Synthetic Approaches to Heterocyclic Ligands for Gd-Based MRI Contrast Agents
Abstract
:Contents
| 1. | Introduction | 1772 |
| 2. | Contrast Agents (CAs) for MRI: Gd-based complexes | 1773 |
| 2.1. CAs derived from acyclic ligands | 1774 | |
| 2.2. CAs derived from macrocyclic ligands | 1784 | |
| 3. | Concluding remarks and future perspectives | 1790 |
1. introduction

2. Contrast Agents (CAs) for MRI: Gd-based complexes

2.1. CAs derived from acyclic ligands
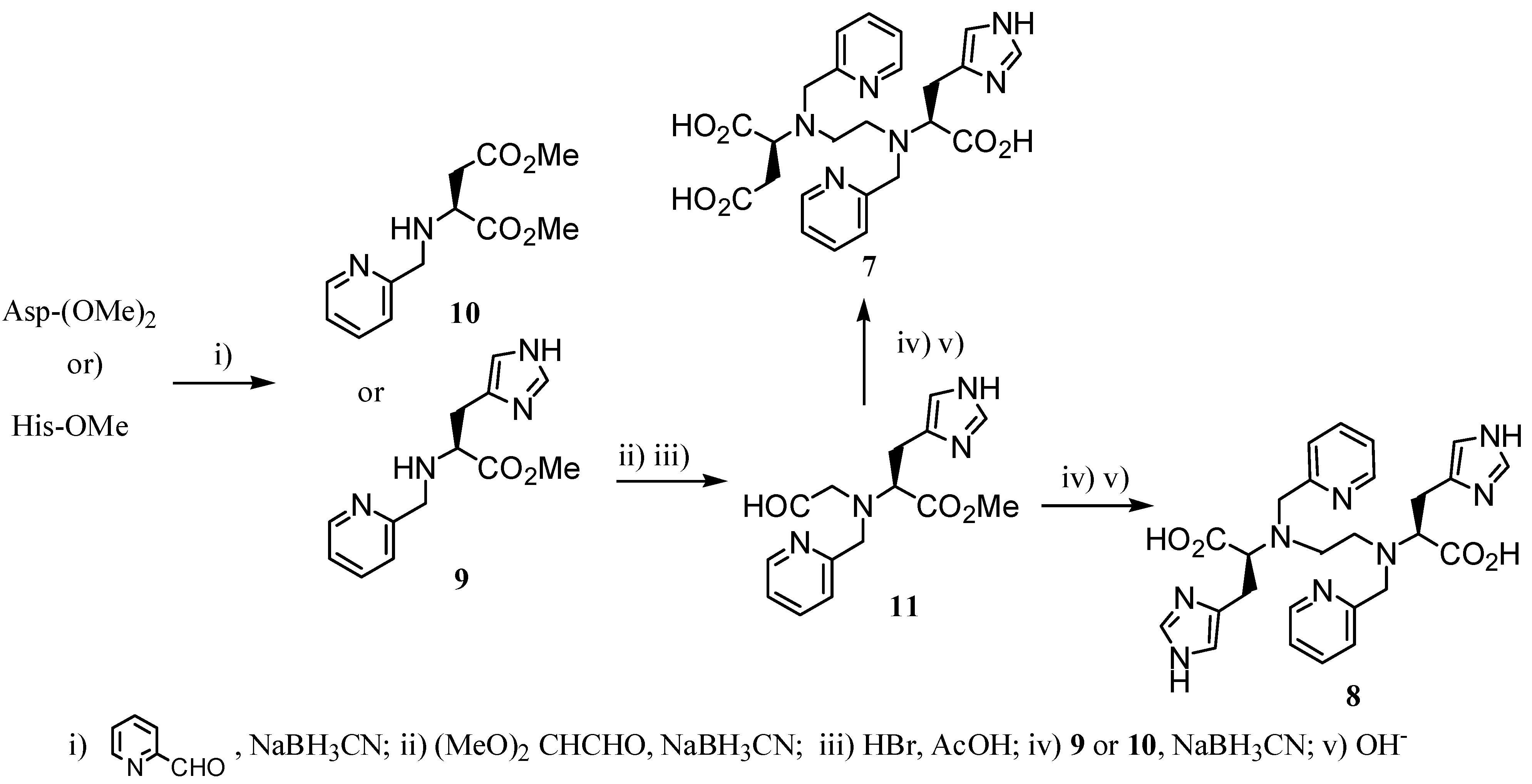
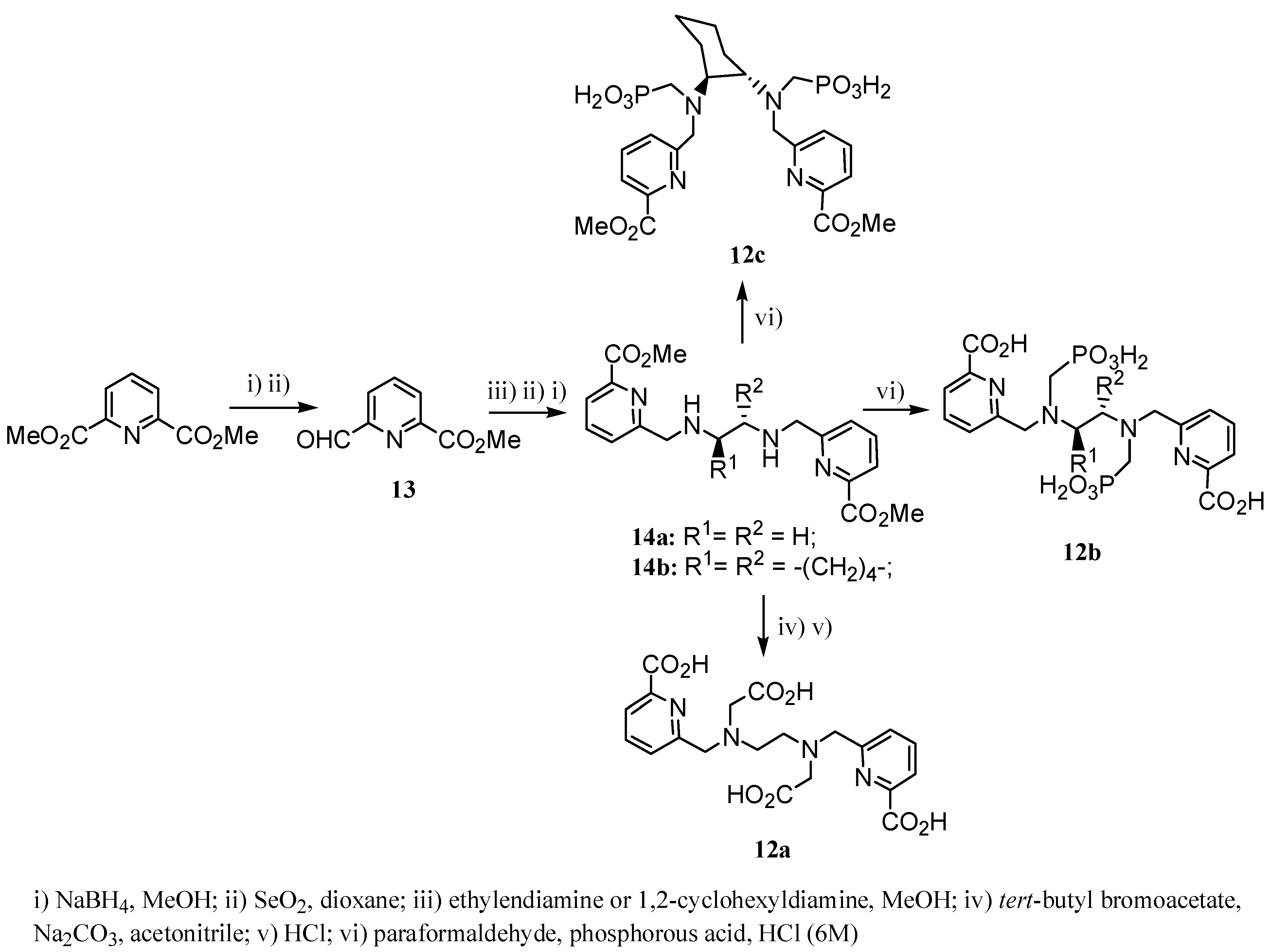

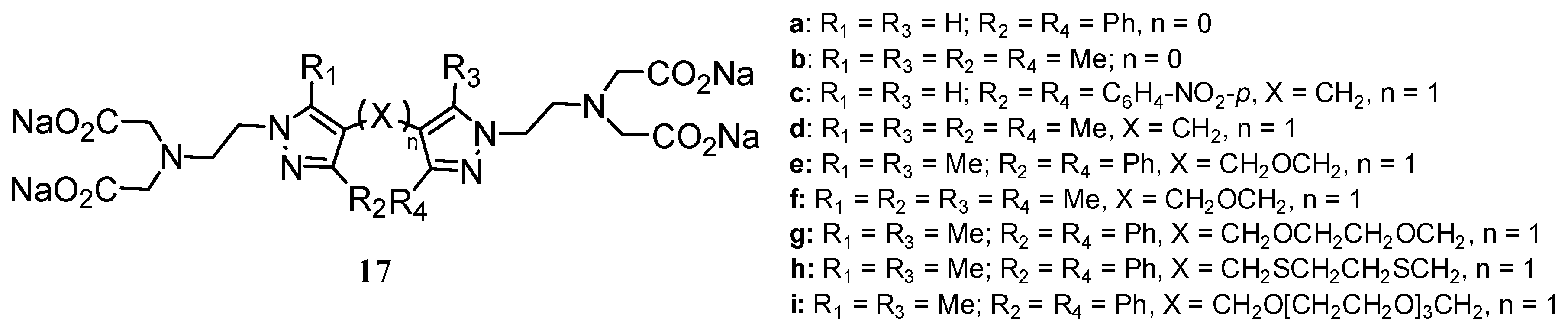

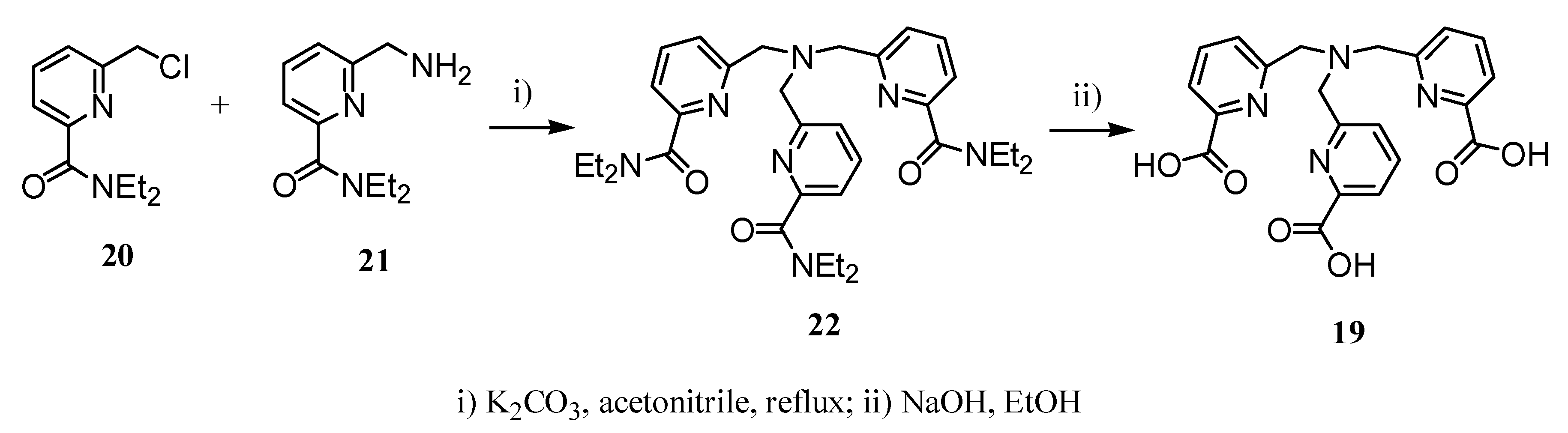
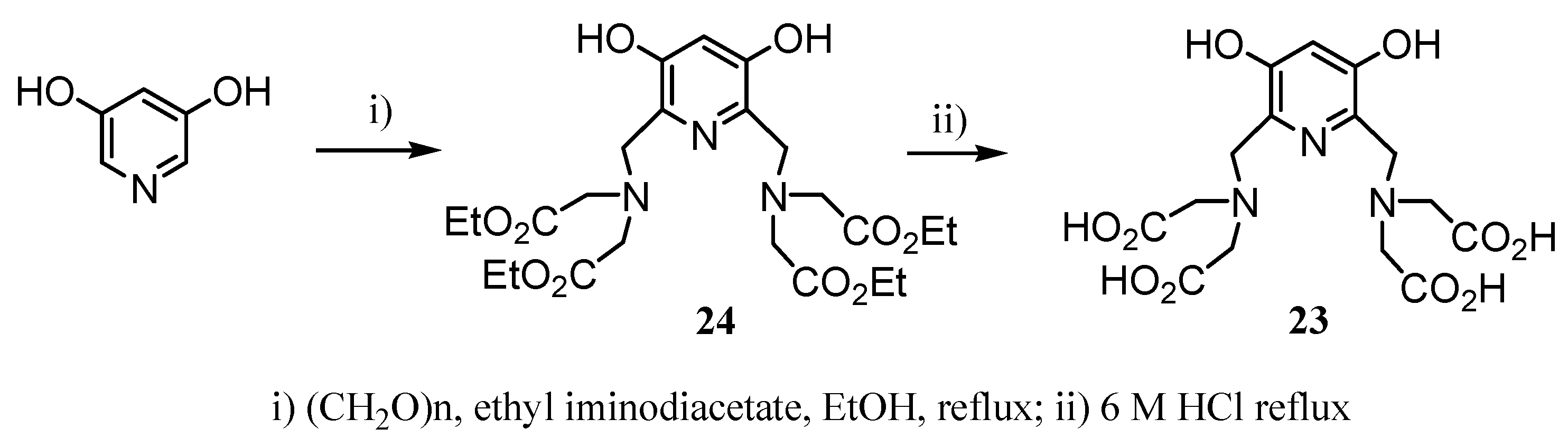


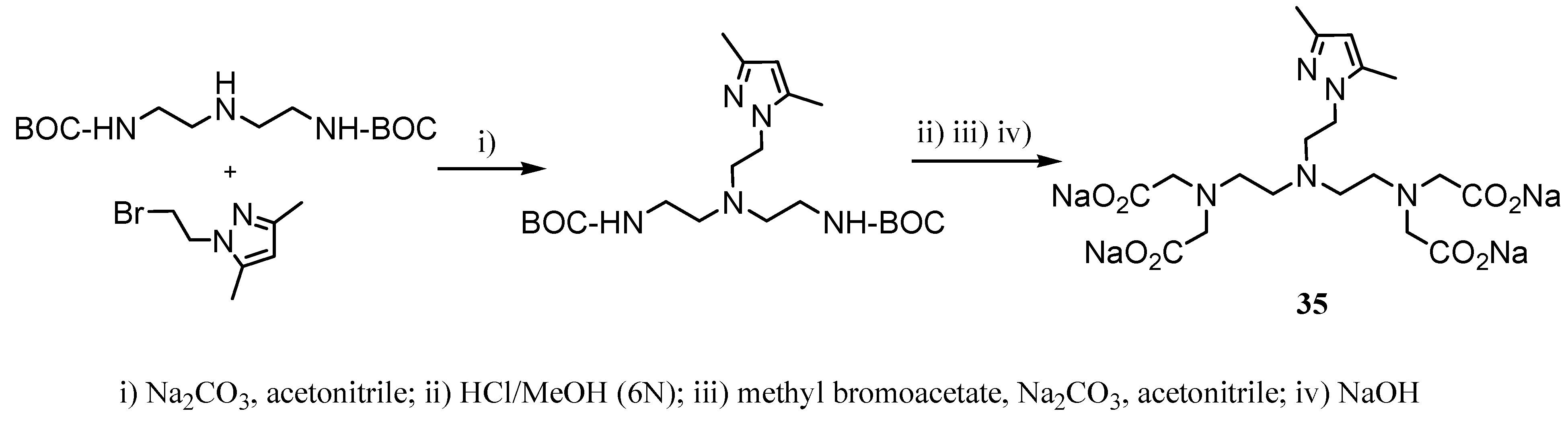


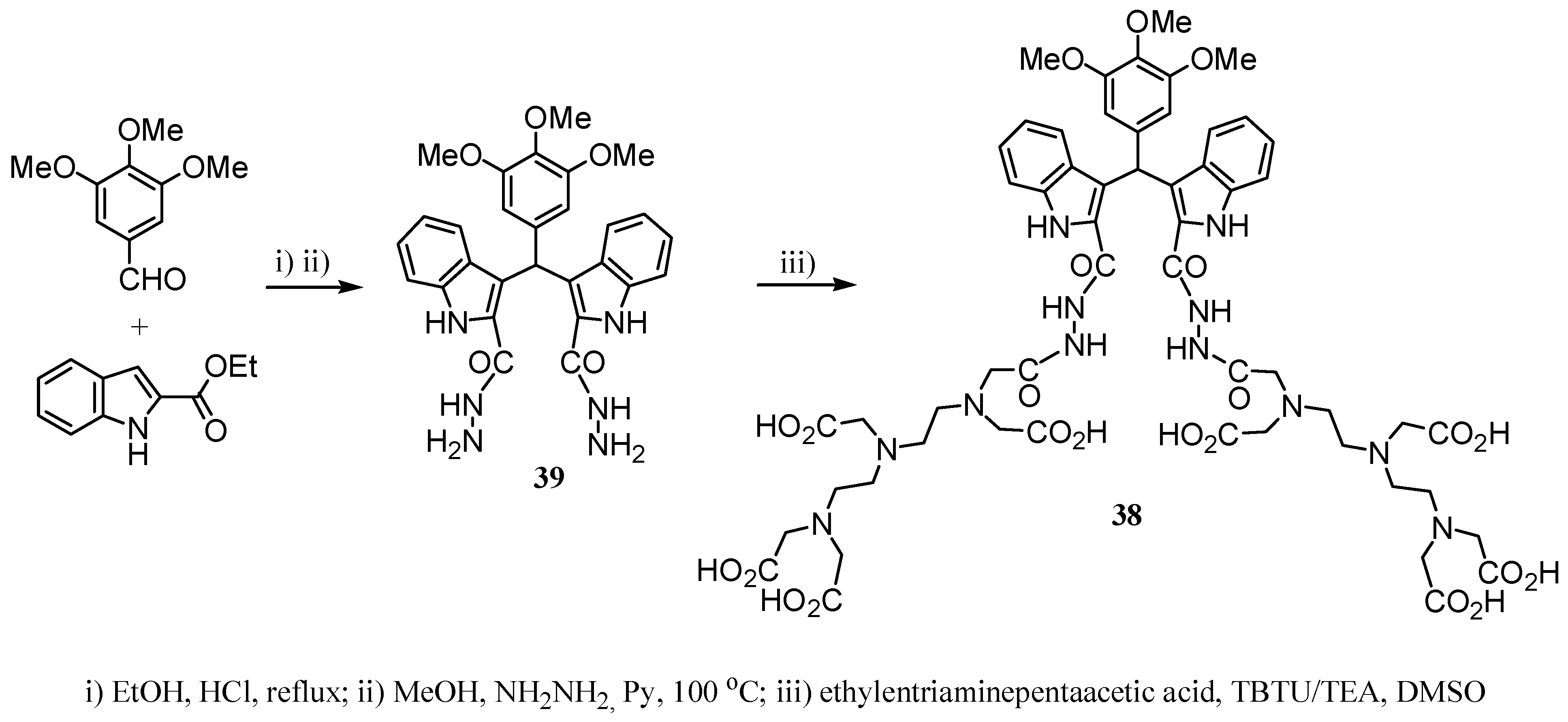

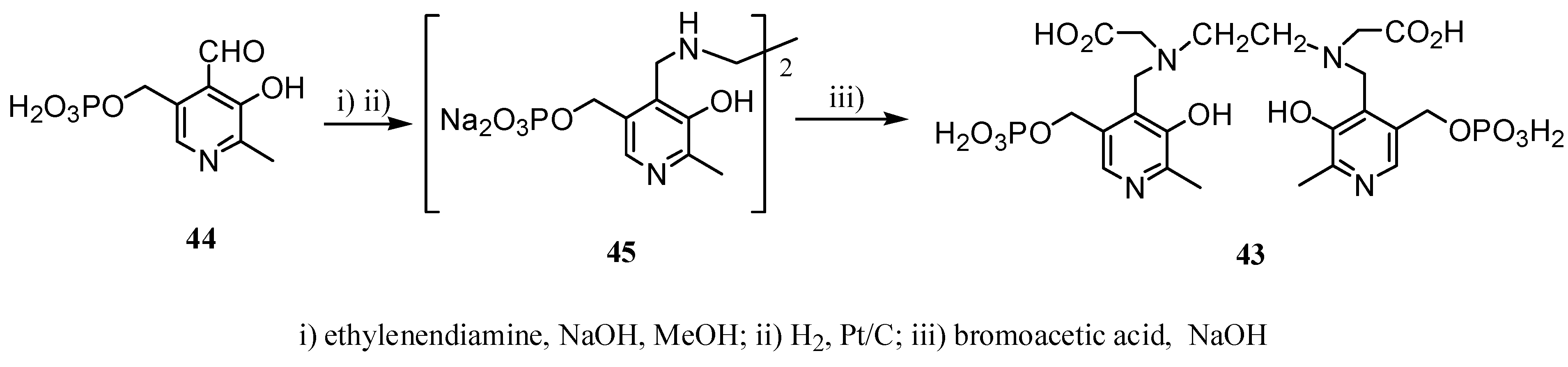
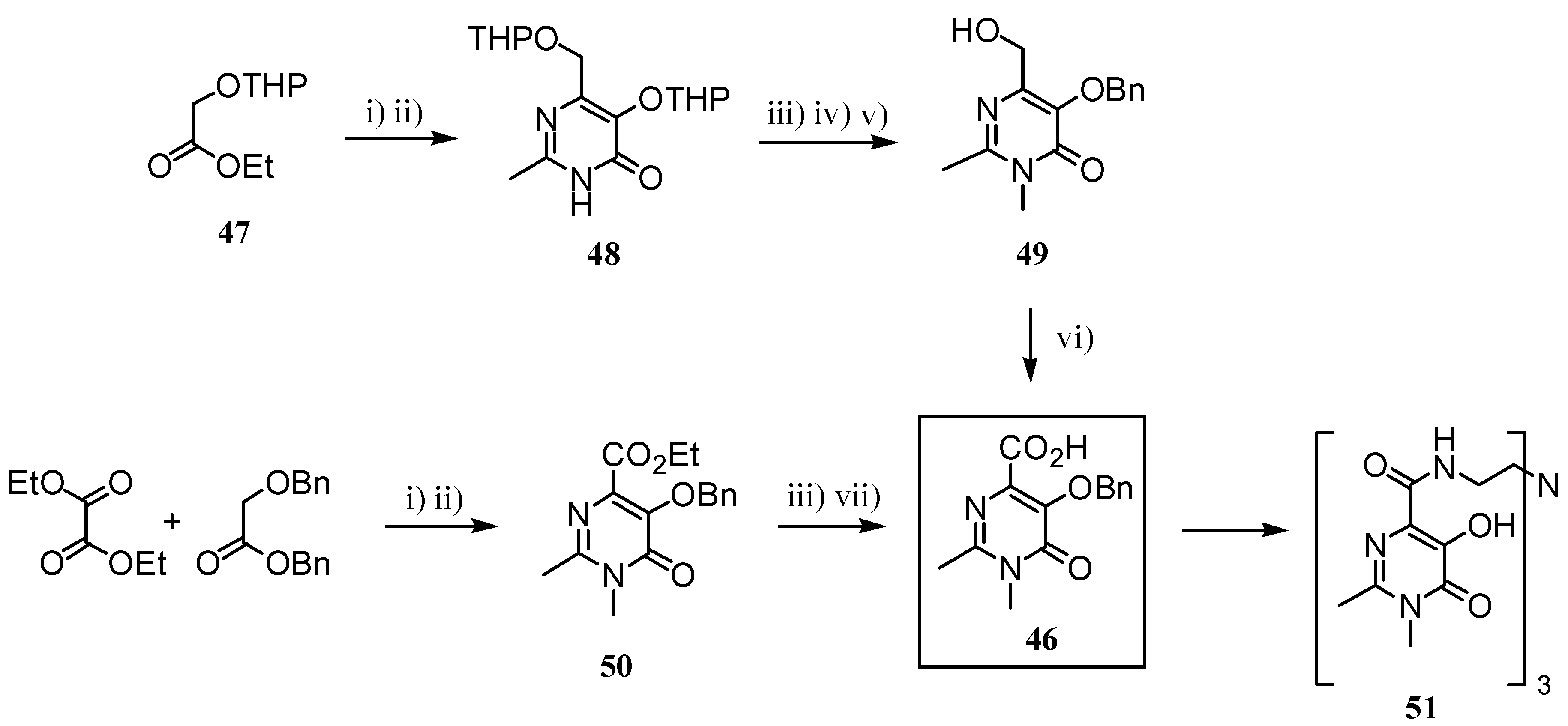
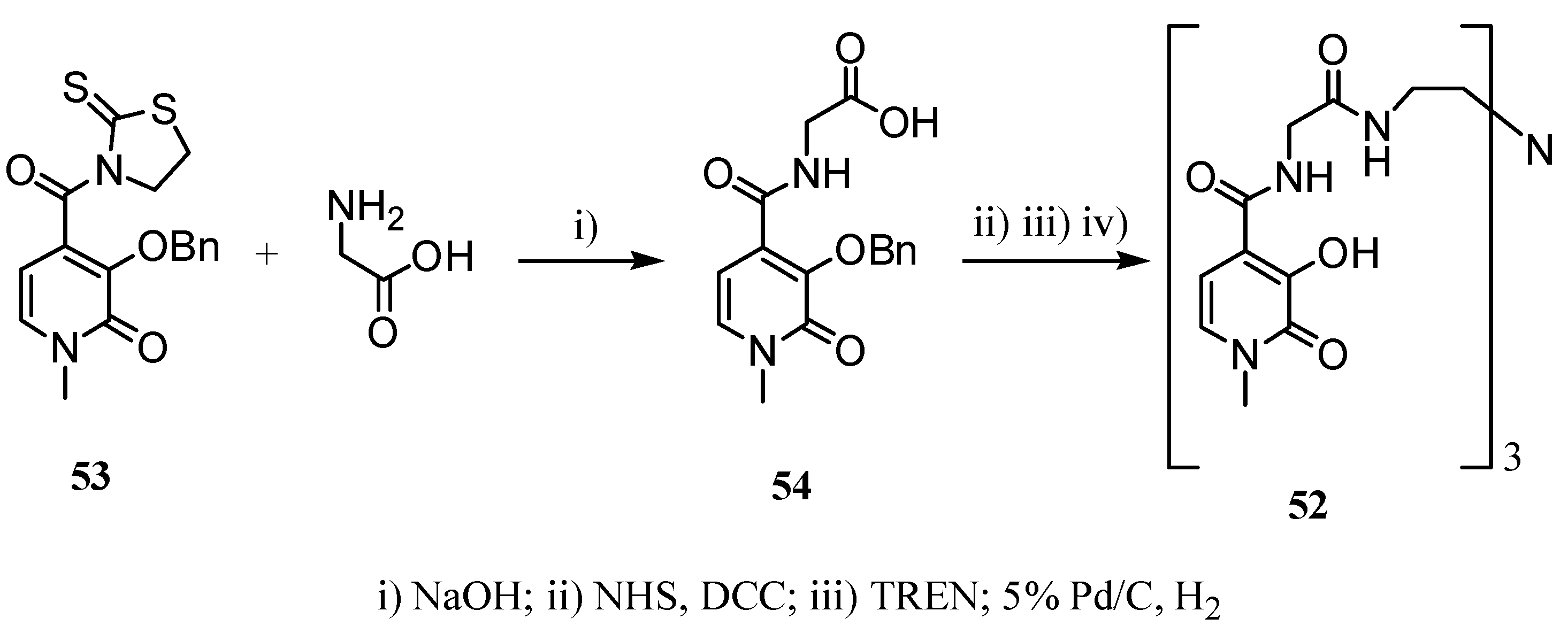

2.2 CAs derived from macrocyclic ligands
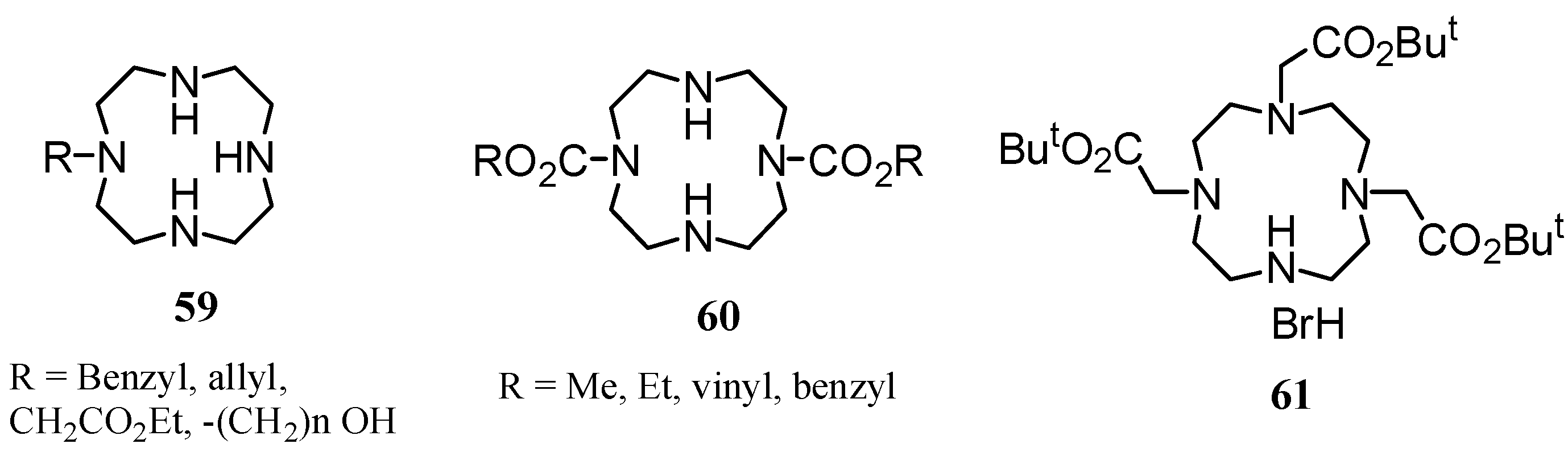
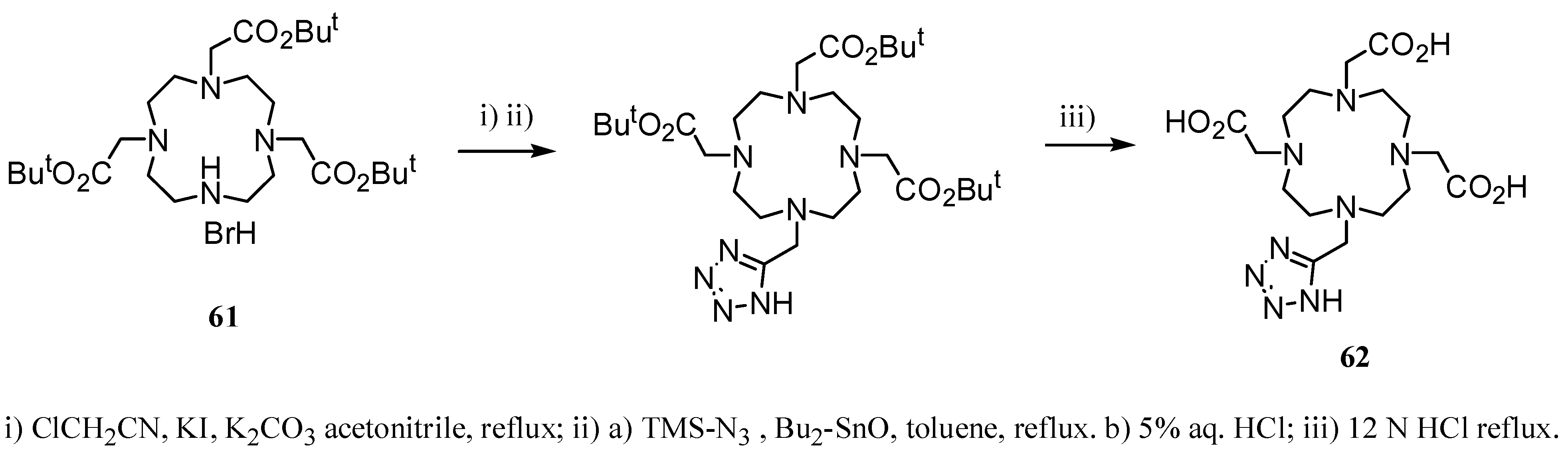

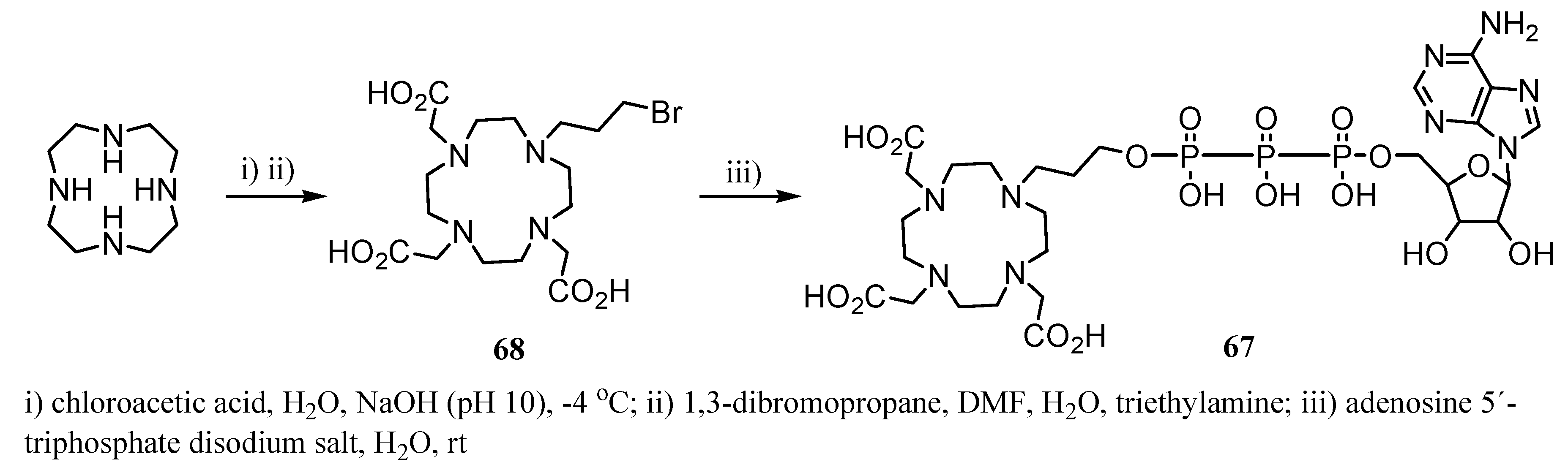
