Two Novel Lanostane Triterpenoids from Ganoderma Sinense
Abstract
:Introduction
Results and Discussion
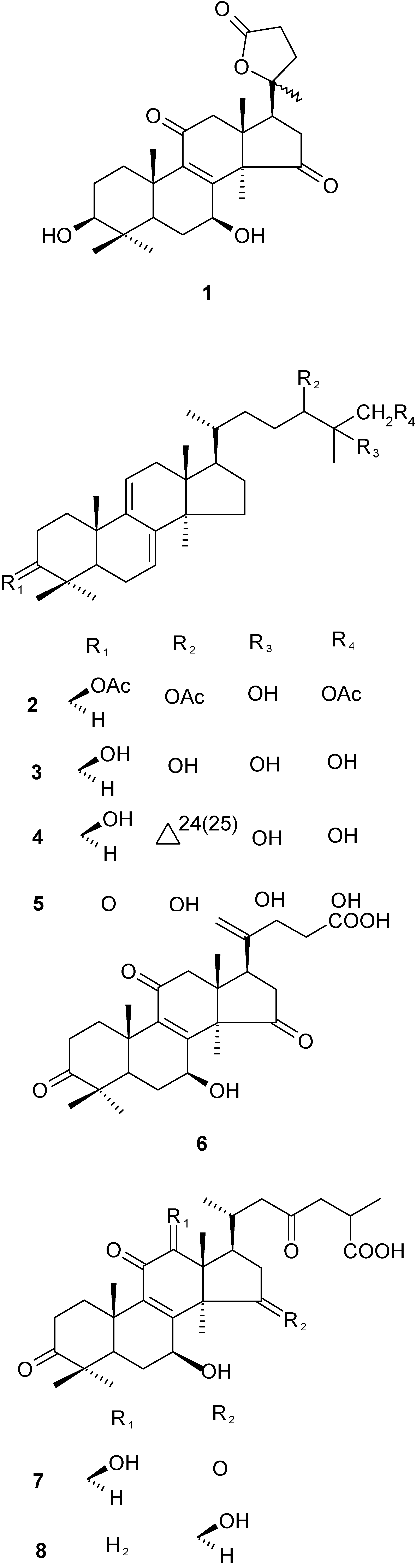
 183°(c=0.3, MeOH). Compound 1 gave a quasi-molecular ion peak at m/z 459 [M+H]+ in its positive FABMS spectrum, and was assigned a molecular formula of C27H38O6, which was confirmed by HRESIMS (C27H38O6Na, 481.2579; calcd. for C27H38O6Na, 481.2566) and NMR spectral data (Table 1). The IR spectrum [3433 (br, OH), 1769, 1719, 1655 (C=O), 1207, 1143 and 1065 (C-O and COOC)] indicated the presence of hydroxyl and carbonyl groups. The UV data [(MeOH) λmax(logε) nm: 255 (0.17)] indicated the presence of an α,β-unsaturated carbonyl group. The 1H-NMR spectrum of 1 (Table 1), analyzed together with the compound’s HMQC spectrum, exhibited singlet signals (3H each) of six methyl groups at δH 1.07, 1.24, 1.36, 1.39, 1.40, 1.44 and two oxygenated methine protons at δH 3.46 (1H, dd, J=5.5, 11.0Hz, 3α-H) and 5.19 (1H, t, 8.4Hz, 7α-H). The 13C-NMR spectrum displayed signals characteristic of six methyl groups, an oxygen-bearing quaternary carbon at δc 86.49 (s), two hydroxyl-bearing methine carbons at δc 77.59 (d) and 66.70 (d), an α,β-unsaturated C=O at δc 197.98 (s), 158.71 (s), 142.65 (s), a ketone carbon at δc 214.96 (s), and a carboxyl group at δc 176.69 (s). These data suggested a polyoxygenated lanostane-type triterpene with a structure very similar to Ganolactone A, including its chemical configuration at C-20 [11]. Ganolactone B (1) had a signal at δc 86.49 (s). This indicated that linking group was at C-20. The connectivities of 1 were established by interpretation of the HMBC spectrum (Figure 2). In the HMBC experiment, δc 86.49 (s, C-20) showed correlation with two secondary methyl group signals δH 2.81, 2.66 (each 1H, H-16), δH 2.68, 2.49 (each 1H, H-22) and one methyl signal δH 1.39 (3H, H-21). From the above observation and on the basis of all the above results the structure of Ganolactone B was established as 3β,7β-dihydroxy-11,15-dioxo-lanosta-8-en-24→20 lactone.
