Isolation, Synthesis and Structures of Cytotoxic Ginsenoside Derivatives
Abstract
:Introduction
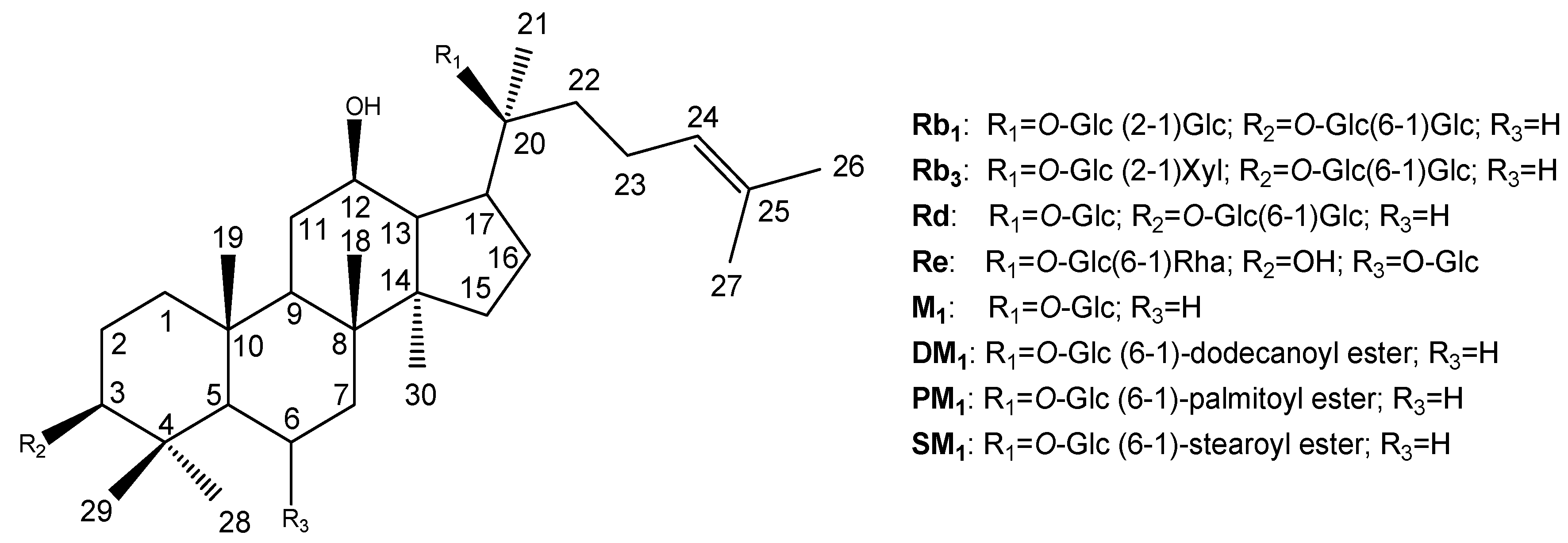
Results and Discussion
Characterizations of compounds 1 – 8
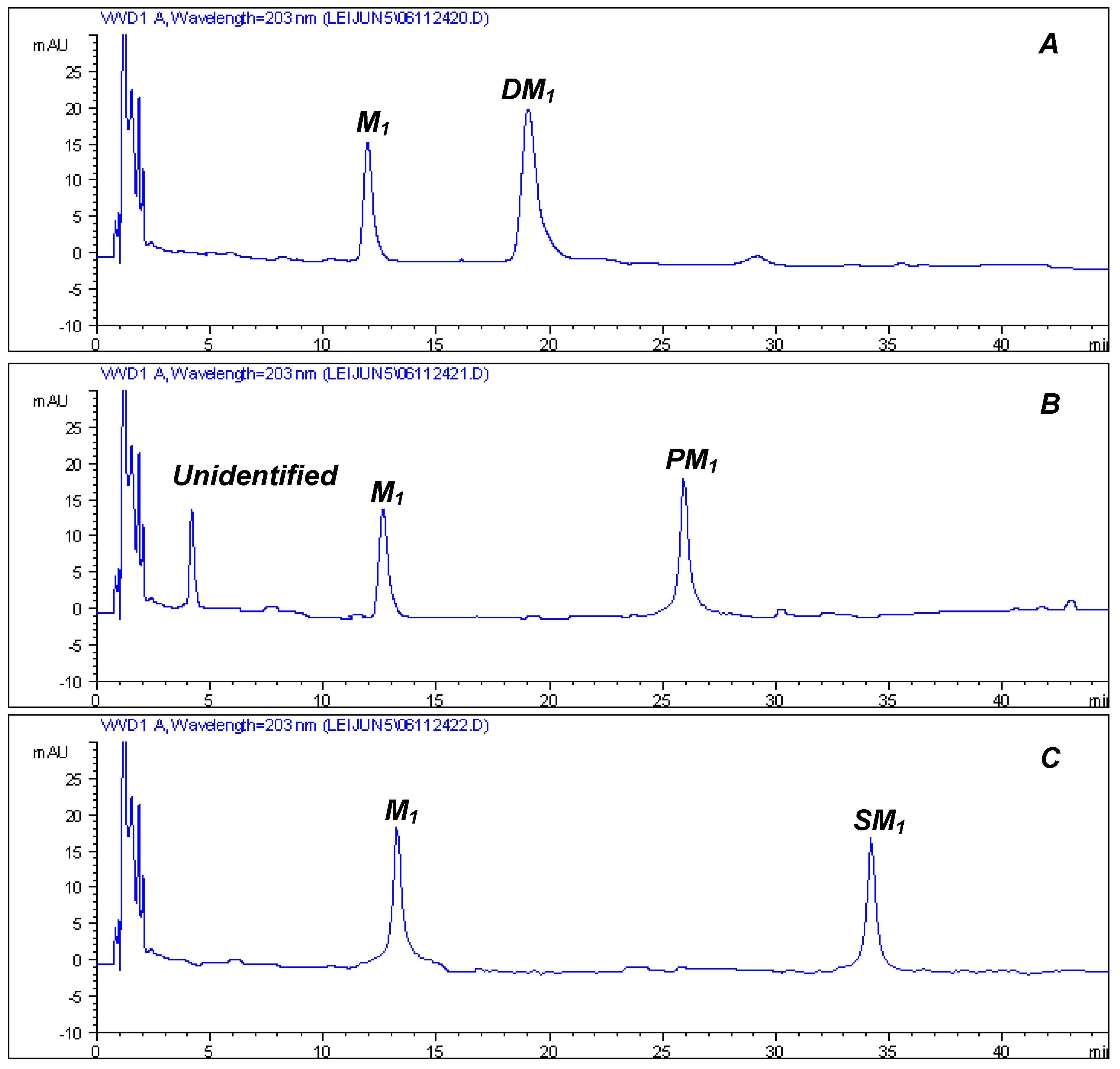
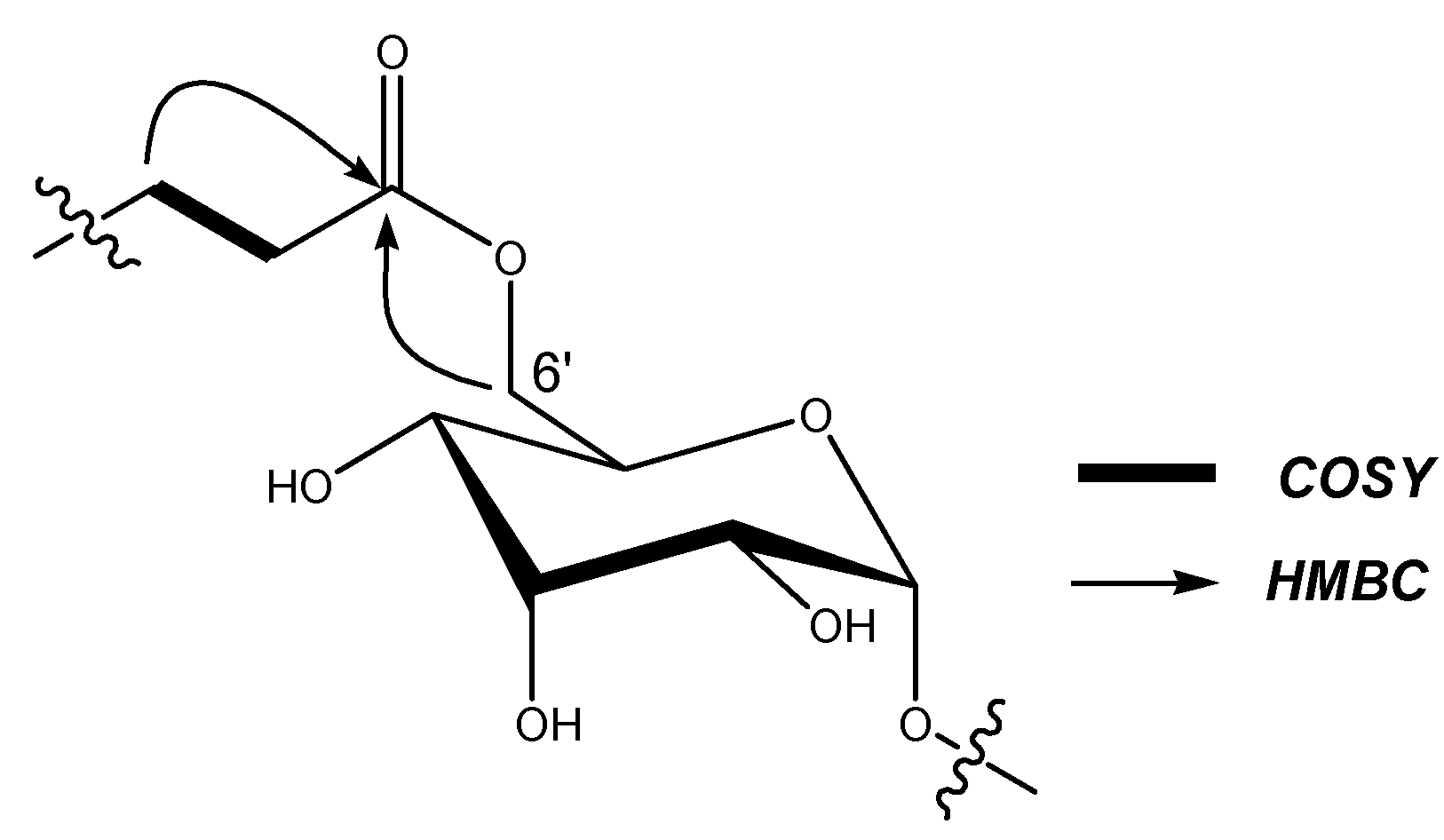
| Position | Rb1 (1) | Rb3 (2) | Rd (3) | Re (4) | M1 (5) | DM1 (6) | PM1 (7) | SM1 (8) |
|---|---|---|---|---|---|---|---|---|
| 1 | 39.2 | 39.3 | 39.2 | 39.7 | 39.3 | 38.9 | 39.5 | 39.1 |
| 2 | 26.3 | 26.3 | 26.7 | 26.6 | 28.1 | 27.3 | 28.2 | 28.0 |
| 3 | 88.9 | 89.0 | 89.0 | 78.1 | 77.9 | 78.8 | 78.0 | 77.8 |
| 4 | 39.6 | 39.6 | 39.1 | 39.9 | 39.4 | 38.9 | 39.4 | 39.3 |
| 5 | 56.1 | 56.7 | 56.3 | 60.7 | 56.2 | 55.8 | 56.4 | 56.1 |
| 6 | 18.6 | 18.6 | 18.5 | 74.2 | 18.6 | 18.2 | 18.7 | 18.5 |
| 7 | 35.1 | 35.2 | 35.1 | 45.8 | 35.0 | 34.7 | 35.2 | 34.9 |
| 8 | 39.8 | 39.9 | 40.4 | 41.3 | 39.9 | 39.7 | 39.5 | 39.8 |
| 9 | 50.0 | 50.2 | 50.2 | 49.5 | 50.2 | 49.8 | 50.3 | 50.0 |
| 10 | 36.7 | 37.0 | 37.1 | 39.6 | 37.2 | 37.0 | 37.3 | 37.1 |
| 11 | 30.8 | 31.0 | 31.1 | 30.7 | 30.7 | 30.3 | 30.7 | 30.7 |
| 12 | 70.1 | 70.1 | 70.2 | 70.1 | 70.1 | 70.6 | 70.1 | 69.8 |
| 13 | 49.3 | 49.5 | 49.6 | 49.4 | 49.3 | 47.8 | 49.5 | 49.3 |
| 14 | 51.3 | 51.4 | 51.5 | 51.3 | 51.3 | 51.7 | 51.4 | 51.3 |
| 15 | 30.8 | 30.8 | 30.7 | 30.9 | 30.7 | 30.6 | 31.0 | 30.5 |
| 16 | 26.6 | 26.8 | 26.6 | 26.6 | 26.5 | 26.6 | 26.7 | 26.5 |
| 17 | 51.5 | 51.6 | 51.6 | 51.6 | 51.5 | 51.3 | 51.6 | 51.2 |