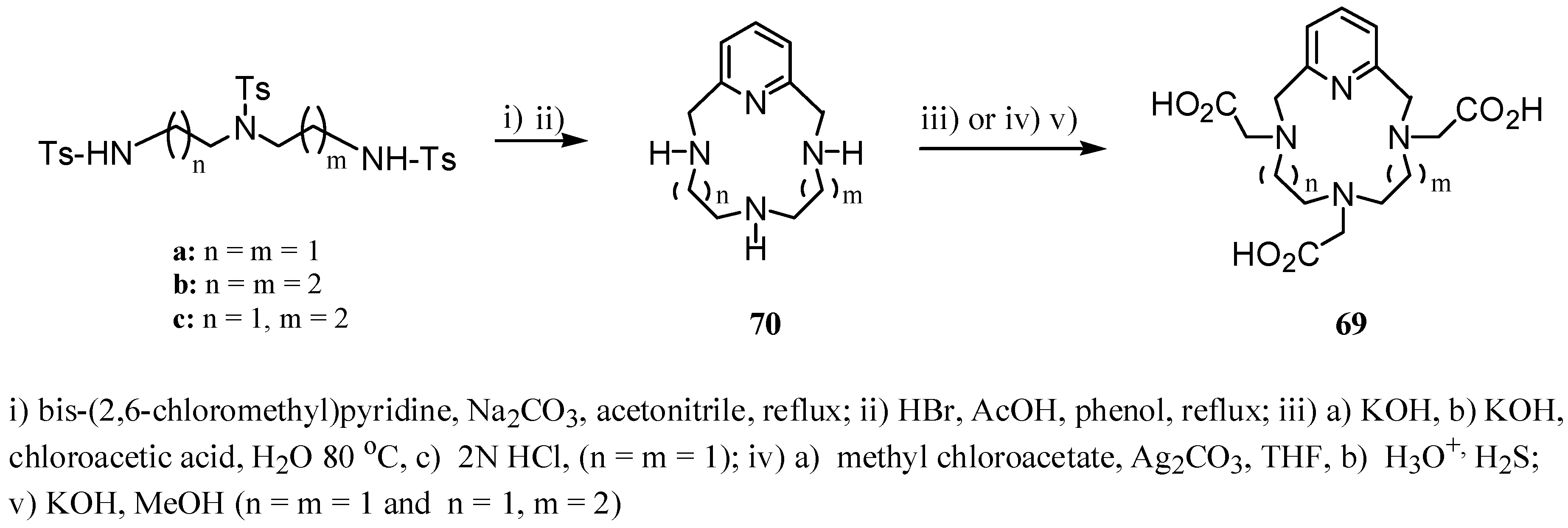
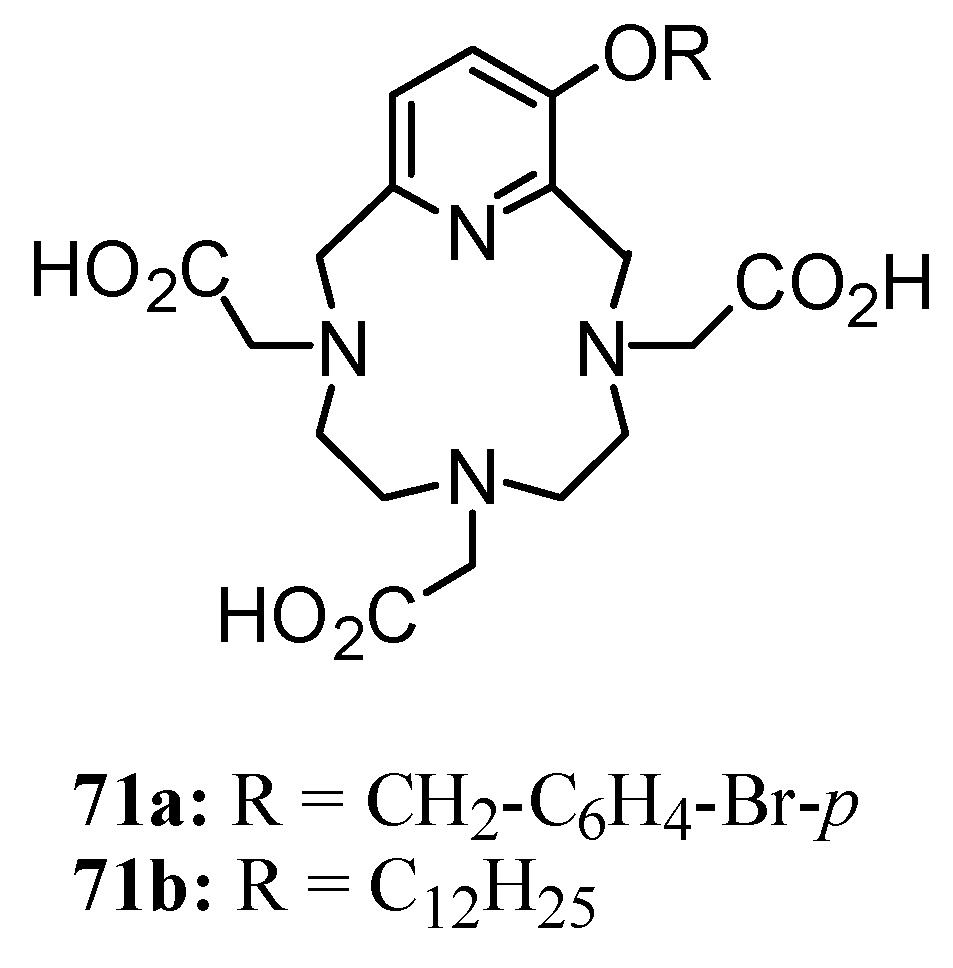



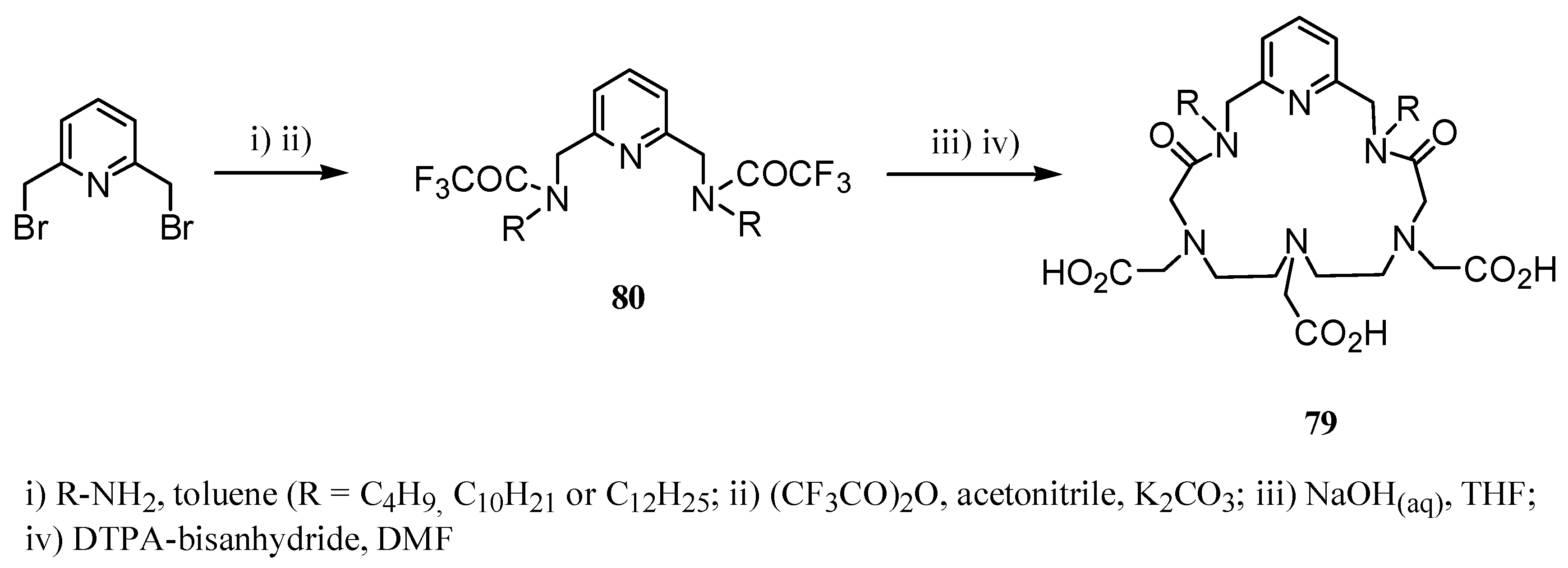
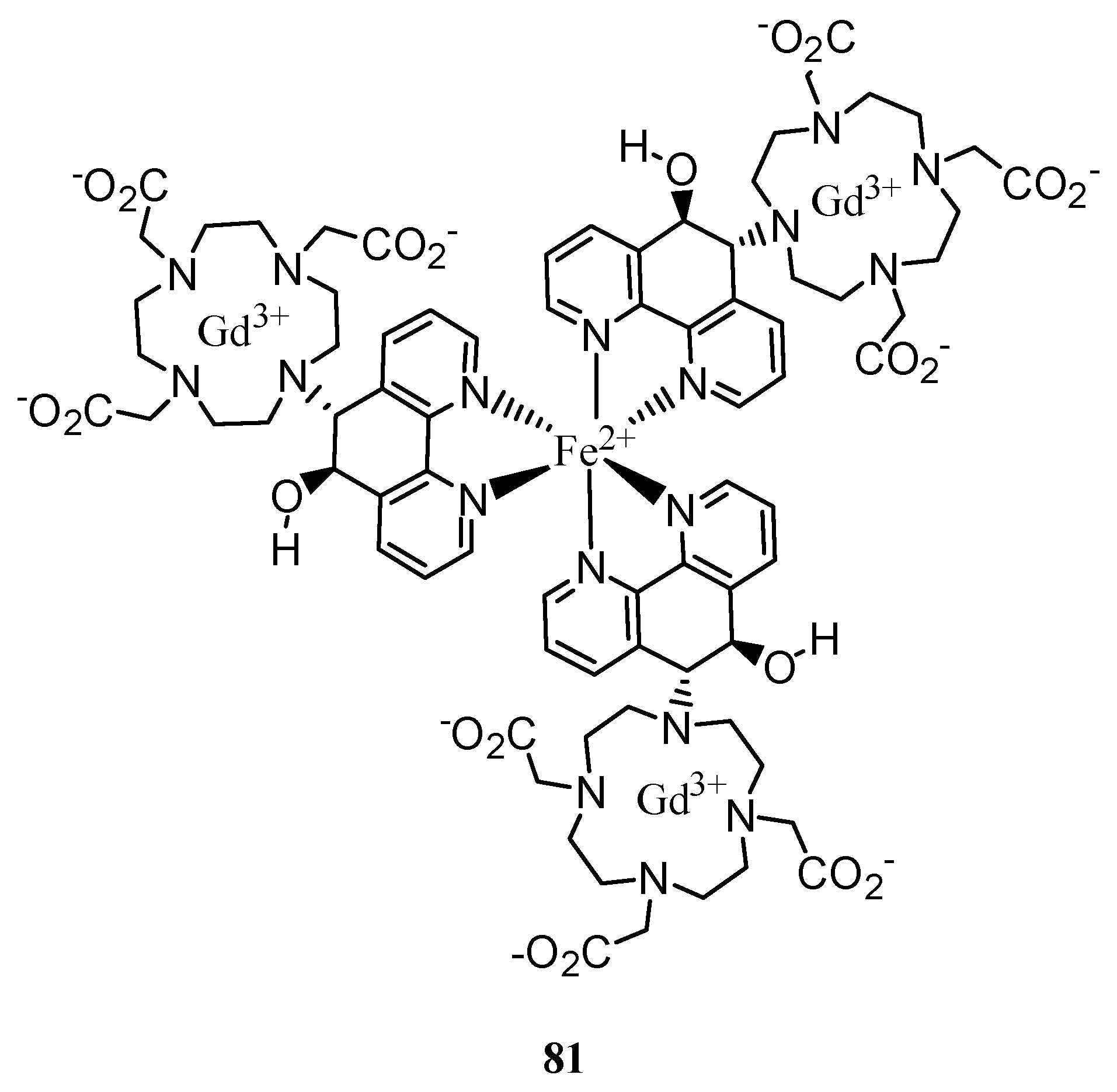

3. Concluding Remarks and Future Perspectives
Acknowledgements
References and Notes
- Mather, S. J. Design of radiolabelled ligands for the imaging and treatment of cancer. Mol. Biosyst. 2007, 3, 30–35. [Google Scholar] [CrossRef]
- Russell, R. R.; Zaret, B. L. Nuclear cardiology: Present and future. Curr Probl. Cardiol. 2006, 31, 557–629. [Google Scholar] [CrossRef]
- Zhang, H.; Maki, J. H.; Prince, M. R. 3D Contrast-enhanced MR angiography. J. Magn. Reson. Imaging 2007, 25, 13–25. [Google Scholar] [CrossRef]
- Deguchi, J-o.; Aikawa, M.; Aikawa, M.; Tung, C.-H.; Aikawa, E.; Kim, D.-E.; Ntziachristos, V.; Weissleder, R.; Libby, P. Inflammation in atherosclerosis. Visualizing matrix metalloproteinase action in macrophages in vivo. Circulation 2006, 114, 55–62. [Google Scholar] [CrossRef]
- Kaufmann, B. A.; Wei, K.; Lindner, J. R. Contrast echocardiography. Curr. Probl. Cardiol. 2007, 32, 51–96. [Google Scholar] [CrossRef]
