Synthesis of Polynucleotide Analogs Containing a Polyvinyl Alcohol Backbone
Abstract
:Introduction
Results and Discussion
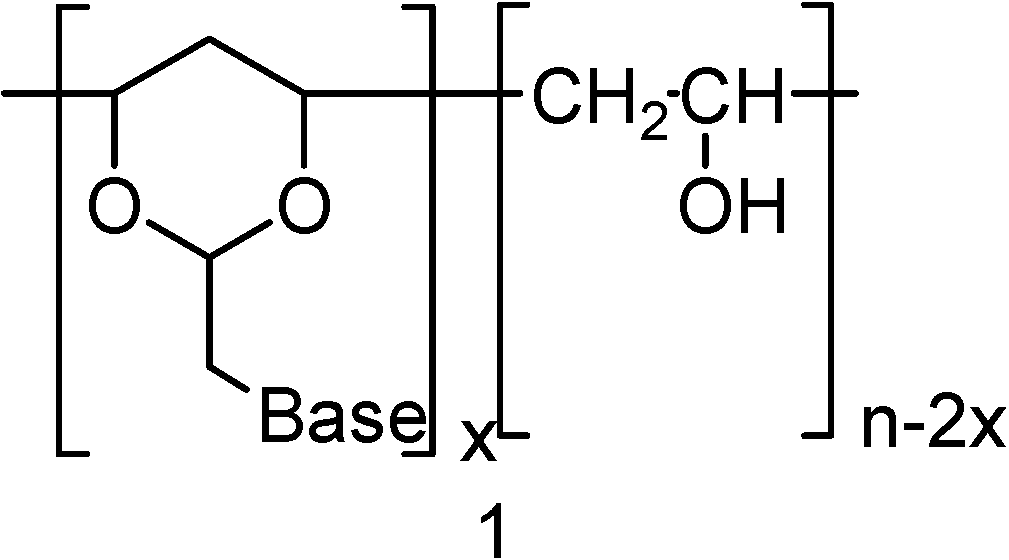
Neutral PVA-polynucleotide Analogs
| Polymer | Base cont. % * | DMSO | H2O | NaH2PO4 0.01 M | Na3PO4 0.01 M |
|---|---|---|---|---|---|
| 1-U8 | 8 | ++ | ++ | ++ | ++ |
| 1-U15 | 15 | + | - | - | - |
| 1-U22 | 22 | + | - | - | - |
| 1- A9 | 9 | ++ | ++ | + | + |
| 1-A16 | 16 | ++ | + | + | + |
| 1-A27 | 27 | ++ | - | - | - |
- * The subscript indicates the content of nucleic base-1,3-dioxane moiety
- ++ Soluble in the solvent, + Partial soluble, - Not soluble
PVA-polynucleotide Phosphate Analogs


| Polymer | Base cont. % * | DMSO | H2O | NaH2PO4 0.01 M | Na3PO4 0.01 M |
|---|---|---|---|---|---|
| PVA-P | - | ++ | ++ | ++ | ++ |
| 7-U8 | 8 | + | ++ | ++ | ++ |
| 7-A6 | 6 | + | ++ | ++ | ++ |
- * The subscript indicates the content of nucleic base-1,3-dioxane moiety
- ++ Soluble in the solvent, + Partial soluble, - Not soluble
Temperature Dependent UV spectra of 1-U8, 1-A9, 7-U8 and 7-A6
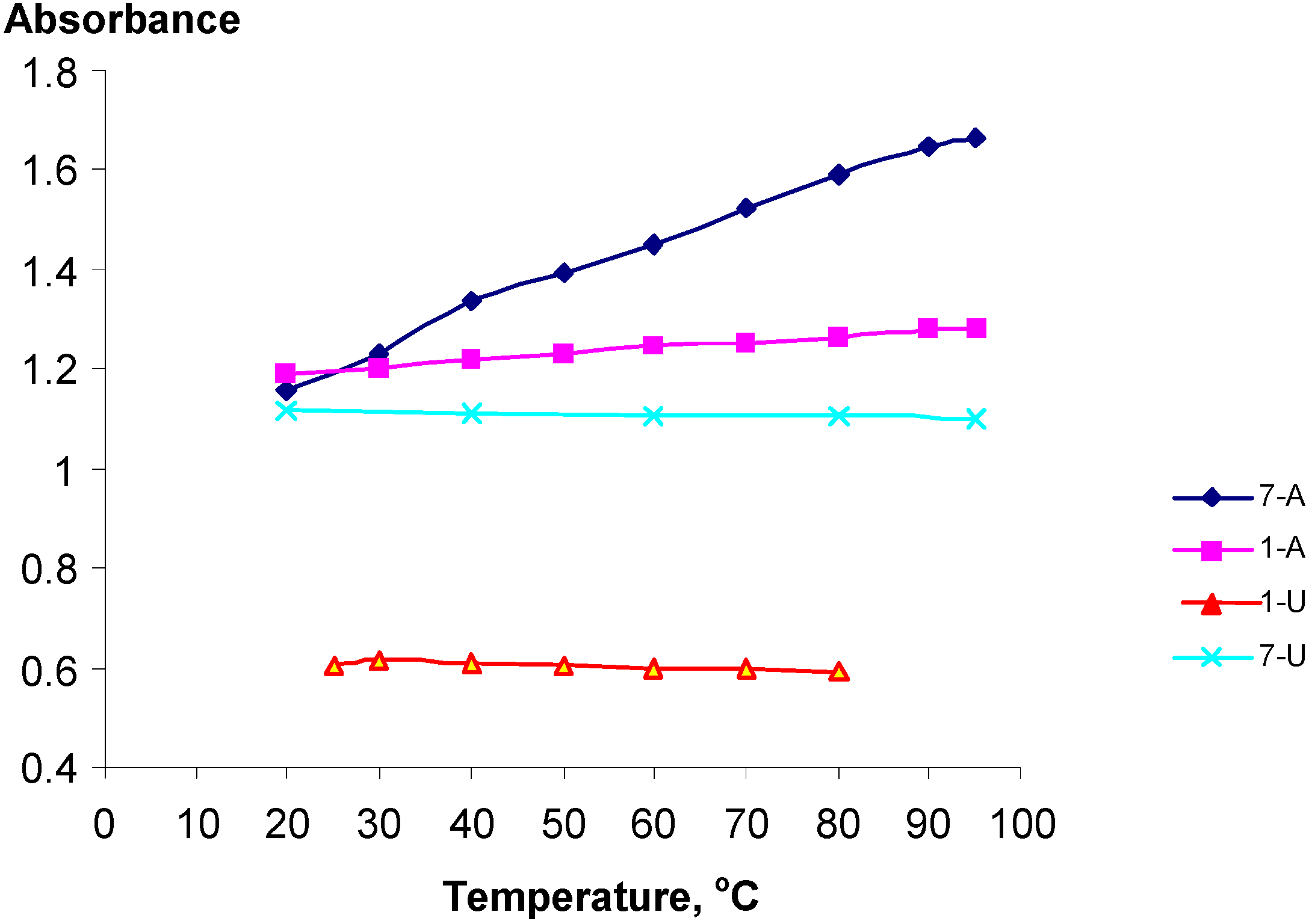
| Polynucleotide analog | 7-A6 | 1-A9 | 1-U8 | 7-U8 | 7-A6 + 7-U8 |
| h (%) | 43 | 7 | -2.0 | -1.5 | 57 |
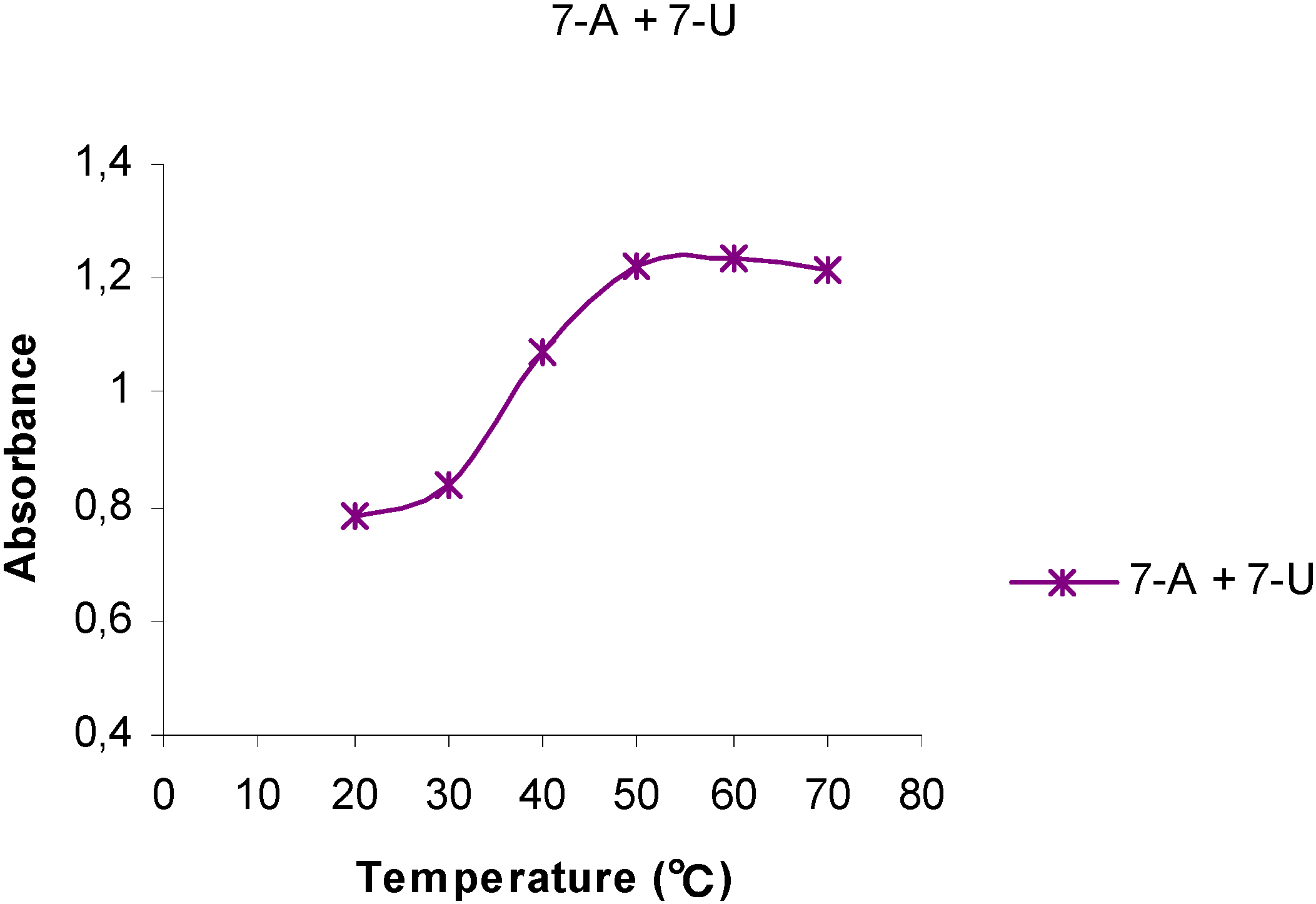
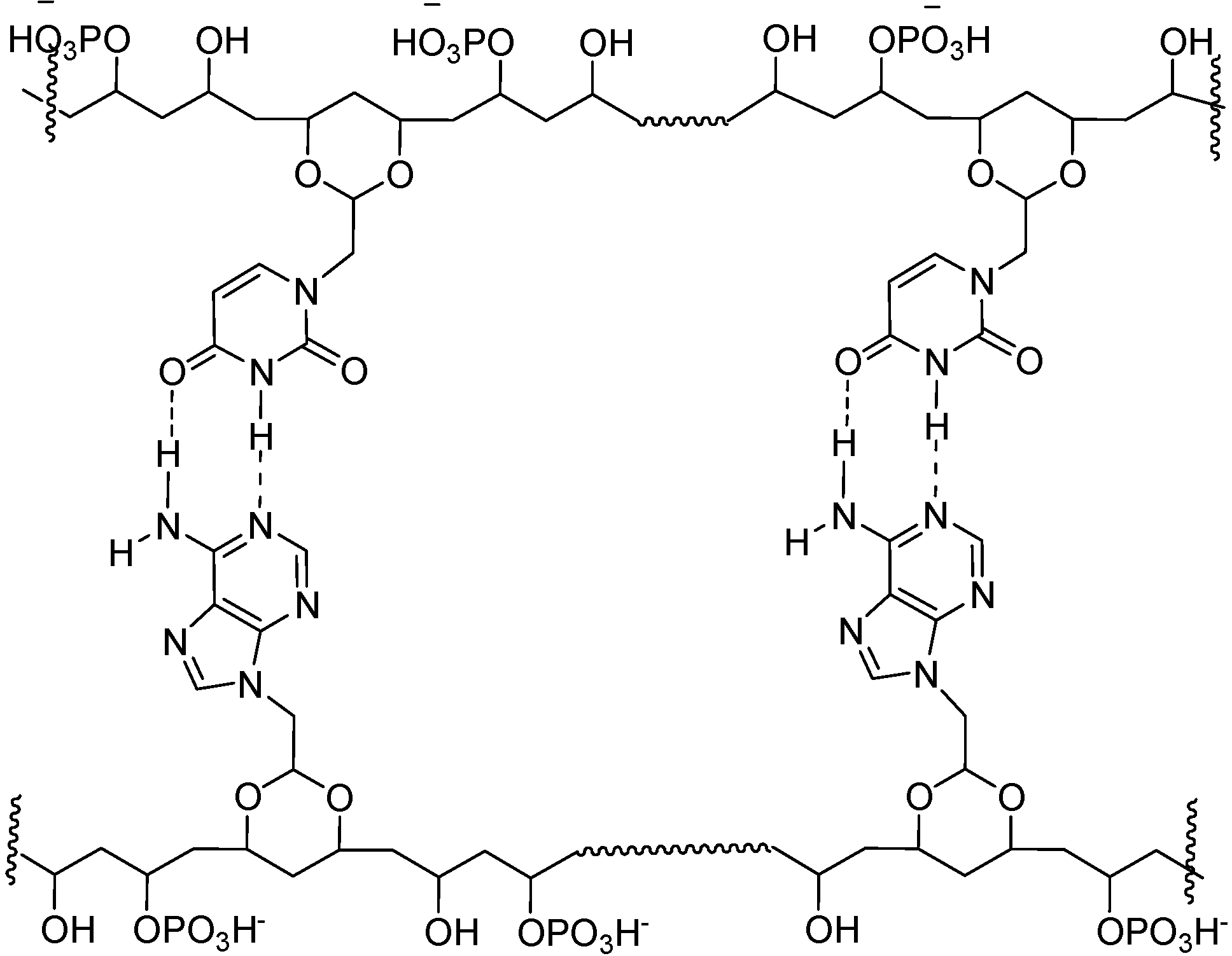
Conclusions
Experimental
General
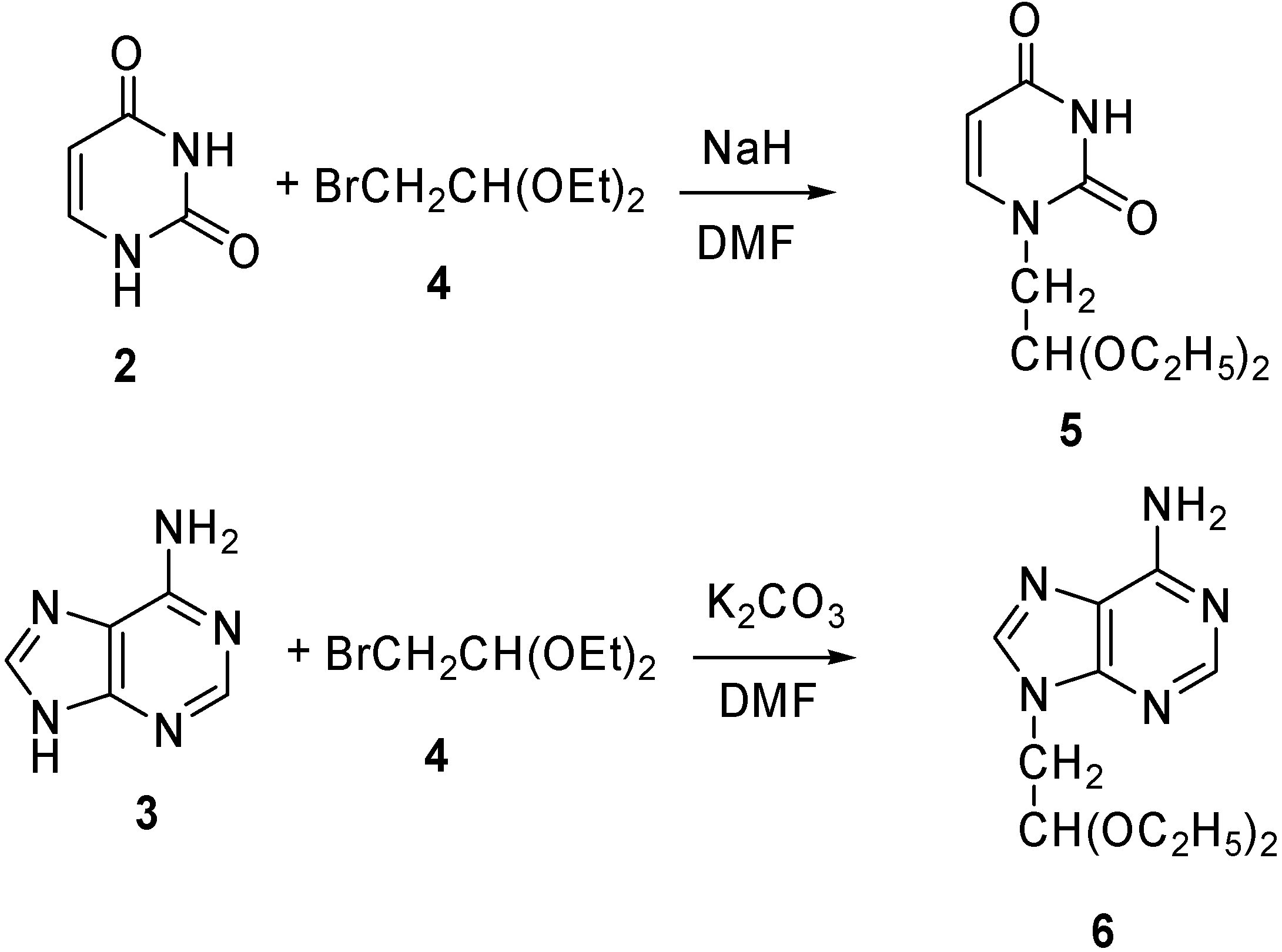
1- (2’,2’-Diethoxyethyl)-uracil (5) [32]
9-(2’,2’-Diethoxyethyl)-adenine (6)
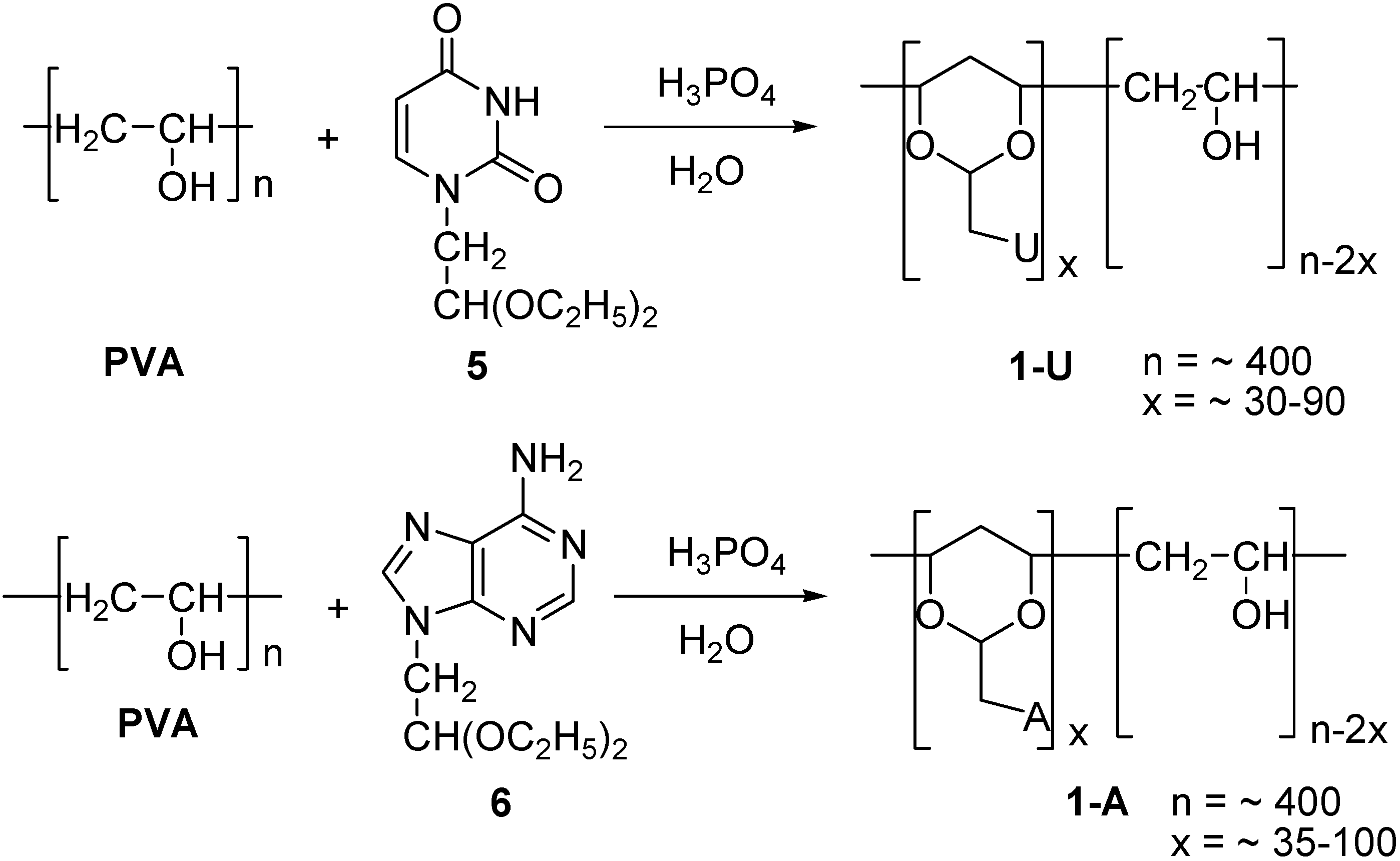
General procedure for the synthesis of adenyl- and uracinyl-1,3-dioxane substituted PVA