183°(c=0.3, MeOH). Compound 1 gave a quasi-molecular ion peak at m/z 459 [M+H]+ in its positive FABMS spectrum, and was assigned a molecular formula of C27H38O6, which was confirmed by HRESIMS (C27H38O6Na, 481.2579; calcd. for C27H38O6Na, 481.2566) and NMR spectral data (Table 1). The IR spectrum [3433 (br, OH), 1769, 1719, 1655 (C=O), 1207, 1143 and 1065 (C-O and COOC)] indicated the presence of hydroxyl and carbonyl groups. The UV data [(MeOH) λmax(logε) nm: 255 (0.17)] indicated the presence of an α,β-unsaturated carbonyl group. The 1H-NMR spectrum of 1 (Table 1), analyzed together with the compound’s HMQC spectrum, exhibited singlet signals (3H each) of six methyl groups at δH 1.07, 1.24, 1.36, 1.39, 1.40, 1.44 and two oxygenated methine protons at δH 3.46 (1H, dd, J=5.5, 11.0Hz, 3α-H) and 5.19 (1H, t, 8.4Hz, 7α-H). The 13C-NMR spectrum displayed signals characteristic of six methyl groups, an oxygen-bearing quaternary carbon at δc 86.49 (s), two hydroxyl-bearing methine carbons at δc 77.59 (d) and 66.70 (d), an α,β-unsaturated C=O at δc 197.98 (s), 158.71 (s), 142.65 (s), a ketone carbon at δc 214.96 (s), and a carboxyl group at δc 176.69 (s). These data suggested a polyoxygenated lanostane-type triterpene with a structure very similar to Ganolactone A, including its chemical configuration at C-20 [11]. Ganolactone B (1) had a signal at δc 86.49 (s). This indicated that linking group was at C-20. The connectivities of 1 were established by interpretation of the HMBC spectrum (Figure 2). In the HMBC experiment, δc 86.49 (s, C-20) showed correlation with two secondary methyl group signals δH 2.81, 2.66 (each 1H, H-16), δH 2.68, 2.49 (each 1H, H-22) and one methyl signal δH 1.39 (3H, H-21). From the above observation and on the basis of all the above results the structure of Ganolactone B was established as 3β,7β-dihydroxy-11,15-dioxo-lanosta-8-en-24→20 lactone. ) correlations of Ganolactone B (1).
) correlations of Ganolactone B (1).
 80°(c=0.1, C5D5N) and a quasi-molecular ion peak at m/z 601 [M+H]+ in its positive FABMS spectrum, and was thus assigned a molecular formula of C36H56O7, which was confirmed by HRESIMS (C36H56O7Na, 623.3923; calcd. for C36H56O7Na, 623.3920) and NMR spectral data (Table 1). Its UV spectrum was similar to that of Ganoderiol A [7], indicating the presence a heteroannular diene system in this molecule. The IR spectrum [3492 (br, OH), 1735, 1719 (C=O), 1233, 1152 and 1031 (C-O and COOC)] indicated the presence of hydroxyl and carbonyl groups. The 1H-NMR spectrum of 2 showed singlets for three acetyl methyl group at δH 2.05, 2.07 and 2.10 (3H each). The 13C-NMR spectrum of 2 verified the presence of three carbons attached to oxygen at δc 68.45 (t), 76.64 (d) and 80.83 (d), three carbonyl carbons for acetyl moieties at δc 170.64 (s), 170.93 (s), and 171.11 (s), and three acetyl methyl carbons at δc 20.83 (q), 20.98 (q), and 21.29 (q). From these spectral data, the structure of this compound was established as 3β,24,26-triacetoxy-5α-lanosta-7, 9(11)-dien-25-ol, for which we propose the name Ganoderiol A triacetate.
