Flavonoids and Strigolactones in Root Exudates as Signals in Symbiotic and Pathogenic Plant-Fungus Interactions
Abstract
:Contents
1. Introduction
2. Flavonoids
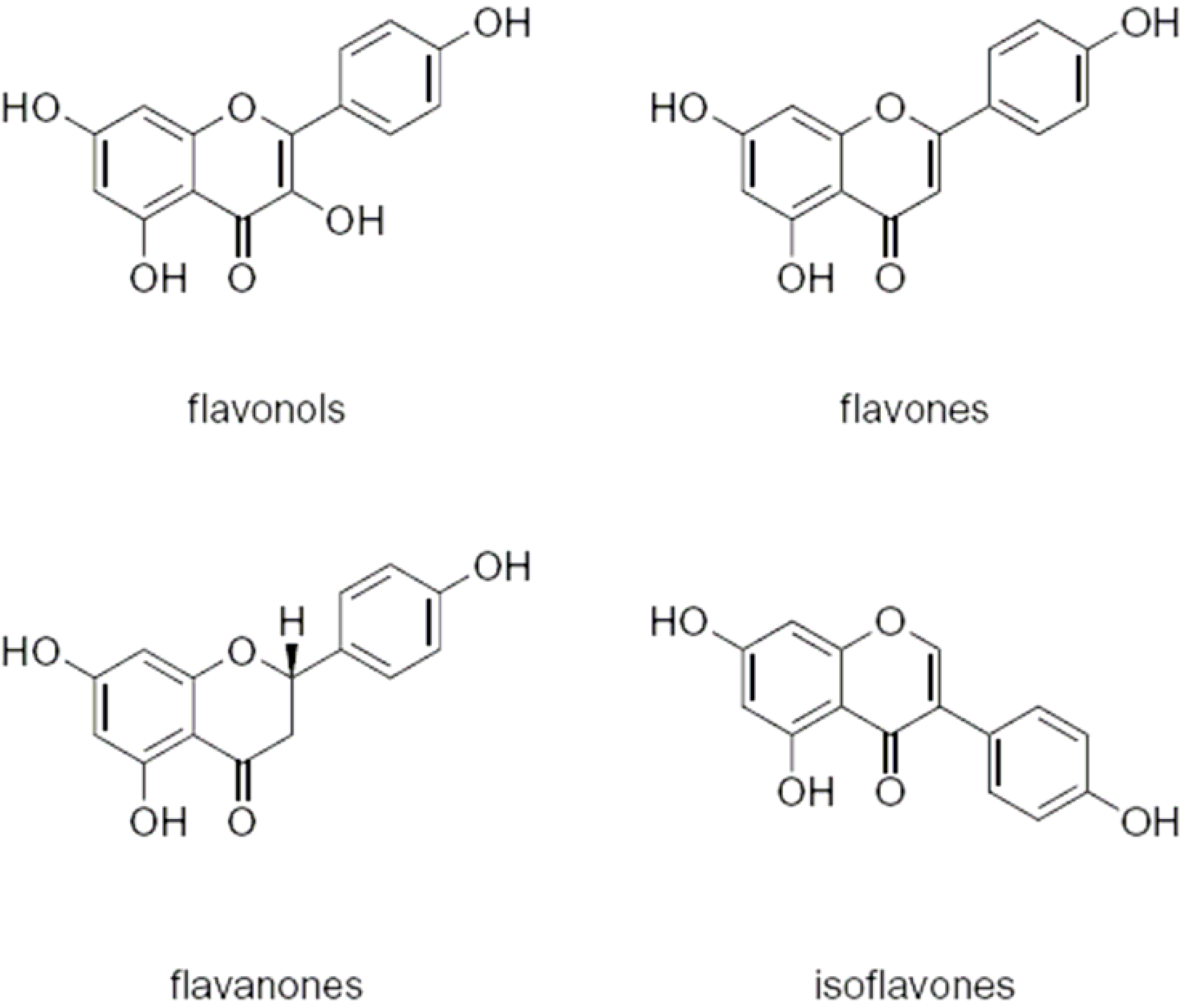
2.1. Flavonoids and AMF
2.2. Flavonoids and pathogenic fungi
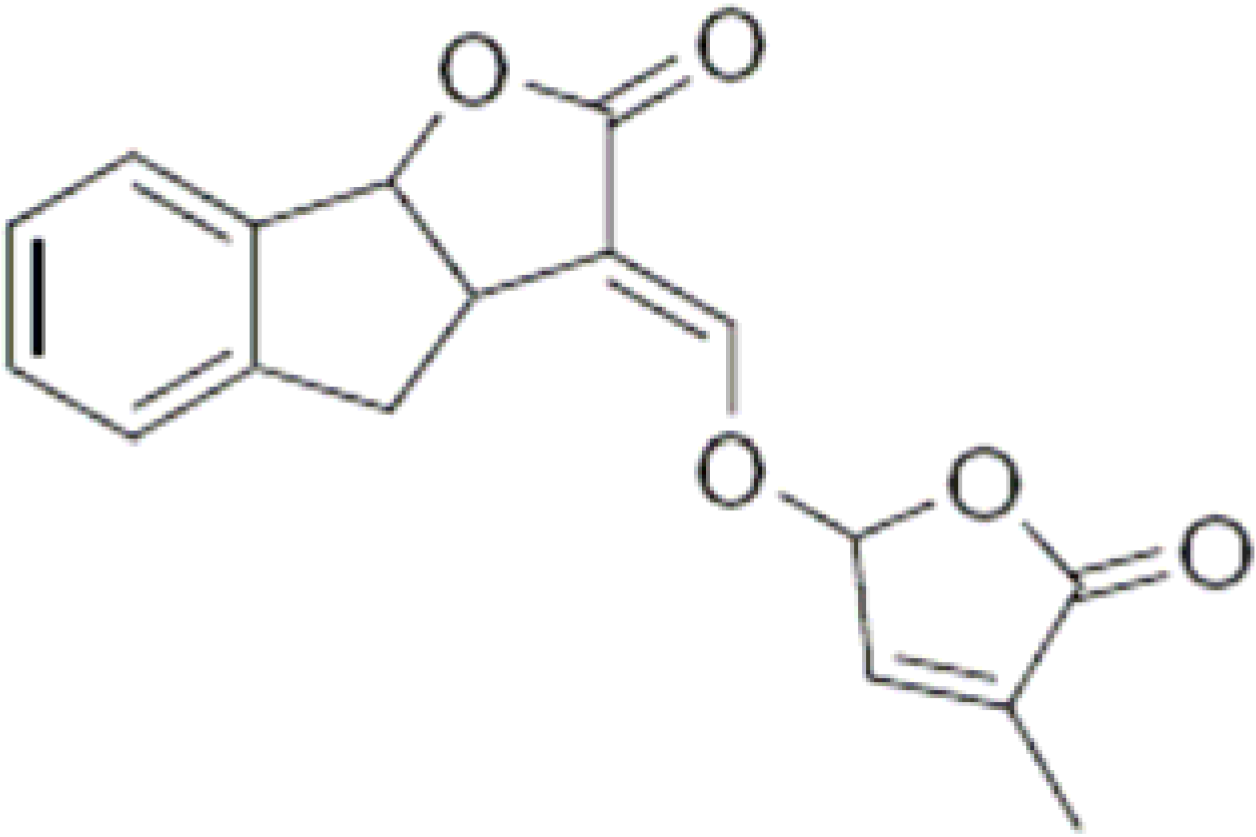
3. Strigolactones
| Family | Plant species | Host for Striga and/or Orobanche | Strigolact. detected in root exudates (1) | Root exudates stimulate Striga/ Orob. germinat. (2) | Root exudat. initiate branching of AMF (3) | Reference |
|---|---|---|---|---|---|---|
| Alliaceae | Onion | Orob. | 2) [38] | |||
| Apiaceae | Celery | Orob. | 2) [39] | |||
| Asteraceae | Lettuce | Orobanche | Orob. | 2) [39,97] | ||
| Sunflower | Orobanche | Orob. | 2) [38,39] | |||
| Brassicaceae | Arabidopsis thaliana | AM nonhost | Orob. | no | 2) [40,41] 3) [42] | |
| Rapeseed | AM nonhost | Orob. | no | 2) [43] 3) [42] | ||
| Cabbage | AM nonhost | Orob. | no | 2) [44] 3) [42] | ||
| Chenopodiaceae | Sugar beet | AM nonhost | no | 3) [42,45] | ||
| Cucurbitaceae | Cucumber | Orob. | 2) [38,39] | |||
| Gramineae | Maize | Striga | Yes | Strig.+Orob. | Yes | 1) [46] 2) [38,39,47] 3) [42] |
| Sorghum | Striga | Yes | Strig.* +Orob | Yes | 1) [46,47,48] 2) [47, 49*] 3) [42,50] | |
| Millet | Striga | Yes | Orob. | 1) [46, 47] 2) [47] | ||
| Barley | Strig.+Orob. | 2) [39,51] | ||||
| Oat | Orob. | 2) [39] | ||||
| Wheat | Orob. | 2) [39] | ||||
| Ryegrass | Orob. | 2) [39] | ||||
| Lamiaceae | Basil | Orob. | 2) [38] | |||
| Leguminoseae | Pea | Orobanche | Orob. | Yes | 2) [53] 3) [42] | |
| Medicago truncatula | Orob. | Yes | 2) [54] 3) [42] | |||
| Alfalfa | Orob. | Yes | 2) [39] 3) [42] | |||
| Red clover | Orobanche | Yes | Orob. | 1) [55,56] 2) [39] | ||
| Leguminoseae | Cowpea | Striga | Yes | Strig. | 1) [57] 2) [58] | |
| Soybean | Yes | Strig.+Orob. | 1) [59] 2) [38,60,61] | |||
| Lotus japonicus | Yes | 1) [59] | ||||
| Linaceae | Flax | Orob. | 2) [39] | |||
| Malvaceae | Cotton | Yes | Strig.+Orob. | 1) [56,62] 2) [56] | ||
| Solanaceae | Tobacco | Striga/ Orobanche | Yes | Strig.+Orob. | Yes | 2) [41,63] 3) [42] |
| Tomato | Orobanche | Yes | Orob. | Yes | 1) [64] 2) [38,39,65] 3) [42,45] | |
| Tropaeolaceae | Tropaelum majus | Orob. | 2) [39] |