| 18 | 16.0 | 16.0 | 17.7 | 17.7 | 16.2 | 16.1 | 16.3 | 16.1 |
| 19 | 16.1 | 16.2 | 16.4 | 17.8 | 15.9 | 15.7 | 16.0 | 15.8 |
| 20 | 83.1 | 83.5 | 83.1 | 83.3 | 83.2 | 84.3 | 83.4 | 83.1 |
| 21 | 22.3 | 22.3 | 22.4 | 22.4 | 22.3 | 21.2 | 22.9 | 22.7 |
| 22 | 36.2 | 36.1 | 36.2 | 35.8 | 36.0 | 35.4 | 36.1 | 35.9 |
| 23 | 23.0 | 23.0 | 23.2 | 23.3 | 23.1 | 22.0 | 23.0 | 22.8 |
| 24 | 125.8 | 126.0 | 125.8 | 126.0 | 125.8 | 124.5 | 126.0 | 125.8 |
| 25 | 131.1 | 131.0 | 131.0 | 130.9 | 130.8 | 131.5 | 130.9 | 130.7 |
| 26 | 25.8 | 25.8 | 25.7 | 25.8 | 25.7 | 25.7 | 25.8 | 25.5 |
| 27 | 17.9 | 17.9 | 18.0 | 17.6 | 17.7 | 17.6 | 17.8 | 17.6 |
| 28 | 28.0 | 28.3 | 28.1 | 32.3 | 28.6 | 28.0 | 28.6 | 28.4 |
| 29 | 16.6 | 16.5 | 16.3 | 17.3 | 16.2 | 15.3 | 16.3 | 16.1 |
| 30 | 17.1 | 17.4 | 17.7 | 17.3 | 17.3 | 16.9 | 17.4 | 17.2 |
| Position | M1 (5) | DM1 (6) | PM1 (7) | SM1 (8) | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | δC | δH | |
| 20-O-Glc | ||||||||
| 1’ | 98.1 | 5.14 (d, 7.5) | 96.8 | 4.51 (d, 7.6) | 98.0 | 5.11 (d, 7.5) | 97.8 | 5.14 (d, 7.5) |
| 2’ | 75.0 | 3.96 (t, 8.0) | 73.3 | 3.36 (m) | 75.0 | 3.95 (d. 8.0) | 74.7 | 3.92 (d. 8.0) |
| 3’ | 79.2 | 4.19 (m) | 76.7 | 3.55 (m) | 79.2 | 4.18 (m) | 79.0 | 4.19 (m) |
| 4’ | 71.5 | 4.12 (dd, 9.0, 8.5) | 70.0 | 3.43 (m) | 71.6 | 4.16 (m) | 71.4 | 4.17 (m) |
| 5’ | 78.2 | 3.88 (m) | 73.4 | 3.44 (m) | 78.0 | 3.98 (m) | 77.8 | 3.98 (m) |