80°(c=0.1, C5D5N) and a quasi-molecular ion peak at m/z 601 [M+H]+ in its positive FABMS spectrum, and was thus assigned a molecular formula of C36H56O7, which was confirmed by HRESIMS (C36H56O7Na, 623.3923; calcd. for C36H56O7Na, 623.3920) and NMR spectral data (Table 1). Its UV spectrum was similar to that of Ganoderiol A [7], indicating the presence a heteroannular diene system in this molecule. The IR spectrum [3492 (br, OH), 1735, 1719 (C=O), 1233, 1152 and 1031 (C-O and COOC)] indicated the presence of hydroxyl and carbonyl groups. The 1H-NMR spectrum of 2 showed singlets for three acetyl methyl group at δH 2.05, 2.07 and 2.10 (3H each). The 13C-NMR spectrum of 2 verified the presence of three carbons attached to oxygen at δc 68.45 (t), 76.64 (d) and 80.83 (d), three carbonyl carbons for acetyl moieties at δc 170.64 (s), 170.93 (s), and 171.11 (s), and three acetyl methyl carbons at δc 20.83 (q), 20.98 (q), and 21.29 (q). From these spectral data, the structure of this compound was established as 3β,24,26-triacetoxy-5α-lanosta-7, 9(11)-dien-25-ol, for which we propose the name Ganoderiol A triacetate.| No. | 1 | 2 | ||
| δc | δH (J = Hz) | δc | δH (J = Hz) | |
| C5D5N | CDCl3 | |||
| 1 | 35.47 t | 3.22 m (α) 1.20 m (β) | 35.41 t | - - |
| 2 | 28.59 t | 1.89 m (α) 1.89 m (β) | 22.77 t | - - |
| 3 | 77.59 d | 3.46 dd(10.96, 5.43) (α) | 80.83 d | 4.51 dd (12.68, 3.56) (α) |
| 4 | 49.68 s | - | 37.62 s | - |
| 5 | 49.65 d | 1.10 t(16.97, 12.99) (α) | 49.26 d | - |
| 6 | 28.14 t | 2.37 m (α) 1.90 m (β) | 24.26 t | - - |
| 7 | 66.70 d | 5.19 t(16.97, 12.99) (α) | 119.97 d | 5.45 m (α) |
| 8 | 158.71 s | - | 142.70 s | - |
| 9 | 142.65 s | - | 146.65 s | - |
| 10 | 39.29 s | - | 37.80 s | - |
| 11 | 197.98 s | - | 116.50 d | 5.30 d (4.64) |
| 12 | 50.94 t | 3.09 d(16.88) (α) 2.95 d(16.78) (β) | 37.24 t | - - |
| 13 | 45.78 s | - | 43.74 s | - |
| 14 | 59.03 s | - | 50.32 s | - |
| 15 | 214.96 s | - | 25.99 t | - |
| 16 | 36.53 t | 2.81 dd(18.30, 10.17) (α) 2.66 dd(18.30, 10.32) (β) | 31.45 t | - - |
| 17 | 49.65 d | 2.65 dd(11.98, 7.26) (α) | 50.77 d | - |
| 18 | 18.64 q | 1.40 s | 16.92 q | 0.54 s |
| 19 | 19.40 q | 1.36 s | 22.82 q | 0.99 s |
| 20 | 86.49 s | - | 36.45 d | - |
| 21 | 25.79 q | 1.39 s | 18.56 q | 0.94 d(5.28) |
| 22 | 27.84 t | 2.28 m (α) 2.49 m (β) | 32.62 t | - - |
| 23 | 34.20 t | 2.08 m (α) 1.85 m (β) | 27.78 t | - - |
| 24 | 176.69 s | - | 76.64 d | 4.89 dd(9.80, 1.96) |
| 25 | - | - | 73.27 s | - |
| 26 | - | - | 68.45 t | - |
| 27 | - | - | 20.19 q | 1.19 s |
| 28 | 25.39 q | 1.44 s | 28.08 q | 0.86 s |
| 29 | 16.47 q | 1.24 s | 15.66 q | 0.86 s |
| 30 | 28.75 q | 1.07 s | 25.53 q | 0.94 s |
| OCOMe | - | - | 170.64 s | - |
| - | - | 170.93 s | - | |
| - | - | 171.11 s | - | |
| OCOMe | - | - | 20.83 q | 2.05(3H, s) |
| - | - | 20.99 q | 2.07(3H, s) | |
| - | - | 21.29 q | 2.10(3H, s) | |
Experimental
General
Plant Material
Extraction and Isolation
 183°(c=0.3, MeOH); positive FABMS m/z 459[M+H]+, HRESIMS (calcd. for C27H38O6: 458.2579); IR(KBr)cm-1: 3433 (br, OH), 1769, 1719, 1655 (C=O), 1207, 1143 and 1065 (C-O and COOC); UV (MeOH) λmax (logε) nm:255(0.17); 1H- and 13C-NMR spectral data, see Table 1.
183°(c=0.3, MeOH); positive FABMS m/z 459[M+H]+, HRESIMS (calcd. for C27H38O6: 458.2579); IR(KBr)cm-1: 3433 (br, OH), 1769, 1719, 1655 (C=O), 1207, 1143 and 1065 (C-O and COOC); UV (MeOH) λmax (logε) nm:255(0.17); 1H- and 13C-NMR spectral data, see Table 1. 80°(c=0.1, C5D5N); positive FABMS m/z 601 [M+H]+; HRESIMS (calcd. for C36H56O7: 600.3920); IR (KBr) cm-1: 3492 (br, OH), 1735, 1719 (C=O), 1233, 1152 and 1031 (C-O and COOC); UV (MeOH) λmax (logε) nm: 243 (0.30); 1H- and 13C-NMR spectral data, see Table 1.