3.1. Strigolactones and AMF
3.2. Strigolactones, Fusarium sp. and other fungi
| Fungal group | Fungus | Growth medium | Observation after treatment | Effect on branching |
|---|---|---|---|---|
| Ectomycorrhiza | Paxillus involutus | ½ modified Melin Norkans medium | 22, 24h | No |
| Laccaria bicolor | ½ modified Melin Norkans medium | 22h | No | |
| Amanita muscaria | ½ modified Melin Norkans medium | 22h | No | |
| Cenococcum geophilum | ½ modified Melin Norkans medium | 16h | No | |
| Beneficial fungi | Trichoderma | Water agar | 16h | No |
| Piriformospora indica | Nutrient agar | 18h | No | |
| Soil-borne pathogen | Rhizoctonia solani | Water agar Potato dextrose agar | 6 , 16, 40 h | No |
| Fusarium oxysporum | Water agar Potato dextrose agar | 16, 38h | No | |
| Verticillium dahliae | Water agar Potato dextrose agar | 23h | No | |
| Pathogen on aerial plant parts | Botrytis cinerea | Water agar | 23h | No |
| Cladosporium sp. | Water agar | 6, 30h | No |
4. Conclusions
Acknowledgments
References
- Neumann, G.; Römheld, V. The release of root exudates as affected by the plant physiological status. In The Rhizosphere: Biochemistry and organic substances at the soil-plant interface; Pinton, R., Varanini, Z., Nannipieri, P., Eds.; Marcel Dekker: New York, 2001. [Google Scholar]
- Uren, N. C. Types, amounts, and possible functions of compounds released into the rhizosphere by soil-grown plants. In The Rhizosphere: Biochemistry and organic substances at the soil-plant interface; Pinton, R., Varanini, Z., Nannipieri, P., Eds.; Marcel Dekker: New York, 2000; pp. 19–40. [Google Scholar]
- Bertin, C.; Yang, X.; Weston, L. A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Nelson, E. B.; Hsu, J. S. T. Nutritional factors affecting responses of sporangia of Pythium ultimum to germination stimulants. Phytopathology 1994, 84, 677–683. [Google Scholar] [CrossRef]
- Nelson, E.B. Exudate molecules initiating fungal response to seed and roots. In The rhizosphere and plant growth; Keister, D. L., Cregan, P. B., Eds.; Kluwer: Dortrecht, 1991; pp. 197–209. [Google Scholar]