- Jacquier, A.; Higgins, C. B.; Saeed, M. MR imaging in assessing cardiovascular interventions and myocardial injury. Contrast Media Mol. Imaging 2007, 2, 1–15. [Google Scholar] [CrossRef]
- Fu, X.; Tang, P-Z.; Kula, N. S.; Baldessarini, R.; Tanagnan, G.; Innis, R. B.; Baldwin, R. M. Synthesis, receptor potency, and selective of halogenated diphenylpiperidines as serotonin 5-HT2A ligands for PET or SPECT brain imaging. J. Med. Chem. 2002, 45, 2319–2324. [Google Scholar] [CrossRef]
- Marinelli, E. R.; Diamantidis, A. G.; Emswiler, J.; Fan, H.; Neubeck, R.; Pillai, K. M. R.; Wagler, T. R.; Chen, C.-K.; Natalie, K.; Soundararajan, N.; Ranganathan, R. S. Heterocyclic non-ionic X-ray contrast agents V: a facile conversion of 2-tetrahydrofuroamidas into α-hydroxy-δ-valerolactams and a general synthesis of lactams conjugated to 2,4,6-triiodoisophthalamides. Tetrahedron 1996, 52, 11177–11214. [Google Scholar] [CrossRef]
- Ntziachristos, V.; Yodh, A. G.; Schnall, M.; Chance, B. Concurrent MRI and diffuse optical tomography of breast following indocyanine green enhancement. Proc. Natl. Acad. Sci. USA 2000, 97, 2767–2772. [Google Scholar] [CrossRef]
- Licha, K.; Riefke, B.; Ntziachristos, V.; Becker, A.; Chance, B.; Semmler, W. Hydrophilic cyanine dyes as contrast agents for near-infrared tumor imaging: synthesis, photophysical properties and spectroscopic in vivo characterization. Photochem. Photobiol. 2000, 73, 392–398. [Google Scholar]
- Merbach, A. E.; Tóth, E. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging; John Wiley & Sons, Ltd.: Chichester, UK, 2001. [Google Scholar] Gries, H. Contrast Agents I. Top. Curr. Chem. 2002, 221, 1–235. [Google Scholar]
- Peters, J. A.; Huskens, J.; Raber, D. J. Lanthanide induced shifts and relaxation rate enhancements. Prog. Nucl. Magn. Reson. Spectrosc. 1996, 28, 283–350. [Google Scholar] [CrossRef] Caravan, P. Strategies for increasing the sensitivity of gadolinium base MRI contrast agent. Chem. Soc. Rev. 2006, 35, 512–523. [Google Scholar]