Acknowledgements
References
- Tokiwa, Y.; Fan, H.; Raku, T.; Kitagawe, M. Chemoenzymatic synthesis of nucleoside-branched poly(vinyl alcohol). ACS Symp. Ser. 2003, 840, 93–98. [Google Scholar] [CrossRef]
- Han, M.J.; Kim, Y.H.; Yoo, K.S.; Son, S.W.; Kang, Y.G.; Chang, J.Y. Poly(cytidylic acid) and poly(adenylic acid) analogs: synthesis and catalytic activity for hydrolysis of dinucleotides and nucleic acids. Polymer 2003, 44, 6475–6482. [Google Scholar] [CrossRef]
- Han, M.J.; Yoo, K.S.; Kim, Y-H.; Chang, J.Y. Polymeric enzyme mimics: catalytic activity of ribose-containing polymers for a phosphate substrate. Org. Biomol. Chem. 2003, 1, 2276–2282. [Google Scholar] [CrossRef]
- Pitha, J. Vinyl polymer analogs of nucleic acids. Polymer 1977, 18, 425–430. [Google Scholar] [CrossRef]
- Eliyahu, H.; Barenholz, Y.; Domb, A.J. Polymers for DNA delivery. Molecules 2005, 10, 34–64. [Google Scholar] [CrossRef]
- Ledley, F.D. Nonviral gene therapy: the promise of genes as pharmaceutical products. Hum. Gene Ther. 1995, 6, 1129–1144. [Google Scholar] [CrossRef]
- Safinya, C.R. Structures of lipid–DNA complexes: supramolecular assembly and gene delivery. Curr. Opin. Struct. Biol. 2001, 11, 440–448. [Google Scholar]
- Inaki, Y. Synthetic nucleic acid analogs. Prog. Polym. Sci. 1992, 17, 515–570. [Google Scholar] [CrossRef]
- Letourneur, D.; Douzon, C.; Jozefowicz, M. Synthesis and characterization of phosphorylated polystyrene derivatives for use in chromatography: DNA-like and phospholipid like behavior. J. Polym. Sci. A: Polymer. Chem. 1991, 29, 1367–1377. [Google Scholar] [CrossRef]