80°(c=0.1, C5D5N); positive FABMS m/z 601 [M+H]+; HRESIMS (calcd. for C36H56O7: 600.3920); IR (KBr) cm-1: 3492 (br, OH), 1735, 1719 (C=O), 1233, 1152 and 1031 (C-O and COOC); UV (MeOH) λmax (logε) nm: 243 (0.30); 1H- and 13C-NMR spectral data, see Table 1. +20°(c=0.10, EtOH); HREIMS m/z 474.3740 (calcd for C30H50O4: 474.3709); UV (EtOH) λmax (logε) nm: 253 (8058), 244 (962), 253 (6518); IR (KBr) cm-1: 3350, 2950, 2900, 2850, 1430, 1360, 1060; 1H-NMR (C5D5N) δH: 5.55 (1H, m, H-7), 5.40 (1H, m, H-12), 3.83, 3.47 (1H each, d, J=10.6Hz, 26-H), 3.46 (1H, t, J=12.1Hz, C-24), 3.24 (1H, dd, J=10.5 and 5.0 Hz, H-3), 1.11 (3H, s, H-27), 1.01 (3H, s, H-19), 0.98 (3H, s, H-28), 0.92 (3H, d, J=6.2Hz H-21), 0.88 (3H, s, H-29), 0.88 (3H, s, H-30), 0.67 (3H, s, H-18); 13C-NMR (C5D5N) δc: 36.40 (t, C-1), 28.86 (t, C-2), 78.12 (d, C-3), 39.37 (s, C-4), 49.83 (d, C-5), 23.56 (t, C-6), 120.99 (d, C-7), 143.03 (s, C-8), 146.61 (s, C-9), 37.85 (s, C-10), 116.58 (s, C-11), 38.14 (t, C-12), 44.13 (s,C-13), 50.68 (s, C-14), 28.17 (t, C-15), 31.91 (t, C-16), 51.50 (d, C-17), 16.64 (q, C-18), 23.11 (q, C-19), 37.15 (d, C-20), 19.06 (q, C-21), 34.40 (t, C-22), 28.94 (t, C-23), 77.23 (d, C-24), 74.79 (s, C-25), 69.28 (t, C-26), 20.15 (q,C-27), 28.94 (q, C-28), 16.07 (q, C-29), 25.89 (q, C- 30).
+20°(c=0.10, EtOH); HREIMS m/z 474.3740 (calcd for C30H50O4: 474.3709); UV (EtOH) λmax (logε) nm: 253 (8058), 244 (962), 253 (6518); IR (KBr) cm-1: 3350, 2950, 2900, 2850, 1430, 1360, 1060; 1H-NMR (C5D5N) δH: 5.55 (1H, m, H-7), 5.40 (1H, m, H-12), 3.83, 3.47 (1H each, d, J=10.6Hz, 26-H), 3.46 (1H, t, J=12.1Hz, C-24), 3.24 (1H, dd, J=10.5 and 5.0 Hz, H-3), 1.11 (3H, s, H-27), 1.01 (3H, s, H-19), 0.98 (3H, s, H-28), 0.92 (3H, d, J=6.2Hz H-21), 0.88 (3H, s, H-29), 0.88 (3H, s, H-30), 0.67 (3H, s, H-18); 13C-NMR (C5D5N) δc: 36.40 (t, C-1), 28.86 (t, C-2), 78.12 (d, C-3), 39.37 (s, C-4), 49.83 (d, C-5), 23.56 (t, C-6), 120.99 (d, C-7), 143.03 (s, C-8), 146.61 (s, C-9), 37.85 (s, C-10), 116.58 (s, C-11), 38.14 (t, C-12), 44.13 (s,C-13), 50.68 (s, C-14), 28.17 (t, C-15), 31.91 (t, C-16), 51.50 (d, C-17), 16.64 (q, C-18), 23.11 (q, C-19), 37.15 (d, C-20), 19.06 (q, C-21), 34.40 (t, C-22), 28.94 (t, C-23), 77.23 (d, C-24), 74.79 (s, C-25), 69.28 (t, C-26), 20.15 (q,C-27), 28.94 (q, C-28), 16.07 (q, C-29), 25.89 (q, C- 30). +9°(c=0.04, EtOH); HREIMS m/z 456.3612 (calcd for C30H48O3: 456.3605); UV (EtOH) λmax (logε) nm: 237 (4740), 245 (5400), 253 (3650); 1H-NMR (C5D5N) δH: 5.56 (1H, J=7.5 , H-24), 5.48 (1H, m, H-7), 5.32 (1H brd, J=6.2Hz, H-11), 4.33 (2H, s, H-27), 4.21 (2H, s, H-26), 3.24 (3H, dd, J=10.6 and 4.8 Hz H-3), 1.01 (3H, s, H-19), 0.98 (3H, s, H-28), 0.91 (3H, d , J=6.2Hz, H-21), 0.88 (3H, s, H-29), 0.88 (3H, s, H-30), 0.67 (3H, s, H-18); 13C-NMR (C5D5N) δc: 36.42 (t, C-1), 28.74 (t, C-2), 78.12 (d, C-3), 39.37 (s, C-4), 49.84 (d, C-5), 23.57 (t, C-6), 121.06 (d,C-7), 142.98 (s, C-8), 146.64 (s, C-9), 37.86 (s, C-10), 116.54 (s, C-11), 38.11 (t, C-12), 44.13 (s,C-13), 50.69 (s, C-14), 28.16 (t, C-15), 31.90 (t,C-16), 51.24 (d, C-17), 16.65 (q, C-18), 23.10 (q, C-19), 36.42 (d, C-20), 18.62 (q, C-21), 36.91 (t, C-22), 24.66 (t, C-23), 127.74 (d, C-24), 140.75 (s, C-25), 65.51 (t, C-26), 58.59 (q,C-27), 28.86 (q, C-28), 16.02 (q, C-29), 25.87 (q, C- 30).