- Caravan, P.; Ellison, J. J.; McMurry, T. J.; Lauffer, R. B. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] Lauffer, R. B. Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: theory and design. Chem. Rev. 1987, 87, 901–927. [Google Scholar]
- Grant, D.; Refsum, H.; Rummeny, E.; Marchal, G. Mangafodipir trisodium, MnDPDP (Teslascan) - A new organ-specific contrast agent for MR imaging. Acta Radiol. 1997, 38, 623–625. [Google Scholar] [CrossRef] Roch, A.; Muller, R. N.; Gillis, P. Theory of proton relaxation induced by super-paramagnetic particles. J. Chem. Phys. 1999, 110, 5403–5411. [Google Scholar] Colet, J. M.; Pierart, C.; Seghi, F.; Gabric, I.; Muller, R. N. Intravascular and intracellular hepatic relaxivities of super-paramagnetic particles: An isolated and perfused organ pharmacokinetics study. J. Magn. Reson. 1998, 134, 199–205. [Google Scholar]
- Miyake, H.; Watanabe, M.; Takemura, M.; Hasegawa, T.; Kojima, Y.; Inoue, M. B.; Inoue, M.; Fernando, Q. Novel optically-active bis(amino acid) ligands and their complexation with gadolinium. J. Chem. Soc., Dalton Trans. 2002, 1119–1125. [Google Scholar]