- Ueno, Y.; Kato, T.; Sato, K.; Ito, Y.; Yoshida, M.; Inoue, T.; Shibata, A.; Ebihara, M.; Kitade, Y. Synthesis and properties of nucleic acid analogues consisting of a benzene-phosphate backbone. J. Org. Chem. 2005, 70, 7925–7935. [Google Scholar] [CrossRef]
- Li, B.S.; Chen, J.; Zhu, C.F.; Leung, K.K.L.; Wan, L.; Bai, C.; Tang, B.Z. Formation of porous films and vesicular fibers via self-organization of an amphiphilic chiral oligomer. Langmuir 2004, 20, 2515–2518. [Google Scholar] [CrossRef]
- Smith, W.T., Jr.; Kluttz III, W.F.; Palmieri, D.; Butterfield, D.A. Adenine and thymine grafts on polyethylenimine. J. Polym. Sci. A: Polym. Chem. 1989, 27, 575–582. [Google Scholar] [CrossRef]
- Egholm, M.; Nielsen, P.E.; Buchard, O.; Berg, R.H. Recognition of guanine and adenine in DNA by cytosine and thymine containing peptide nucleic acids (PNA). J. Am. Chem. Soc. 1992, 114, 9677–9678. [Google Scholar] [CrossRef]
- Kim, S.K.; Nielsen, P.E.; Egholm, M.; Buckhard, O.; Berg, R.H.; Norden, B. Right-handed triplex formed between peptide nucleic acid PNA-T8 and poly(dA) shown by linear and circular dichroism spectroscopy. J. Am. Chem. Soc. 1993, 115, 6477–6481. [Google Scholar] [CrossRef]
- Wittung, P.; Erikson, M.; Lyng, R.; Nielsen, P.E.; Norden, B. Induced Chirality in PNA-PNA Duplexes. J. Am. Chem. Soc. 1995, 117, 10167–10173. [Google Scholar] [CrossRef]
- Egholm, M.; Buchard, O.; Nielsen, P.E.; Berg, R.H. Peptide nucleic acids (PNA). Oligonucleo- tide analogs with an achiral peptide backbone. J. Am. Chem. Soc. 1992, 114, 1895–1897. [Google Scholar] [CrossRef]