- Bais, H. P.; Weir, T. L.; Perry, L. G.; Gilroy, S.; Vivanco, J. M. The role of root exudates in rhizosphere interactions with plants and other organisms. Ann. Rev. Plant Biology 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Perret, X.; Staehelin, C.; Broughton, W. J. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 2000, 64, 180–201. [Google Scholar] [CrossRef]
- Recourt, K.; Van Tunen, A. J.; Mur, L. A.; Van Brussel, A. A. N.; Lugtenberg, B.; Kijne, J. W. Activation of flavonoid biosynthesis in roots of Vicia sativa subsp. nigra plants by inoculation with Rhizobium leguminosarum biovar viciae. Plant Mol. Biol. 1992, 19, 411–420. [Google Scholar] [CrossRef]
- Dakora, F. D.; Joseph, C. M.; Phillips, D. A. Alfalfa (Medicago sativa L.) root exudates contain isoflavonoids in the presence of Rhizobium meliloti. Plant Physiol. 1993, 101, 819–824. [Google Scholar]
- Schmidt, P. E.; Broughton, W. J.; Werner, D. Nod factors of Bradyrhizobium japonicum and Rhizobium sp. NGR234 induce flavonoid accumulation in soybean root exudates. Mol. Plant-Microbe Interact. 1994, 7, 384–390. [Google Scholar] [CrossRef]
- Bolanos-Vasquez, M. C.; Werner, D. Effect of Rhizobium tropici, R. etli, and R. leguminosarum bv. phaseoli on nod gene-inducing flavonoids in root exudates of Phaseolus vulgaris. Mol. Plant-Microbe Interact. 1997, 10, 339–346. [Google Scholar] [CrossRef]
- Morandi, D. Occurrence of phytoalexins and phenolic compounds on endomycorrhizal interactions, and their potential role in biological control. Plant Soil 1996, 185, 241–251. [Google Scholar] [CrossRef]
- Vierheilig, H.; Bago, B.; Albrecht, C.; Poulin, M.-P.; Piché, Y. Flavonoids and arbuscular-mycorrhizal fungi. In Flavonoids in the Living System; Manthey, J.A., Buslig, B. S., Eds.; Plenum Press: New York, 1998; pp. 9–33. [Google Scholar]
- Bécard, G.; Douds, D. D.; Pfeffer, P. E. Extensive in vitro hyphal growth of vesicular-arbuscular mycorrhizal fungi in the presence of CO2 and flavonols. Appl. Environ. Microbiol. 1992, 68, 1260–1264. [Google Scholar]
- Chabot, S.; Bel-Rhlid, R.; Chênevert, R.; Piché, Y. Hyphal growth promotion in vitro of the VA mycorrhizal fungus, Gigaspora margarita Becker & Hall, by the activity of structurally specific flavonoids compounds under CO2-enriched conditions. New Phytol. 1992, 122, 461–467. [Google Scholar] [CrossRef]
