 30 | 1.03, d (7.0) | 18.6 | 1.02, d (7.0) | 19.4 | |
| Glc | |||||
| 1' | 6.27, d (7.8) | 95.9 | 6.29, d (8.3) | 95.9 | |
| 2' | 4.18, dd (8.3, 7.8) | 74.0 | 4.21, dd (8.8, 8.3) | 74.0 | |
| 3' | 4.27, m | 78.8 | 4.28, m | 78.9 | |
| 4' | 4.35, m | 71.1 | 4.34, dd (9.3, 9.2) | 71.1 | |
| 5' | 3.98, m | 79.1 | 4.03, m | 79.3 | |
| 6' | 4.37, m; 4.46, m | 62.2 | 4.37, m; 4.47, m | 62.2 | |
Biological activity
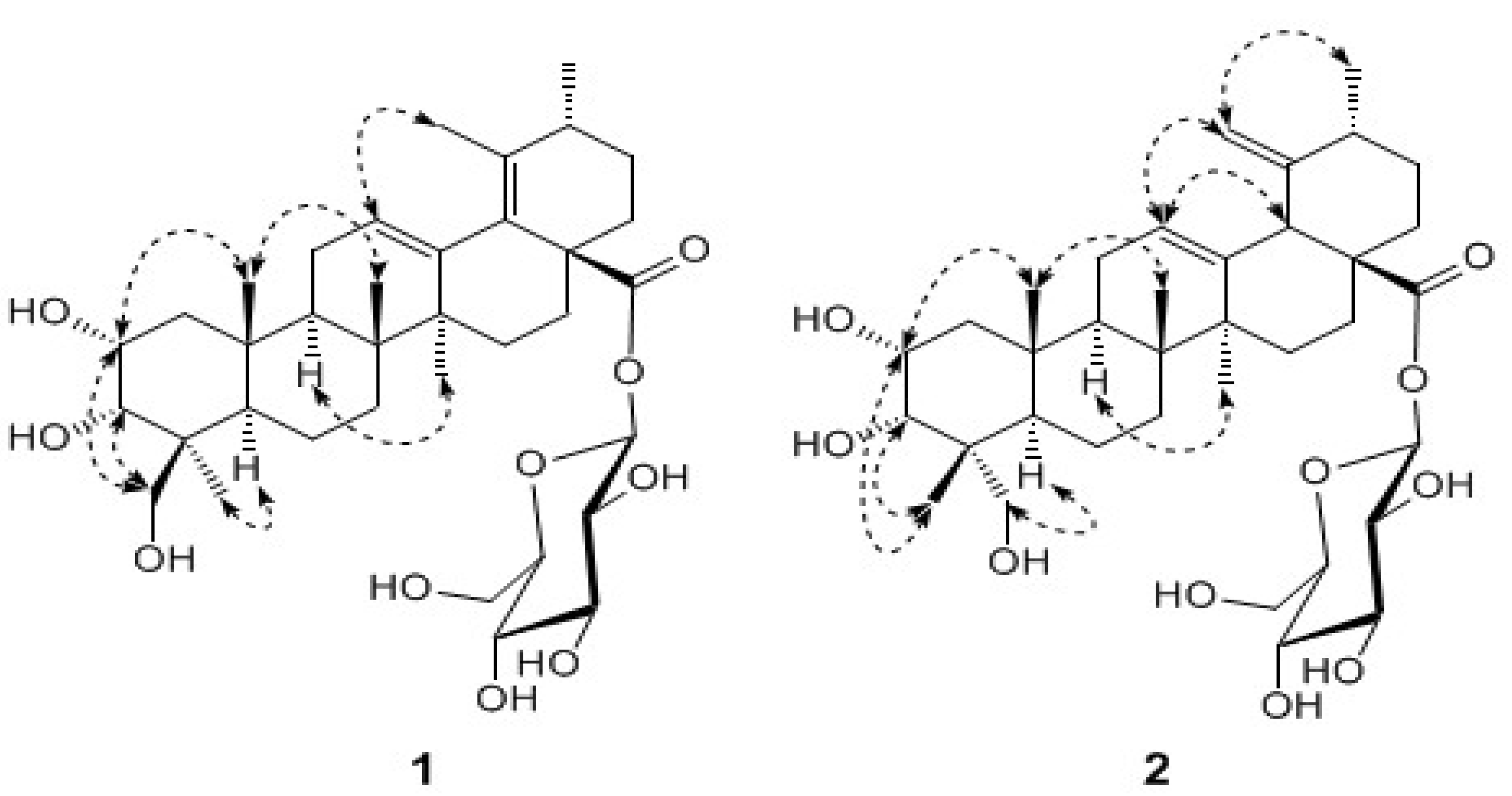
| 1 | 2 | 3 | 4 | 5 | Chloroamphenicol | Fluconazole | |
|---|---|---|---|---|---|---|---|
| S. aureus | 100 | 100 | >200 | >200 | >200 | 4.0 | |
| S. epidermidis | >200 | >200 | 100 | >200 | >200 | 4.0 | |
| B. subfitis | 100 | 50 | 100 | >200 | >200 | 8.0 | |
| E. coli | >200 | 100 | >200 | >200 | >200 | 2.0 | |
| K. pneumoniae | >200 | 100 | >200 | >200 | >200 | 1.5 | |
| C.albicans | 100 | 12.5 | 100 | 100 | 25 | 1.56 | |
| C. krusei | 50 | 12.5 | 50 | >200 | 12.5 | 50 | |
| C. parapsilosis | >200 | 50 | 100 | >200 | 200 | 1.56 | |
| T. glabrata | >200 | >200 | >200 | >200 | >200 | 6.25-12.5 | |
| C. neoformans | >200 | >200 | >200 | >200 | >200 | 50 |
Experimental
General
Plant Material
Extraction and Isolation
 + 82.1 (c = 0.5, MeOH); IR (KBr) cm-1: 3428, 2935, 1731, 1645, 1457, 1073, 1030; HR-ESI-MS m/z: 671.3779 [M+Na]+ (Calcd for C36H56 NaO10, 671.3771), 1H- and 13C-NMR: see Table 1.