- Overberger, C.G.; Lu, C. Synthesis of poly(vinyl alcohol) containing asymmetric nucleic acid base derivatives as grafted pendants. J. Polym. Sci. A. Polym. Chem. 1985, 23, 1321–1332. [Google Scholar] [CrossRef]
- Overberger, C.G.; Chang, J.Y. Synthesis of optically active polynucleotide analogs with poly (vinyl alcohols) as backbones and adenine and 5-bromouracil derivatives as pendants. J. Polym. Sci A. Polym. Chem. 1989, 27, 3589–3602. [Google Scholar] [CrossRef]
- Overberger, C.G.; Chang, J.Y.; Gunn, V.E. Synthesis of optically active polynucleotide analogs with polytrimethylenimine backbones and thymine moieties. J. Polym. Sci. A. Polym. Chem. 1989, 27, 99–106. [Google Scholar]
- Seita, T.; Yamauchi, K.; Kinoshita, M.; Imoto, M. Synthesis of poly(vinyl alcohol) with pending N-derivatives of nucleic acid bases. Makromol. Chem. 1972, 154, 263–269. [Google Scholar] [CrossRef]
- Seita, T.; Kinoshita, M.; Imoto, M. Synthesis of poly(vinyl alcohol) combined with two kinds of nucleic acid bases and its optical behavior. Makromol. Chem. 1973, 164, 345–347. [Google Scholar] [CrossRef]
- Hatanaka, K.; Takeshige, H.; Kanno, K-I.; Maruyama, A.; Oishi, J.; Kajihara, Y.; Hashimoto, H. New polymeric inhibitor of galactosyl transferase. J. Carbohydr. Chem. 1997, 16, 667–672. [Google Scholar] [CrossRef]
- Efimtseva, E.V.; Mikhailov, S.N.; Meshkov, S.V.; Lönneberg, H. Synthesis and physico- chemical properties of dioxolane nucleoside analogs. Acta Chem. Scand. 1992, 46, 1122–1126. [Google Scholar] [CrossRef]