+9°(c=0.04, EtOH); HREIMS m/z 456.3612 (calcd for C30H48O3: 456.3605); UV (EtOH) λmax (logε) nm: 237 (4740), 245 (5400), 253 (3650); 1H-NMR (C5D5N) δH: 5.56 (1H, J=7.5 , H-24), 5.48 (1H, m, H-7), 5.32 (1H brd, J=6.2Hz, H-11), 4.33 (2H, s, H-27), 4.21 (2H, s, H-26), 3.24 (3H, dd, J=10.6 and 4.8 Hz H-3), 1.01 (3H, s, H-19), 0.98 (3H, s, H-28), 0.91 (3H, d , J=6.2Hz, H-21), 0.88 (3H, s, H-29), 0.88 (3H, s, H-30), 0.67 (3H, s, H-18); 13C-NMR (C5D5N) δc: 36.42 (t, C-1), 28.74 (t, C-2), 78.12 (d, C-3), 39.37 (s, C-4), 49.84 (d, C-5), 23.57 (t, C-6), 121.06 (d,C-7), 142.98 (s, C-8), 146.64 (s, C-9), 37.86 (s, C-10), 116.54 (s, C-11), 38.11 (t, C-12), 44.13 (s,C-13), 50.69 (s, C-14), 28.16 (t, C-15), 31.90 (t,C-16), 51.24 (d, C-17), 16.65 (q, C-18), 23.10 (q, C-19), 36.42 (d, C-20), 18.62 (q, C-21), 36.91 (t, C-22), 24.66 (t, C-23), 127.74 (d, C-24), 140.75 (s, C-25), 65.51 (t, C-26), 58.59 (q,C-27), 28.86 (q, C-28), 16.02 (q, C-29), 25.87 (q, C- 30). +41°(c=0.20, MeOH); HREIMS m/z 472.3540 (calcd. for C30H48O4: 472.3550); 1H-NMR (C5D5N) δH: 5.51 (1H, d, J=6.2Hz, H-7), 5.39 (1H, d, J=5.9Hz, H-11), 3.83, 3.48 (1H each, d, J=11.3Hz, 26-H), 3.46 (1H, t, J=11.0Hz, C-24), 1.20 (3H, s, H-19), 1.13 (3H, s, H-28), 1.11 (3H, s, H-27), 1.09 (3H, s, H-29), 0.92 (3H, d, J=6.2Hz, H-21), 0.88 (3H, s, H-30), 0.60 (3H, s, H-18); 13C-NMR (C5D5N) δc: 36.86 (t, C-1), 34.39 (t, C-2), 215.18 (s, C-3), 47.52 (s, C-4), 51.11 (d, C-5), 23.92 (t, C-6), 120.43 (d,C-7), 143.14 (s, C-8), 146.97 (s, C-9), 37.13 (s, C-10), 117.69 (s, C-11), 37.50 (t, C-12), 44.08 (s,C-13), 50.60 (s, C-14), 31.39 (t, C-15), 28.14 (t, C-16), 51.11 (d, C-17), 16.06 (q, C-18), 22.11 (q, C-19), 36.55 (d, C-20), 19.06 (q, C-21), 34.39 (t, C-22), 28.94 (t, C-23), 77.24 (d, C-24), 74.79 (s, C-25), 69.28 (t, C-26), 20.14 (q,C-27), 25.66 (q, C-28), 22.39 (q, C-29), 25.66 (q, C- 30).