- Platas-Iglesias, C.; Mato-Iglesias, M.; Djanashvili, K.; Muller, R. N.; Vander Elst, L.; Peters, J. A.; de Blas, A.; Rodríguez-Blas, T. Lanthanide chelates containing pyridine units with potential applications as contrast agents in magnetic resonance imaging. Chem. Eur. J. 2004, 10, 3579–3590. [Google Scholar] [CrossRef]
- Mato-Iglesias, M.; Platas-Iglesias, C.; Djanashvili, K.; Peters, J. A.; Tóth, E.; Balogh, E.; Muller, R. N.; Vander Elst, L.; de Blas, A.; Rodríguez-Blas, T. The highest water exchange rate ever measured for a Gd(III) chelate. J. Chem. Soc., Chem. Commun. 2005, 4729–4731. [Google Scholar]
- Mato-Iglesias, M.; Balogh, E.; Platas-Iglesias, C.; Tóth, E.; de Blas, A.; Rodríguez-Blas, T. Pyridine and phosphonate containing ligands for stable lanthanide complexation. An experimental and theoretical study to assess the solution structure. J. Chem. Soc., Dalton Trans. 2006, 5404–5415. [Google Scholar] Balogh, E.; Mato-Iglesias, M.; Platas-Iglesias, C.; Tóth, E.; Djanashvili, K.; Peters, J. A.; de Blas, A.; Rodríguez-Blas, T. Inorg. Chem. 2006, 45, 8719–8728.