- Bera, S.; Malik, L.; Bhat, B.; Carroll, S.S.; Hrin, R.; MacCoss, M.; McMasters, D.R.; Miller, M.D.; Moyer, G.; Olsen, D.B.; Schleif, W.A.; Tomassini, J.E.; Eldrup, A.B. Synthesis and biological evaluation of 5R- and 5S-methyl substituted D- and L-configuration 1,3-dioxolane nucleoside analogs. Bioorg. Med. Chem. 2004, 12, 6237–6247. [Google Scholar] [CrossRef]
- Kim, H.O.; Ahn, S.K.; Alves, A.J.; Beach, J.W.; Jeong, L.S.; Choi, B.G.; van Roey, P.; Schinazi, R.F.; Chu, C.K. Aymmetric synthesis of 1,3-dioxolane-pyrimidine nucleosides and their anti-HIV activity. J. Med. Chem. 1992, 35, 1987–1995. [Google Scholar] [CrossRef]
- Bednarski, K.; Dixit, D.M.; Mansour, T.S.; Colman, S.G.; Walcott, S.M.; Ashman, C. Synthesis of racemic 2-phosphonomethyl-1,3-dioxolane nucleoside analogs as potential antiviral agents. Bioorg. Med. Chem. Lett. 1995, 15, 1741–1744. [Google Scholar]
- Eschenmoser, A.; Dobler, M. Chemistry of α-amino nitriles. 5. Why pentose and not hexose nucleic acids? Part I. Introduction to the problem, conformational analysis of oligonucleotide single strands containing 2',3'-dideoxyglucopyranosyl building blocks (homo-DNA), and reflections on the conformation of A- and B-DNA. Helv. Chim. Acta 1992, 75, 218–259. [Google Scholar] [CrossRef]
- Böhringer, M.; Roth, H-J.; Hunziker, J.; Göbel, M.; Krishnan, R.; Giger, A.; Schweizer, B.; Schreiber, J.; Leumann, C.; Eschenmoser, A. Why pentose and not hexose nucleic acids? Part II. Preparation of oligonucleotides containing 2',3'-dideoxy-⌷ -D-glucopyranosyl building blocks. Helv. Chim. Acta 1992, 75, 1416–1477. [Google Scholar] [CrossRef]