| 6’ | 62.7 | 4.44, 4.27 (m) | 63.3 | 4.30, 4.38 (m) | 64.6 | 4.63, 5.02 (m) | 64.4 | 4.65, 5.05 (m) |
| Glc-6’-O-ester | ||||||||
| 1’’ | 174.1 | 173.5 | 173.3 | |||||
| 2’’ | 34.2 | 2.27, 2.35 (m) | 34.4 | 2.43, 2.48 (m) | 34.2 | 2.42, 2.49 (m) | ||
| 3’’ | 24.8 | 1.27c | 25.3 | 1.42 | 25.0 | 1.45 | ||
| 4’’ | 29.6 c | 1.27c | 29.4 | 1.27c | 30.7 | 1.27c | ||
| 5’’ | 29.1 c | 1.27c | 28.2 | 1.27c | 29.2 | 1.27c | ||
| 6’’ | 29.2 c | 1.27c | 29.6 | 1.27c | 29.3 | 1.27c | ||
| 7’’ | 29.3 c | 1.27c | 30.0c | 1.27c | 29.4 | 1.27c | ||
| 8’’ | 29.4 c | 1.27c | 30.0 c | 1.27c | 29.7 c | 1.27c | ||
| 9’’ | 29.6 c | 1.27c | 30.0 c | 1.27c | 29.7 c | 1.27c | ||
| 10’’ | 31.9 | 1.36 (m) | 30.0 c | 1.27c | 29.7 c | 1.27c | ||
| 11’’ | 22.6 | 1.56 (m) | 30.0 c | 1.27c | 29.7 c | 1.27c | ||
| 12’’ | 14.1 | 0.85 (t, 6.0) | 29.6 | 1.27c | 29.7 c | 1.27c | ||
| 13’’ | 29.8 | 1.27c | 29.5 | 1.27c | ||||
| 14’’ | 32.1 | 1.30 (m) | 30.5 | 1.27c | ||||
| 15’’ | 22.1 | 1.48 (m) | 29.7 | 1.27c | ||||
| 16’’ | 14.2 | 0.83 (t, 5.6) | 31.9 | 1.36 (m) | ||||
| 17’’ | 22.7 | 1.48 (m) | ||||||
| 18’’ | 14.0 | 0.85 (t, 0.56) | ||||||
Bioactivity Results
| M1 (5) | DM1 (6) | PM1 (7) | SM1 (8) | |
|---|---|---|---|---|
| MCF-7 | 8.48 | 0.50 | 2.31 | 1.65 |
| SK-MEL-2 | 14.71 | 1.46 | 1.88 | 0.17 |
| B16 | 6.10 | 6.13 | 5.73 | 0.33 |
| COR-L23 | 33.0 | 5.68 | 4.86 | 7.76 |
Conclusions
Experimental
General
Chemicals and reagents
Extraction and isolation
Enzymatic reaction
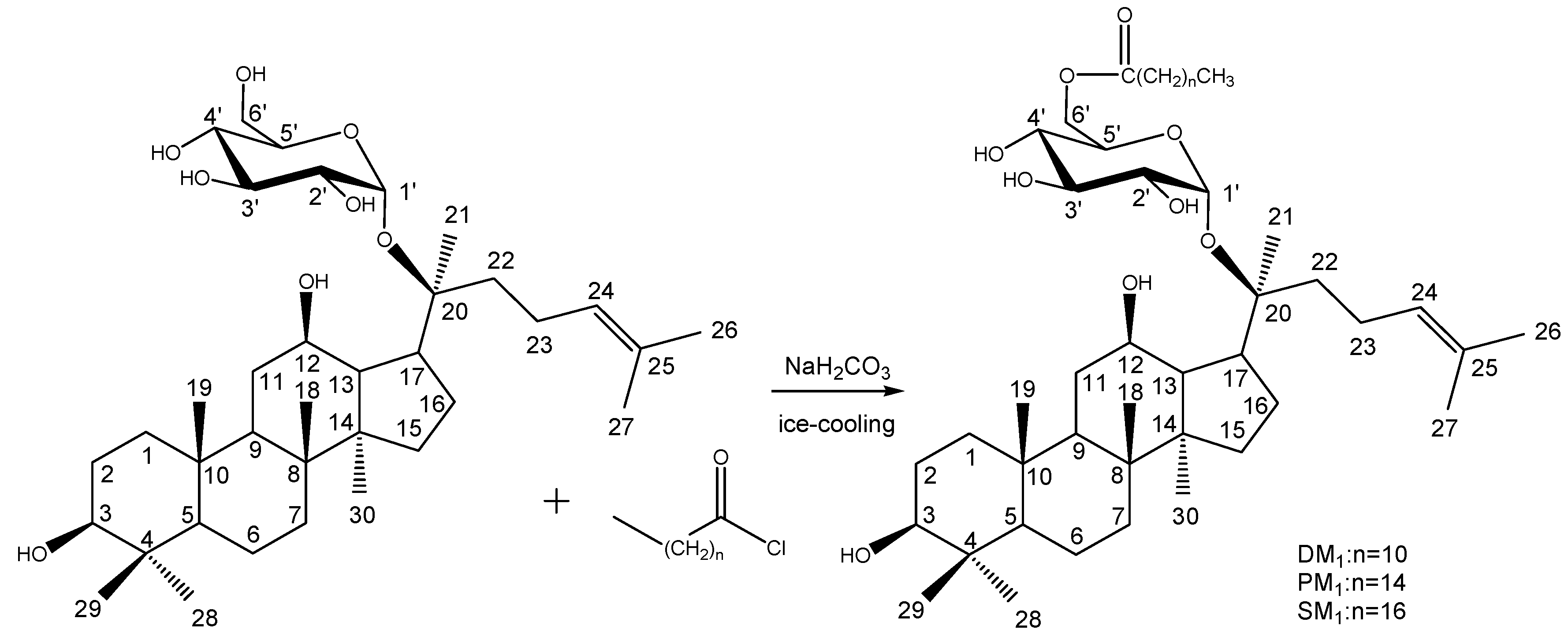