+41°(c=0.20, MeOH); HREIMS m/z 472.3540 (calcd. for C30H48O4: 472.3550); 1H-NMR (C5D5N) δH: 5.51 (1H, d, J=6.2Hz, H-7), 5.39 (1H, d, J=5.9Hz, H-11), 3.83, 3.48 (1H each, d, J=11.3Hz, 26-H), 3.46 (1H, t, J=11.0Hz, C-24), 1.20 (3H, s, H-19), 1.13 (3H, s, H-28), 1.11 (3H, s, H-27), 1.09 (3H, s, H-29), 0.92 (3H, d, J=6.2Hz, H-21), 0.88 (3H, s, H-30), 0.60 (3H, s, H-18); 13C-NMR (C5D5N) δc: 36.86 (t, C-1), 34.39 (t, C-2), 215.18 (s, C-3), 47.52 (s, C-4), 51.11 (d, C-5), 23.92 (t, C-6), 120.43 (d,C-7), 143.14 (s, C-8), 146.97 (s, C-9), 37.13 (s, C-10), 117.69 (s, C-11), 37.50 (t, C-12), 44.08 (s,C-13), 50.60 (s, C-14), 31.39 (t, C-15), 28.14 (t, C-16), 51.11 (d, C-17), 16.06 (q, C-18), 22.11 (q, C-19), 36.55 (d, C-20), 19.06 (q, C-21), 34.39 (t, C-22), 28.94 (t, C-23), 77.24 (d, C-24), 74.79 (s, C-25), 69.28 (t, C-26), 20.14 (q,C-27), 25.66 (q, C-28), 22.39 (q, C-29), 25.66 (q, C- 30). +69.9°(c 0.20, CHCl3); HREIMS m/z456.2512 (calcd. for C27H36O6 [M], 456.2511); UV (MeOH) λmax (logε) nm: 253 (3.78); IR (KBr) cm-1: 3445, 1735, 1702, 1659, 897; 1H-NMR (CHCl3) δH: 5.06 (1H, s, H-21), 4.91 (1H, s, H-21), 1.39 (3H, s, H-30), 1.25 (3H, s, H-19), 1.13 (3H, s, H-28), 1.10 (3H, s, H-29), 0.89 (3H, s, H-19); 13C-NMR (CHCl3) δc: 35.64 (t, C-1), 34.27 (t, C-2), 216.57 (s, C-3), 46.78 (s, C-4),49.07 (d, C-5), 27.66 (t, C-6), 66.34 (d,C-7), 157.75 (s, C-8), 141.15 (s, C-9), 38.31 (s, C-10), 197.49 (s, C-11), 49.07 (t, C-12), 45.26 (s,C-13), 58.82 (s, C-14), 217.67 (s, C-15), 38.64 (t,C-16), 46.24 (d, C-17), 18.82 (q, C-18), 18.14 (q, C-19), 143.91 (s, C-20), 112.35 (t, C-21), 31.28 (t, C-22), 32.26 (t, C-23), 176.88 (s, C-24), 27.03(q, C-28), 20.78(q, C-29), 24.55(q, 30).
+69.9°(c 0.20, CHCl3); HREIMS m/z456.2512 (calcd. for C27H36O6 [M], 456.2511); UV (MeOH) λmax (logε) nm: 253 (3.78); IR (KBr) cm-1: 3445, 1735, 1702, 1659, 897; 1H-NMR (CHCl3) δH: 5.06 (1H, s, H-21), 4.91 (1H, s, H-21), 1.39 (3H, s, H-30), 1.25 (3H, s, H-19), 1.13 (3H, s, H-28), 1.10 (3H, s, H-29), 0.89 (3H, s, H-19); 13C-NMR (CHCl3) δc: 35.64 (t, C-1), 34.27 (t, C-2), 216.57 (s, C-3), 46.78 (s, C-4),49.07 (d, C-5), 27.66 (t, C-6), 66.34 (d,C-7), 157.75 (s, C-8), 141.15 (s, C-9), 38.31 (s, C-10), 197.49 (s, C-11), 49.07 (t, C-12), 45.26 (s,C-13), 58.82 (s, C-14), 217.67 (s, C-15), 38.64 (t,C-16), 46.24 (d, C-17), 18.82 (q, C-18), 18.14 (q, C-19), 143.91 (s, C-20), 112.35 (t, C-21), 31.28 (t, C-22), 32.26 (t, C-23), 176.88 (s, C-24), 27.03(q, C-28), 20.78(q, C-29), 24.55(q, 30). +185°(c=0.10, EtOH); HREIMS m/z 530.2875 (calcd. for C30H42O8: 530.2881); UV (EtOH) λmax (logε) nm: 253 (8800); IR (KBr) cm-1: 3410, 3400~2500 (br), 1730, 1720, 1660; 1H-NMR (C5D5N) δH: 5.16 (1H, dd, J=8.4 and 8.4Hz, H-7), 4.69 (1H, s, H-12), 1.47 (3H, s, H-28), 1.43 (3H, s, H-19), 1.41 (3H, d, J=6.7Hz, C-21), 1.36 (3H, d, J=7.3Hz, C-27), 1.22 (3H, s, H-18), 1.15 (3H, s, H-30), 1.07 (3H, s, H-29); 13C-NMR (C5D5N) δc: 35.47 (t, C-1), 34.59 (t, C-2), 216.64 (s, C-3), 46.93 (s, C-4), 49.34 (d, C-5), 28.94 (t, C-6), 65.72 (d, C-7), 159.83 (s, C-8), 140.90 (s, C-9), 38.23 (s, C-10), 201.07 (s, C-11), 79.26 (d, C-12), 51.78 (s, C-13), 59.91 (s, C-14), 215.26 (s, C-15), 39.24 (t,C-16), 46.93 (d, C-17), 13.03 (q, C-18), 18.37 (q, C-19), 29.09 (d, C-20), 21.89 (q, C-21), 49.02 (t, C-22), 209.04 (s, C-23), 46.93 (t, C-24), 46.93 (d, C-25), 178.26 (s, C-26), 17.66 (q,C-27), 24.01 (q, C-28), 21.14 (q, C-29), 26.60 (q, C- 30).