- Hunziker, J.; Roth, H-J.; Böhringer, M.; Geiger, A.; Diederichsen, U.; Göbel, M.; Krishnan, R.; Jaun, B.; Leuman, C.; Eschenmoser, A. Why pentose- and not hexose-nucleic acids? Part III. Oligo(2',3'-dideoxy-α-D-glucopyranosyl)nucleotides. ('Homo-DNA'): base-pairing properties. Helv. Chim. Acta 1993, 76, 259–352. [Google Scholar] [CrossRef]
- Pitsch, S.; Wendeborn, S.; Jaun, B.; Eschenmoser, A. Why pentose- and not hexose-nucleic acids? Part VII. Pyranosyl-RNA('p-RNA'). Helv. Chim. Acta 1993, 76, 2161–2183. [Google Scholar] [CrossRef]
- Wilds, C.J.; Wawrzak, Z.; Krisnamurthy, R.; Eschenmoser, A.; Egli, M. Crystal Structure of a B-Form DNA Duplex Containing (L)-Threofuranosyl (3', 2') Nucleosides: A Four-Carbon Sugar Is Easily Accommodated into the Backbone of DNA. J. Am. Chem. Soc. 2002, 124, 13716–13721. [Google Scholar] [CrossRef]
- Martinez, A.P.; Lee, W.W. An improved synthesis of willardiine and 1-(2',2'-diethoxyethyl) Uracil. J. Org. Chem. 1965, 30, 317–318. [Google Scholar] [CrossRef]
- Holy, A.; Votruba, I.; Clercq, E. D. Studies on S-adenosyl-L-homocysteine hydrolase. Part V. Synthesis and antiviral activity of stereoisomeric eritadenines. Coll. Czech. Chem. Commun. 1982, 47, 1392–1407. [Google Scholar] [CrossRef]
- Ichimura, K. Preparation of water-soluble photoresist derived from poly(vinyl alcohol). J. Polym. Sci. 1982, 20, 1411–1417. [Google Scholar]