Synthesis of DM1, PM1 and SM1
In vitro cytotoxicity assays
Acknowledgments
References
- Ligor, T.; Ludwiczuk, A.; Wolski, T.; Buszewski, B. Isolation and determination of ginsenosides in American ginseng leaves and root extracts by LC-MS. Anal. Bioanal. Chem. 2005, 383, 1098–1105. [Google Scholar] [CrossRef]
- Liu, W.; Xu, S.; Che, C. Anti-proliferative efect of ginseng saponins on human prostate cancer cell line. Life Sci. 2000, 67, 1297–130. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Park, J.; Lee, S. Ginsenoside-Rs4, a new type of ginseng saponin concurrently induces apoptosis and selectively elevates protein levels of p53 and p21WAF1 in human hepotoma SK-HEP-1 cells. Eur. J. Cancer 1999, 35, 507–511. [Google Scholar] [CrossRef]
- Lee, K.; Lee, Y.; Kim, S.; Park, J.; Lee, S. Ginsenoside-Rg5 suppresses cyclin E-dependent protein kinase activity via up-regulation of p21 Cip/WAF1 and down-regulating cyclin E in SK-HEP-1 cells. Anticancer Res. 1997, 17, 1067–1072. [Google Scholar]
- Lee, Y.; Lee, H.; Lee, Y.; Chung, H.; Kim, S.; Lee, S.; Park, B.; Kim, K. Involvement of glucocorticoid receptor in the induction of differentiation by ginsenoside in F9 teratocarcinoma cells. J Steroid Biochem. Mol. Biol. 1998, 67, 105–111. [Google Scholar]
- Cho, W.; Chung, W.; Lee, S.; Leung, A.; Cheng, C.; Yue, K. Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2006, 550, 173–179. [Google Scholar] [CrossRef]