+185°(c=0.10, EtOH); HREIMS m/z 530.2875 (calcd. for C30H42O8: 530.2881); UV (EtOH) λmax (logε) nm: 253 (8800); IR (KBr) cm-1: 3410, 3400~2500 (br), 1730, 1720, 1660; 1H-NMR (C5D5N) δH: 5.16 (1H, dd, J=8.4 and 8.4Hz, H-7), 4.69 (1H, s, H-12), 1.47 (3H, s, H-28), 1.43 (3H, s, H-19), 1.41 (3H, d, J=6.7Hz, C-21), 1.36 (3H, d, J=7.3Hz, C-27), 1.22 (3H, s, H-18), 1.15 (3H, s, H-30), 1.07 (3H, s, H-29); 13C-NMR (C5D5N) δc: 35.47 (t, C-1), 34.59 (t, C-2), 216.64 (s, C-3), 46.93 (s, C-4), 49.34 (d, C-5), 28.94 (t, C-6), 65.72 (d, C-7), 159.83 (s, C-8), 140.90 (s, C-9), 38.23 (s, C-10), 201.07 (s, C-11), 79.26 (d, C-12), 51.78 (s, C-13), 59.91 (s, C-14), 215.26 (s, C-15), 39.24 (t,C-16), 46.93 (d, C-17), 13.03 (q, C-18), 18.37 (q, C-19), 29.09 (d, C-20), 21.89 (q, C-21), 49.02 (t, C-22), 209.04 (s, C-23), 46.93 (t, C-24), 46.93 (d, C-25), 178.26 (s, C-26), 17.66 (q,C-27), 24.01 (q, C-28), 21.14 (q, C-29), 26.60 (q, C- 30). +150°(c=0.13, CHCl3); HREIMS m/z 516.3070 (calcd. for C30H42O8: 516.3087); UV (EtOH) λmax (logε) nm: 254 (3.70); IR (KBr) cm-1: 3400, 2700~2300 (br), 1700, 1655, 1270, 1000; 1H-NMR (C5D5N) δH: 5.23 (1H, dd, J=9.2 and 7.8Hz, H-15), 4.94 (1H, dd, J=9.7 and 7.6Hz, H-7), 1.51 (3H, s, H-28), 1.41 (3H, s, H-19), 1.33 (3H, d, J=7.3Hz, C-27), 1.15 (3H, s, H-30), 1.11 (3H, s, H-29), 1.07 (3H, s, H-18), 0.95 (3H, d, J=6.2Hz, C-21); 13C-NMR (C5D5N) δc: 36.07 (t, C-1), 34.63 (t, C-2), 216.11 (s, C-3), 47.10 (s, C-4), 49.04 (d, C-5), 29.69 (t, C-6), 68.76 (d,C-7), 161.51 (s, C-8), 139.98 (s, C-9), 38.35 (s, C-10), 199.70 (s, C-11), 52.44 (t, C-12), 46.78 (s, C-13), 54.76 (s, C-14), 72.22 (d, C-15), 36.96 (t, C-16), 48.68 (d, C-17), 17.54 (q, C-18), 19.58 (q, C-19), 33.10 (d, C-20), 19.77 (q, C-21), 49.97 (t, C-22), 208.95 (s, C-23), 47.15 (t, C-24), 35.60 (d, C-25), 176.23 (s, C-26), 17.65 (q,C-27), 20.29 (q, C-28), 20.87 (q, C-29), 27.26 (q, C- 30).