- Ferrel, R. E.; Olcott, H. S.; Fraenkel-Conrat, H. Phosphorylation of proteins with phosphoric acid containing excess phosphorus pentoxide. J. Am. Chem. Soc. 1948, 70, 2101–2117. [Google Scholar] [CrossRef]
- Daul, G.C.; Reid, J.D.; Reinhardt, R.M. Cation-exchange materials from cotton and polyvinyl phosphate. Ind. Eng. Chem. 1954, 46, 1042–1045. [Google Scholar] [CrossRef]
- Fazakerley, G.V.; Sowers, L.C.; Eritja, R.; Kaplan, B.E.; Goodman, M.F. NMR studies on an oligodeoxynucleotide containing 2-aminopurine opposite adenine. Biochemistry 1987, 26, 5641–5646. [Google Scholar] [CrossRef]
- Shapiro, R. The prebiotic role of adenine: A critical analysis. Origins Life Evol B. 1995, 25, 83–98. [Google Scholar] [CrossRef]
- Newcomb, L.F.; Gellman, S.H. Aromatic Stacking Interactions in Aqueous Solution: Evidence that neither Classical Hydrophobic Effects nor Dispersion Forces Are Important. J. Am. Chem. Soc. 1994, 116, 4993–4994. [Google Scholar] [CrossRef]
- Marmur, J.; Doty, P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J. Mol. Biol. 1962, 5, 109–118. [Google Scholar] [CrossRef]
- Jaeger, J.A.; Turner, D.H.; Zuker, M. Improved predictions of secondary structures for RNA. Proc. Natl. Acad. Sci. U.S.A. 1989, 86, 7706–7710. [Google Scholar] [CrossRef]
- Ts’o, P.O.P. Basic Principles in Nucleic Acid Chemistry; Academic Press: N.Y., 1974; pp. 453–584. [Google Scholar]
- Lai, T.F.; Marsh, R.E. The Crystal Structure of Adenosine. Acta Cryst. B. 1972, B28, 1982–1989. [Google Scholar]
- Caillet, J.; Claverie, P. Differences of nucleotide stacking patterns in a crystal and in binary complexes. Case of adenine. Biopolymers 1974, 13, 601–614. [Google Scholar] [CrossRef]