- Lee, W.; Kao, S.; Liu, I.; Cheng, J. Ginsenoside Rh2 is one of the active principles of Panax ginseng root to improve insulin sensitivity in fructose-rich chow-fed rats. Horm. Metab. Res. 2007, 39, 347–354. [Google Scholar] [CrossRef]
- Akao, T.; Kida, H.; Kanaoka, M.; Hattori, M.; Kobashi, K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J. Pharm. Pharmacol. 1998, 50, 1155–1160. [Google Scholar] [CrossRef]
- Wakabayashi, C.; Hasegawa, H.; Murata, J.; Saiki, I. In vivo anti-metastatic action of ginseng proto-panaxadiol saponins is based on their intestinal metabolites after oral administration. Oncol. Res. 1997, 9, 411–417. [Google Scholar]
- Wakabayashi, C.; Hasegawa, H.; Murata, J. The expression of in vivo anti-metastatic effect of ginseng protopanaxadiol saponins is mediated by their intestinal metabolites after oral administration. J. Trad. Med. 1997, 14, 180–185. [Google Scholar]
- Hasegawa, H.; Sung, J.; Huh, J. Ginseng intestinal bacterial metabolite IH901 as a new anti-metastatic agent. Arch. Pharm. Res. 1997, 20, 539–544. [Google Scholar]
- Hasegawa, H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with Fatty Acid. J. Pharmacol. Sci. 2004, 95, 153–157. [Google Scholar]
- Tawab, M.; Bahr, U.; Karas, M.; Wurglics, M. Degeneration of ginsenosides in humans after oral administration. Drug Metab. Dispos. 2003, 31, 1065–1071. [Google Scholar] [CrossRef]