- Scervino, J. M.; Ponce, M. A.; Erra-Bassels, R.; Vierheilig, H.; Ocampo, J. A.; Godeas, A. Glycosidation of apigenin results in a loss of activity on different growth parameters of arbuscular mycorrhizal fungi from the genus Glomus and Gigaspora. Soil Biol. Biochem. 2006, 38, 2919–2922. [Google Scholar] [CrossRef]
- Poulin, M.-J.; Bel-Rhlid, R.; Piché, Y.; Chênevert, R. Flavonoids released by carrot (Daucus carota) seedlings stimulate hyphal development of vesicular-arbuscular mycorrhizal fungi in the presence of optimal CO2 enrichment. J. Chem. Ecol. 1993, 19, 2317–2327. [Google Scholar] [CrossRef]
- Scervino, J. M.; Ponce, M. A.; Erra-Bassels, R.; Vierheilig, H.; Ocampo, J. A.; Godeas, A. Flavonoids exhibit fungal species and genus specific effects on the presymbiotic growth of Gigaspora and Glomus. Mycol. Res. 2005, 109, 789–794. [Google Scholar] [CrossRef]
- Scervino, J.M.; Ponce, M. A.; Erra-Bassels, R.; Vierheilig, H.; Ocampo, J.A.; Godeas, A. Flavonoids exclusively present in mycorrhizal roots of white clover exhibit different effects on arbuscular mycorrhizal fungi than flavonoids exclusively present in non-mycorrhizal roots of white clover. J. Plant Interact. 2005, 15, 22–30. [Google Scholar]
- Scervino, J. M.; Ponce, M. A.; Erra-Bassels, R.; Vierheilig, H.; Ocampo, J. A.; Godeas, A. Arbuscular mycorrhizal colonization of tomato by Gigaspora and Glomus species in presence of roots flavonoids. J. Plant Physiol. 2005, 162, 625–633. [Google Scholar] [CrossRef]
- Morandi, D.; Branzanti, B.; Gianinazzi-Pearson, V. Isoflavonoid accumulation in soybean roots infected with vesicular-arbuscular mycorrhizal fungi. Physiol. Plant Pathol. 1984, 24, 357–364. [Google Scholar] [CrossRef]
- Larose, G.; Chenevert, R.; Moutoglis, P.; Gagne, S.; Piché, Y.; Vierheilig, H. Flavonoid levels in roots of Medicago sativa are modulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus. J. Plant Physiol. 2002, 159, 1329–1339. [Google Scholar] [CrossRef]
- Harrison, M.; Dixon, R. Isoflavonoid accumulation and expression of defense gene transcripts during the establishment of vesicular arbuscular mycorrhizal associations in roots of Medicago truncatula. Mol. Plant-Microbe Interact. 1993, 6, 643–659. [Google Scholar] [CrossRef]