- SantaLucia, J., Jr.; Turner, D.H. Structure of (rGGCGAGCC)2 in solution from NMR and restrained molecular dynamics. Biochemistry 1993, 32, 12612–12623. [Google Scholar] [CrossRef]
- Leininger, M.L.; Nielsen, I.M.B.; Colvin, M.E.; Janssen, C.L. Accurate Structures and Binding Energies for Stacked Uracil Dimers. J. Phys. Chem. A. 2002, 106, 3850–3854. [Google Scholar] [CrossRef]
- Hobza, P.; Sponer, J. Toward true DNA base-stacking energies: MP2, CCSD(T), and complete basis set calculations. J. Am. Chem. Soc. 2002, 124, 11802–11808. [Google Scholar] [CrossRef]
- SantaLucia, J., Jr.; Kierzek, R.; Turner, D.H. Stabilities of consecutive A.C, C.C, G.G, U.C, and U.U mismatches in RNA internal loops: evidence for stable hydrogen-bonded U.U and C.C+ pairs. Biochemistry 1991, 30, 8242–8251. [Google Scholar] [CrossRef]
- Samples Availability: Contact the author.
© 2008 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Yu, Q.; Carlsen, P. Synthesis of Polynucleotide Analogs Containing a Polyvinyl Alcohol Backbone. Molecules 2008, 13, 701-715. https://doi.org/10.3390/molecules13030701
Yu Q, Carlsen P. Synthesis of Polynucleotide Analogs Containing a Polyvinyl Alcohol Backbone. Molecules. 2008; 13(3):701-715. https://doi.org/10.3390/molecules13030701
Chicago/Turabian StyleYu, Qiang, and Per Carlsen. 2008. "Synthesis of Polynucleotide Analogs Containing a Polyvinyl Alcohol Backbone" Molecules 13, no. 3: 701-715. https://doi.org/10.3390/molecules13030701