- Lee, J.; Shin, J.; Chun, K.; Park, K.; Chung, W.; Bang, Y.; Sung, J.; Surh, Y. Antitumor promotional effects of a novel intestinal bacterial metabolite (IH-901) derived from the protopanaxadiol-type ginsenosides in mouse skin. Carcinogenesis 2005, 26, 359–367. [Google Scholar]
- Hasegawa, H.; Lee, K.; Nagaoka, T.; Tezuka, Y.; Uchiyama, M.; Kadota, S.; Saiki, I. Pharmacokinetics of ginsenoside deglycosylated by intestinal bacteria and its transformation to biologically active fatty acid esters. Biol. Pharm. Bull. 2000, 23, 298–304. [Google Scholar]
- Li, X.; Li, X.; Lei, J. Studies on chemical components and their pharmacological activities of Panax ginseng root. Drug Discovery and Traditional Chinese Medicine: Science, Regulation, and Globalization; Kluwer Academic Publishers: Maryland, 2000; pp. 95–109. [Google Scholar]
- Wang, J.; Li, X.; Zheng, Y.; Yang, X. Isoginsenoside-Rh3, a new triterpenoid saponin from the fruits of Panax ginseng C. A. Mey. J. Asian Nat. Prod. Res. 2004, 6, 289–293. [Google Scholar] [CrossRef]
- Sun, G.; Liu, Z.; Li, X.; Zheng, Y.; Wang, J. Isolation and identification of two malonyl-ginsenosides from the fresh root of Panax Ginseng. Chin. J. Anal. Chem. 2005, 33, 1783–1786. [Google Scholar]
- Gong, X. Study on chemical modification of the enzyme of the enzyme metabolites and its anti-tumor activities. PHD dissertation of Jilin Agricultural University, 2004; pp. 96–103. [Google Scholar]