- Catford, J. G.; Staehelin, C.; Larose, G.; Piché, Y.; Vierheilig, H. Systemically suppressed isoflavonoids and their stimulating effects on nodulation and mycorrhization in alfalfa split-root systems. Plant Soil 2006, 285, 257–266. [Google Scholar] [CrossRef]
- Bécard, G.; Taylor, L. P.; Douds, D. D.; Pfeffer, P. E.; Doner, L. W. Flavonoids are not necessary plant signal compounds in arbuscular mycorrhizal symbiosis. Mol. Plant-Microbe Interact. 1995, 8, 252–258. [Google Scholar] [CrossRef]
- Morris, P. F.; Ward, E. W. B. Chemoattraction of zoospores of the soybean pathogen, P. sojae, by isoflavones. Physiol. Mol. Plant Pathol. 1992, 40, 17–22. [Google Scholar] [CrossRef]
- Tyler, B. M.; Wu, M.; Wang, J.; Cheung, W.; Morris, P. F. Chemotactic preferences and strain variation in the response of Phytophthora sojae zoospores to host isoflavonones. Appl. Environ. Microbiol. 1996, 62, 2811–2817. [Google Scholar]
- Morris, P. F.; Bone, E.; Tyler, B. M. Chemotropic and contact responses of Phytophthora sojae hyphae to soybean isoflavonoids and artificial substrates. Plant Physiol. 1998, 117, 1171–1178. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, A. M.; Dietz Bauer, W.; Bird, D. M.; Cullimore, J.; Tyler, B.; Yoder, J. I. Molecular signals and receptors: controlling rhizosphere interactions between plants and other organisms. Ecology 2003, 84, 858–868. [Google Scholar] [CrossRef]
- Ruan, Y.; Kotraiah, V.; Straney, D. C. Flavonoids stimulate spore germination in Fusarium solani pathogenic on legumes in a manner sensitive to inhibitors of cAMP-dependent protein kinase. Mol. Plant Microb. Interact. 1995, 8, 929–938. [Google Scholar] [CrossRef]
- Bagga, S.; Straney, D. C. Modulation of cAMP and phosphodiesterase activity by flavonoids which induce spore germination of Nectria haematococa. Physiol. Molec. Plant Pathol. 2000, 56, 51–61. [Google Scholar] [CrossRef]
- Steinkellner, S.; Mammerler, R. Effect of flavonoids on the development of Fusarium oxysporum f. sp. lycopersici. J. Plant Interact. 2007. accepted. [Google Scholar]
- Straney, D.; Khan, R.; Tan, R.; Bagga, S. Host recognition by pathogenic fungi through plant flavonoids. In Flavonoids in cell function; Buselig, B., Manthey, J., Eds.; Kluwer Academic/Plenum Publishers: New York, 2002; pp. 9–22. [Google Scholar]