+150°(c=0.13, CHCl3); HREIMS m/z 516.3070 (calcd. for C30H42O8: 516.3087); UV (EtOH) λmax (logε) nm: 254 (3.70); IR (KBr) cm-1: 3400, 2700~2300 (br), 1700, 1655, 1270, 1000; 1H-NMR (C5D5N) δH: 5.23 (1H, dd, J=9.2 and 7.8Hz, H-15), 4.94 (1H, dd, J=9.7 and 7.6Hz, H-7), 1.51 (3H, s, H-28), 1.41 (3H, s, H-19), 1.33 (3H, d, J=7.3Hz, C-27), 1.15 (3H, s, H-30), 1.11 (3H, s, H-29), 1.07 (3H, s, H-18), 0.95 (3H, d, J=6.2Hz, C-21); 13C-NMR (C5D5N) δc: 36.07 (t, C-1), 34.63 (t, C-2), 216.11 (s, C-3), 47.10 (s, C-4), 49.04 (d, C-5), 29.69 (t, C-6), 68.76 (d,C-7), 161.51 (s, C-8), 139.98 (s, C-9), 38.35 (s, C-10), 199.70 (s, C-11), 52.44 (t, C-12), 46.78 (s, C-13), 54.76 (s, C-14), 72.22 (d, C-15), 36.96 (t, C-16), 48.68 (d, C-17), 17.54 (q, C-18), 19.58 (q, C-19), 33.10 (d, C-20), 19.77 (q, C-21), 49.97 (t, C-22), 208.95 (s, C-23), 47.15 (t, C-24), 35.60 (d, C-25), 176.23 (s, C-26), 17.65 (q,C-27), 20.29 (q, C-28), 20.87 (q, C-29), 27.26 (q, C- 30).Acknowledgements
Reference
- Cao, Q. Z.; Lin, Z. B. Antitumor and anti-angiogenic activity of Ganoderma lucidum polysaccharides peptide. Acta. Pharm. Sin. 2004, 25, 833–838. [Google Scholar]
- Morigiwa, A.; Kitabatake, K.; Fujjimoto, Y. Angitensin convetting enzymeinbibitory triterpenes from Ganoderma lucidum. Chem. Pharm. Bull. 1986, 34, 3025–3018. [Google Scholar] [CrossRef]
- Zhang, W. M.; Sun, X. M.; Wu, S. L. Studies on the regulation of the blood fat function of Ganoderma lucidum spores powder. Chin. Wild Plant Resour. 2001, 20, 14–16. [Google Scholar]
- Zeng, X. L.; Bao, H.Y. Advances of Researches on triterpene constituents and pharmacology of Ganoderma lucidum. J. Fung. Res. 2004, 2, 68–77. [Google Scholar]
- Li, P. Z.; Zhang, K. C. Studies on pH controlled fermentation of bioactive exopolysaccharides by Ganoderma lucidum. Microbiology 2000, 27, 5–8. [Google Scholar]
- Min, B. S.; Nakamura, N.; Miyashio, H. Triterpenes from the spores of Ganoderma lucidum and their inhibitory activity against HIV-1 protease. Chem. Pharm. Bull. 1998, 46, 1607–1612. [Google Scholar] [CrossRef]
- Sato, H.; Nishitoba, T.; Shirasu, S. Ganoderiol A and Ganoderiol B, new triterpenoids from the fungus Ganoserma Lucidum(Reishi). Agric. Biol. Chem. 1986, 50, 2887–2890. [Google Scholar] [CrossRef]
- Akihisa, T.; Tagata, M.; Ukiya, M.; Tokuda, H.; Suzuki, T.; Kimura, Y. Oxygenated lanostane-type triterpenoids from the fungus Ganoserma Lucidum. J. Nat. Prod. 2005, 68, 559–563. [Google Scholar] [CrossRef]
- Nishitoba, T.; Sato, H.; Sakamura, S. New terpenoids from Ganoserma Lucidum and their bitterness. Agric. Boil.Chem. 1985, 49, 1547–1549. [Google Scholar] [CrossRef]
- Kohda, H.; Tokumoto, W.; Sakamoto, K.; Fujii, M.; Hirai, Y.; Yamasaki, K.; Komoda, Y.; Nakamura, H.; Ishihara, S. The biologically active constituents of Ganoserma Lucidum(FR.)Karst. Histamine release-inhibitory triterpenes. Chem. Pharm. Bull. 1985, 33, 1367–1374. [Google Scholar] [CrossRef] [Green Version]
- Wang, F. S.; Cai, H.; Yang, J. S. Triterpenoids from the fruiting body of Ganoderma lucidum. Acta. Pharm. Sin. 1997, 32, 447–450. [Google Scholar]
- Sample Availability: Contact the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Qiao, Y.; Zhang, X.-m.; Qiu, M.-h. Two Novel Lanostane Triterpenoids from Ganoderma Sinense. Molecules 2007, 12, 2038-2046. https://doi.org/10.3390/12082038
Qiao Y, Zhang X-m, Qiu M-h. Two Novel Lanostane Triterpenoids from Ganoderma Sinense. Molecules. 2007; 12(8):2038-2046. https://doi.org/10.3390/12082038
Chicago/Turabian StyleQiao, Yin, Xian-min Zhang, and Ming-hua Qiu. 2007. "Two Novel Lanostane Triterpenoids from Ganoderma Sinense" Molecules 12, no. 8: 2038-2046. https://doi.org/10.3390/12082038