- Chen, J.; Peng, H.; Pu, S.; Guo, Y. Apoptosis induced by ginsenoside Rg3 in a human bladder carcinoma cell line. Chin. J. Clin. Oncol. 2006, 4, 283–287. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, S.; Huang, C.; Chen, J.; Chang, W.; Hsu, S. Molecular mechanisms of ginsenoside Rh2-mediated G1 growth arrest and apoptosis in human lung adenocarcinoma A549 cells. Cancer Chemother. Pharmacol. 2005, 55, 531–540. [Google Scholar] [CrossRef]
- Connolly, T.; Carruthers, A.; Melchior, D. Effects of bilayer cholesterol on human erythrocyte hexose transport protein activity in synthetic lecithin bilayers. Biochemistry 1985, 24, 2865–2873. [Google Scholar] [CrossRef]
- Baruch, B.; Lina, W.; Einat, B.; Jardena, N.; Jacob, S.; Haim, G.; Eyal, F. Variable cytotoxicity of amifostine in malignant and non-malignant cell lines. Oncol. Rep. 2003, 10, 1609–1613. [Google Scholar]
- Sample availability: From the author.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Lei, J.; Li, X.; Gong, X.-j.; Zheng, Y.-n. Isolation, Synthesis and Structures of Cytotoxic Ginsenoside Derivatives. Molecules 2007, 12, 2140-2150. https://doi.org/10.3390/12092140
Lei J, Li X, Gong X-j, Zheng Y-n. Isolation, Synthesis and Structures of Cytotoxic Ginsenoside Derivatives. Molecules. 2007; 12(9):2140-2150. https://doi.org/10.3390/12092140
Chicago/Turabian StyleLei, Jun, Xiang Li, Xiao-jie Gong, and Yi-nan Zheng. 2007. "Isolation, Synthesis and Structures of Cytotoxic Ginsenoside Derivatives" Molecules 12, no. 9: 2140-2150. https://doi.org/10.3390/12092140