- López, P.; Seipelt, C. G.; Merkling, P.; Sturz, L.; Álvarez, J.; Dölle, A.; Zeidler, M. D.; Cerdán, S.; Ballesteros, P. N-2-(Azol-1(2)-yl)ethyliminodiacetic acids: a novel series of Gd(III) chelators as T2 relaxation agents for magnetic resonance imaging. Bioorg. Med. Chem. 1999, 7, 517–527. [Google Scholar] [CrossRef]
- Mayoral, E. P.; García-Amo, M.; López, P.; Soriano, E.; Cerdán, S.; Ballesteros, P. A novel series of complexones with bis- or bi structure as mixed ligands of paramagnetic contrast agents for MRI. Bioorg. Med. Chem. 2003, 11, 5555–5567. [Google Scholar] [CrossRef]
- Pérez-Mayoral, E.; García-Amo, M.; López-Larrubia, P.; Cerdán, S.; Ballesteros, P. Synthesis of new family of ligands with bispyrazole structure. Reactivity of bispyrazolylmethyl ethers. Heterocycles 2005, 65, 1691–1704. [Google Scholar] [CrossRef]
- Bretonnière, Y.; Mazzanti, M.; Pécaut, J.; Dunand, F. A.; Merbach, A. E. A new heptadentate tripodal ligand leading to a gadolinium complex with improved relaxation efficiency. J. Chem Soc., Chem. Commun. 2001, 621–622. [Google Scholar] Bretonnière, Y.; Mazzanti, M.; Pécaut, J.; Dunand, F. A.; Merbach, A. E. Solid-state and solution properties of the lanthanide complexes of a new heptadentate tripodal ligand: a route to gadolinium complexes with an improved relaxation. Inorg. Chem. 2001, 40, 6737–6745. [Google Scholar]
- Aime, S.; Cavallotti, C.; Gianolio, E.; Giovenzana, G. B.; Palmisano, G.; Sisti, M. Mannich reaction as a new route to pyridine-based polyaminocarboxylic ligands. Org. Lett. 2004, 6, 1201–1204. [Google Scholar] [CrossRef]
- Chong, H.-S.; Garmestani, K.; Bryant, H.; Brechbiel, M. W. Synthesis of DTPA analogues derived from piperidine and azepane: potential contrast enhancement agents for magnetic resonance imaging. J. Org. Chem. 2001, 66, 7745–7750. [Google Scholar] [CrossRef]
- Cheng, T.-H.; Wang, Y.-M.; Lee, W.-T.; Liu, G.-C. Synthesis of two N’-2-pyridylmethyl and N’-2-hydroxypropyl derivatives of diethylentriaminepentaacetic acid and the stabilities of their complexes with Ln3+, Ca2+, Cu2+ and Zn2+. Polyhedron 2000, 19, 2027–2037. [Google Scholar] [CrossRef]
- Pérez-Mayoral, E.; Soriano, E.; Cerdán, S.; Ballesteros, P. Experimental and theoretical study of lanthanide complexes based on linear and macrocyclic polyaminopolycarboxylic acids containing pyrazolylethyl arms. Molecules 2006, 11, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Ruloff, R.; van Koten, G.; Merbach, A. E. Novel heteroditopic chelated for self-assembled gadolinium(III) complex with high relaxivity. J. Chem. Soc., Chem. Commun. 2004, 842–843. [Google Scholar] [CrossRef]
- Livramento, J. B.; Tóth, E.; Sour, A.; Borel, A.; Merbach, A. E.; Ruloff, R. High relaxiviy confined to a smart molecular space: a metallostar-based, potential MRI contrast agent. Angew. Chem. Int. Ed. 2005, 44, 1480–1484. [Google Scholar] [CrossRef]
- Parac-Vogt, T. N.; Kimpe, K.; Laurent, S.; Vander Els, L.; Burtea, C.; Chen, F.; Muller, R. N.; Ni, Y.; Verbruggen, A.; Binnemans, K. Synthesis, characterization, and pharmacokinetic evaluation of a potential MRI contrast agents containing two paramagnetic centers with albumin binding affinity. Chem. Eur. J. 2005, 11, 3077–3086. [Google Scholar] [CrossRef]
- Frullano, L.; Rohovec, J.; Aime, S.; Maschmeyer, T.; Prata, M. I.; Predroso de Lima, J. J.; Geraldes, C. F. C. G.; Peters, J. A. Towards targeted MRI: new MRI contrast agents for sialic acid detection. Chem. Eur. J. 2004, 10, 5205–5217. [Google Scholar] [CrossRef]
- Federle, M. P.; Chezmar, J. L.; Rubin, D. L.; Weinreb, J. C.; Freeny, P. C.; Semelka, R. C.; Brown, J. J.; Borrello, J. A.; Lee, J. K. T.; Mattrey, R.; Dachman, A. H.; Saini, S.; Harmon, B.; Fenstermacher, M.; Pelsang, R. E.; Harms, S. E.; Mitchell, D. G.; Halford, H. H.; Anderson, M. W.; Johnson, C. D.; Francis, I. R.; Bova, J. G.; Kenney, P. J-; Klippenstein, D. L.; Foster, G. S.; Turner, D. A.; Stillman, A. E.; Nelson, R. C.; Young, S. W.; Patt, R. H.; Rifkin, M.; Seltzer, S. E.; Gay, S. B.; Robinson, R. O.; Sherwin, P. F.; Ballerini, R. Safety and efficacy of Mangafodipir trisodium (MnDPDP) injection for hepatic MRI in adults: results of the U.S. multicenter phase III clinical trials (safety). J. Mag. Reson. Imaging 2000, 12, 186–197. [Google Scholar] [CrossRef]
- Rocklage, S. M.; Cacheris, W. P.; Quay, S. C.; Hahn, E. Manganese(II) N,N´-dipyridoxylethylenediamine-N,N´-diacetate 5,5´-bis(phosphonate). Synthesis and characterization of paramagnetic chelate for magnetic resonance imaging enhancement. Inorg. Chem. 1989, 28, 477–485. [Google Scholar] [CrossRef] Schmidt, P. P.; Toft, K.; Skotland, T. Stability and transmetallation of the magnetic resonance contrast agent MnDPDP measured by EPR. J. Biol. Inorg. Chem. 2002, 7, 241–248. [Google Scholar] Halavaara, J.; Abo-Ramadan, U.; Makkola, A.; Aronen, H.; Häkkinen, A.-M. MnDPDP-enhanced magnetization transfer MR imaging: implications for effective liver imaging. Mag. Reson. Imaging 2003, 21, 47–50. [Google Scholar]
- Sunderland, C. J.; Botta, M.; Aime, S.; Raymond, K. N. 6-Carboxamido-5,4-hydroxypyrimidinones: a new class of heterocyclic ligands and their evaluation as gadolinium chelating agents. Inorg. Chem. 2001, 40, 6746–6756. [Google Scholar] [CrossRef]
- O´Sullivan, B.; Doble, D. M. J.; Thompson, M. K.; Siering, C.; Xu, J.; Otta, M.; Aime, S.; Raymond, K. N. The effect of ligand scaffold size on the stability of tripodal hydroxypyridonate gadolinium complexes. Inorg. Chem. 2003, 42, 2577–2583. [Google Scholar] [CrossRef]
- Thompson, M. K.; Misselwitz, B.; Tso, L. S.; Doble, D. M. J.; Schmitt-Willich, H.; Raymond, K. N. In vivo evaluation of gadolinium hydroxypyridonate chelates: initial experience as contrast media in magnetic resonance imaging. J. Med. Chem. 2005, 48, 3874–3877. [Google Scholar] [CrossRef]
- Pierre, V. C.; Botta, M.; Raymond, K. N. Dendrimeric gadolinium chelate with fast water exchange and high relaxivity at high magnetic field strength. J. Am. Chem. Soc. 2005, 127, 504–505. [Google Scholar] [CrossRef]
- Ballesteros, P.; Soriano, E.; Pérez-Mayoral, E.; García-Amo, M.; Cerdán, S. Flexible tetra-azamacrocycles as metal ligands. Their relevance in magnetic resonance imaging. In Strutural analysis of cyclic systems; Iriepa, I., Ed.; Research Singpost: Trivandrum, India, 2005; pp. 69–86. [Google Scholar]
- Li, C.; Wong, W.-T. A convenient method for the preparation of mono N-alkylated cyclams and cyclens in high yields. Tetrahedron Lett. 2002, 43, 3217–3220. [Google Scholar] [CrossRef]
- Kovacs, Z.; Sherry, A. D. A general synthesis of 1,7-disubstituted 1,4,7,10-tetraazacyclo-dodecanes. J. Chem. Soc., Chem. Commun. 1995, 185–186. [Google Scholar] [CrossRef]