+ 82.1 (c = 0.5, MeOH); IR (KBr) cm-1: 3428, 2935, 1731, 1645, 1457, 1073, 1030; HR-ESI-MS m/z: 671.3779 [M+Na]+ (Calcd for C36H56 NaO10, 671.3771), 1H- and 13C-NMR: see Table 1. + 72.4 (c = 0.5, MeOH); UV λmax (MeOH) nm: 220; IR (KBr) cm-1: 3417, 2945, 1716, 1632, 1442, 1064, 1029; HR-ESI-MS m/z: 671.3768 [M+Na]+ (Calcd for C36H56 NaO10, 671.3771); 1H- and 13C-NMR: see Table 1.
+ 72.4 (c = 0.5, MeOH); UV λmax (MeOH) nm: 220; IR (KBr) cm-1: 3417, 2945, 1716, 1632, 1442, 1064, 1029; HR-ESI-MS m/z: 671.3768 [M+Na]+ (Calcd for C36H56 NaO10, 671.3771); 1H- and 13C-NMR: see Table 1. Determination of the Sugar Components [10]
Antimicrobial activity
Acknowledgements
References
- Xu, G.J.; He, H.X.; Xu, L.S.; Jin, R.Y. Chinese Materia Medica (“Zhonghua Benchao”); Chinese Medicinal Science & Technology Publishing House: Beijing, P. R. China, 1998; Vol. 7, p. 223. [Google Scholar]
- State Pharmacopoeia Commission of the Ministry of Public Health. Pharmacopoeia of the People’s Republic of China 2005; Chemical Industry Press: Beijing, P. R. China, 2005; Vol. 1, p. 153. [Google Scholar]
- Department of Health of Guangxi Zhuang Autonomous Region. Guangxi Standard of the Traditional Chinese Medicinal Materials; Guangxi Science & Technology Press: Nanning, P. R. China, 1992; p. 68. [Google Scholar]
- Fang, J.M.; Wang, K.C.; Cheng, Y.S. Steroids and triterpenoids from Rosa laevigata. Phytochemistry 1991, 30, 3383–3387. [Google Scholar] [CrossRef]
- Yoshida, T.; Tanaka, K.; Chang, X.M; Okuda, T. Dimeric ellagitannins, Laevigatins E, F and G, from Rosa Laevigata. Phytochemistry 1989, 28, 2451–2454. [Google Scholar] [CrossRef]
- Durham, D.G.; Liu, X.J.; Richards, R.M.E. A triterpene from Rubus pinfaensis. Phytochemistry 1994, 36, 1469–1472. [Google Scholar] [CrossRef]
- Seto, T.; Tanaka, T.; Tanaka, O.; Naruhashi, N. β-glucosyl esters of 19α-hydroxyursolic acid derivatives in leaves of Rubus species. Phytochemistry 1984, 23, 2829–2834. [Google Scholar] [CrossRef]
- Zhou, X.H.; Kasai, R.; Ohtani, K.; Tanaka, O.; Nie, R.L.; Yang, C.R.; Zhou, J.; Yamasaki, K. Oleanane and ursane glucosides from Rubus species. Phytochemistry 1992, 31, 3642–3644. [Google Scholar] [CrossRef]
- Adnyana, I.K.; Tezuka, Y.; Banskota, A.H.; Xiong, Q.B.; Tran, K.Q.; Kadota, S. Quadranosides I-V, new triterpene glucosides from the seeds of Combretum quadrangulare. J. Nat. Prod. 2000, 63, 496–500. [Google Scholar] [CrossRef]
- Adnyana, I.K.; Tezuka, Y.; Awale, S.; Banskota, A.H.; Tran, K.Q.; Kadota, S. Quadranosides VI-XI, six new triterpene glucosides from the seeds of Combretum quadrangulare. Chem. Pharm. Bull. 2000, 48, 1114–1120. [Google Scholar] [CrossRef]
- Barrero, A.F.; Moral, J.F.; Lara, A.; Herrado, M.M. Antimicrobial activity of sesquiterpenes from the essential oil of Juniperus thurifera wood. Planta Med. 2005, 71, 67–71. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yuan, J.-Q.; Yang, X.-Z.; Miao, J.-H.; Tang, C.-P.; Ke, C.-Q.; Zhang, J.-B.; Ma, X.-J.; Ye, Y. New Triterpene Glucosides from the Roots of Rosa laevigata Michx. Molecules 2008, 13, 2229-2237. https://doi.org/10.3390/molecules13092229
Yuan J-Q, Yang X-Z, Miao J-H, Tang C-P, Ke C-Q, Zhang J-B, Ma X-J, Ye Y. New Triterpene Glucosides from the Roots of Rosa laevigata Michx. Molecules. 2008; 13(9):2229-2237. https://doi.org/10.3390/molecules13092229
Chicago/Turabian StyleYuan, Jing-Quan, Xin-Zhou Yang, Jian-Hua Miao, Chun-Ping Tang, Chang-Qiang Ke, Ji-Bao Zhang, Xiao-Jun Ma, and Yang Ye. 2008. "New Triterpene Glucosides from the Roots of Rosa laevigata Michx" Molecules 13, no. 9: 2229-2237. https://doi.org/10.3390/molecules13092229