- Dadabhoy, A.; Faulkner, S.; Sammes, P. G. Long wavelength for europium(III) luminescence based on acridone derivatives. J. Chem. Soc., Perkin Trans. 2 2002, 348–357. [Google Scholar] [CrossRef]
- Aime, S.; Cravotto, G.; Geninatti Crich, S.; Giovenzana, G. B.; Ferrari, M.; Palmisano, G.; Sisti, M. Synthesis of the Gd(III) complex with tetrazole-armed macrocyclic ligand as a potential MRI contrast agent. Tetrahedron Lett. 2002, 43, 783–786. [Google Scholar] [CrossRef]
- Woods, M.; Sherry, A. D. Synthesis and luminescence studies of aryl substituted tetraamide complexes of europium(III): a new approach to pH responsive luminescent europium probes. Inorg. Chem. 2003, 42, 4401–4408. [Google Scholar] [CrossRef] Woods, M.; Zhang, S.; Ebron, V. H.; Sherry, A. D. pH-sensitive modulation of the second hydration sphere in lanthanide(III) tetraamide-DOTA complexes: a novel approach to smart MR contrast media. Chem. Eur. J. 2003, 9, 4634–4640. [Google Scholar]
- Ratnakar, S. J.; Alexander, V. Synthesis and relaxivity studies of a gadolinium(III) complex of ATP-conjugated DO3A as a contrast enhancing agent for MRI. Eur. J. Inorg. Chem. 2005, 3918–3927. [Google Scholar] [CrossRef]
- Aime, S.; Botta, M.; Geninatti Crich, S.; Giovenzana, G. B.; Jommi, G.; Pagliarin, R.; Sisti, M. MRI Contrast agents: macrocyclic lanthanide(III) complexes with improved relaxation efficiency. J. Chem. Soc., Chem. Commun. 1995, 1885–1886. [Google Scholar] Aime, S.; Botta, M.; Geninatti Crich, S.; Giovenzana, G. B.; Jommi, G.; Pagliarin, R.; Sisti, M. Synthesis and NMR studies of three pyridine-containing triaza macrocyclic triacetate ligands and their complexes with lanthanide ions. Inorg. Chem. 1997, 36, 2992–3000. [Google Scholar] Siaugue, J.-M.; Segat-Dioury, F.; Favre-Réguillon, A.; Madic, C.; Foos, J.; Guy, A. An efficient synthesis of pyridine containing triaza-macrocyclic triacetate ligand and luminescence properties of its europium(III) complex. Tetrahedron Lett. 2000, 41, 7443–7446. [Google Scholar]
- Aime, S.; Botta, M.; Frullano, L.; Geninatti Crich, S.; Giovenzana, G. B.; Pagliarin, R.; Palmisano, G.; Sisti, M. Contrast agents for magnetic resonance imaging: a novel route to enhanced relaxivities based on the interaction of a Gd(II) chelate with poly-β-cyclodextrins. Chem. Eur. J. 1999, 5, 1253–1260. [Google Scholar] [CrossRef] Hovland, R.; Glogard, C.; Aasen, A. J.; Klaveness, J. Preparation and in vitro evaluation of a novel amphiphilic GdPCTA-[12] derivative; a micelar MRI contrast agent. Org. Biomol. Chem. 2003, 1, 644–647. [Google Scholar]
- Aime, S.; Botta, M.; Frullano, L.; Geninatti Crich, S.; Giovenzana, G.; Pagliarin, R.; Palmisano, G.; Sirtori, F. R.; Sisti, M. GdPCP2A(H2O)2]-: a paramagnetic contrast agent designed for improved applications in magnetic resonance imaging. J. Med. Chem. 2000, 43, 4017–4024. [Google Scholar] [CrossRef]
- Aime, S.; Gianolio, E.; Corpillo, D.; Cavallotti, C.; Palmisano, G.; Sisti, M.; Giovenzana, G. B.; Pagliarin, R. Designing novel contrast agents for magnetic resonance imaging. Synthesis and relaxometric characterization of three gadolinium(III) complexes based on functionalized pyridine-containing macrocyclic ligands. Helv. Chim. Acta 2003, 86, 615–632. [Google Scholar] [CrossRef]
- Zheng, Q.; Dai, H.; Merritt, M. E.; Malloy, C.; Pan, C. Y.; Li, W.-H. A new class of macrocyclic lanthanide complexes for cell labeling and magnetic resonance applications. J. Am. Chem. Soc. 2005, 127, 16178–16188. [Google Scholar] [CrossRef]
- Comblin, V.; Gilsoul, D.; Hermann, M.; Humblet, V.; Jacques, V.; Mesbahi, M.; Sauvage, C.; Desreux, J. F. Designing new MRI contrast agents: a coordination chemistry challenge. Coord. Chem. Rev. 1999, 185–186, 451–470. [Google Scholar] [CrossRef] Paris, J.; Gameiro, C.; Humblet, V.; Mohapatra, P. K.; Jacques, V.; Desreux, J. F. Auto-assembling of ditopic macrocyclic lanthanide chelates with transition-metal ions. Rigid multimetallic high relaxivity contrast agents for magnetic resonance imaging. Inorg. Chem. 2006, 45, 5092–5102. [Google Scholar]
- Aime, S.; Geninatti Crich, S.; Gianolio, E.; Giovenzana, G. B.; Tei, L.; Terreno, E. High sensitivity lanthanide(III) based probes for MR-medical imaging. Coord. Chem. Rev. 2006, 250, 1562–1579. [Google Scholar] [CrossRef] Lowe, M. P. Activated MR contrast agents. Curr. Pharm. Biotech. 2004, 5, 519–528. [Google Scholar]
- Woods, M.; Woessner, D. E.; Sherry, A. D. Paramagnetic lanthanide complexes as PARACEST agents for medical imaging. Chem. Soc. Rev. 2006, 35, 500–511. [Google Scholar] [CrossRef]
- García-Martín, M. L.; Herigault, G.; Remy, C.; Flarion, R.; Ballesteros, P.; Coles, J.; Cerdán, S.; Ziegler, A. Mapping extracellular pH in rat brain gliomas in vivo by 1H Magnetic Resonance Spectroscopy: comparison with maps of other metabolites. Cancer Res. 2001, 61, 6524–6531. [Google Scholar]
- Pacheco-Torres, J.; Pérez-Mayoral, E.; Soriano, E.; López-Larrubia, P.; Ouari, O.; González-Cortés, A.; Cerdán, S.; Ballesteros, P. A convenient and efficient synthesis of the first nitroimidazolylsuccinic esters and their diacids. Synthesis 2006, 22, 3859–3864. [Google Scholar]
- Soler-Padrós, J.; Pérez-Mayoral, E.; Domínguez, L.; López-Larrubia, P.; Soriano, E.; Marco-Contelles, J. L.; Cerdán, S.; Ballesteros, P. A novel generation of pH indicators for proton magnetic resonance spectroscopic imaging. J. Med. Chem. 2007, (in press). [Google Scholar]
- Provent, P.; Benito, M.; Hiba, B.; Farion, R.; López-Larrubia, P.; Ballesteros, P.; Rémy, C.; Segebarth, C.; Cerdán, S.; Coles, J. A.; García-Martín, M. L. Serial in vivo spectroscopic NMR imaging of lactate and extracellular pH in rat gliomas shows redistribution of protons away from sites of glycolysis. Cancer Res. 2007, (in press). [Google Scholar]
- Bhujwala, Z.; Artemov, D.; Ballesteros, P.; Cerdán, S.; Gillies, R.; Solaiyappan, M. Combined vascular and extracellular pH imaging of solid tumors. NMR Biomed. 2002, 15, 114–119. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Pérez-Mayoral, E.; Soler-Padrós, J.; Negri, V.; Cerdán, S.; Ballesteros, P. Synthetic Approaches to Heterocyclic Ligands for Gd-Based MRI Contrast Agents. Molecules 2007, 12, 1771-1795. https://doi.org/10.3390/12081771
Pérez-Mayoral E, Soler-Padrós J, Negri V, Cerdán S, Ballesteros P. Synthetic Approaches to Heterocyclic Ligands for Gd-Based MRI Contrast Agents. Molecules. 2007; 12(8):1771-1795. https://doi.org/10.3390/12081771
Chicago/Turabian StylePérez-Mayoral, Elena, Jordi Soler-Padrós, Viviana Negri, Sebastián Cerdán, and Paloma Ballesteros. 2007. "Synthetic Approaches to Heterocyclic Ligands for Gd-Based MRI Contrast Agents" Molecules 12, no. 8: 1771-1795. https://doi.org/10.3390/12081771