- Steinkellner, S.; Mammerler, R.; Vierheilig, H. Microconidia germination of the tomato pathogen Fusarium oxysporum in the presence of root exudates. J. Plant Interact. 2005, 1, 23–30. [Google Scholar] [CrossRef]
- Curir, P.; Dolci, M.; Lanzotti, V.; Taglialata-Scafati, O. Kaempferide triglycoside: a possible factor of resistance of carnation (Dianthus caryophyllus) to Fusarium oxysporum f. sp. dianthi. Phytochemistry 2001, 56, 717–721. [Google Scholar] [CrossRef]
- Curir, P.; Dolci, M.; Galeotti, F. A phytoalexin-like flavonol involved in the carnation (Dianthus caryophyllus)-Fusarium oxysporum f.sp. dianthi pathosystem. J. Phytopathology 2005, 153, 65–67. [Google Scholar] [CrossRef]
- Bouwmeester, H. J.; Roux, C.; Lopez-Raez, J. A.; Bécard, G. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci. 2007, 12, 224–230. [Google Scholar] [CrossRef]
- Yoneyama, K.; Takeuchi, Y.; Yokota, T. Production of clover broomrape seed germination stimulants by red clover root requires nitrate but is inhibited by phosphate and ammonium. Physiol. Planta. 2001, 112, 25–30. [Google Scholar] [CrossRef]
- Ross, K. C.; Colquhoun, J. B.; Mallory-Smith, C. A. Small broom rape (Orobanche minor) germination and early development in response to plant species. Weed Sci. 2004, 52, 260–266. [Google Scholar] [CrossRef]
- Goldwasser, Y.; Yodder, J. I. Differential induction of Orobanche seed germination by Arabidopsis thaliana. Plant Sci. 2001, 160, 951–959. [Google Scholar] [CrossRef]
- Westwood, J. H. Characterization of the Orobanche-Arabidopsis system for studying parasite-host interactions. Weed Sci. 2000, 48, 742–748. [Google Scholar] [CrossRef]
- Buée, M.; Rossignol, M.; Jauneau, A.; Ranjeva, R.; Bécard, G. The pre-symbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates. Mol. Plant-Microbe Interact. 2000, 13, 693–698. [Google Scholar] [CrossRef]
- Benharrat, H.; Boulet, C.; Veronesi, C.; Thalouarn, P. An overview of ongoing laboratory and field studies carried out on Orobanche ramosa: a pest for rape seed, hemp and tobacco. Phytoma 2003, 564, 24–26. [Google Scholar]
- Jacobsohn, R.; Levy, D. Glyphosate for Orobanche control in various crops: problems and promises. In Proceedings of Workshop on Biology and Control of Orobanche; ter Borg, S.J., Ed.; Wageningen, The Netherlands, 1986; pp. 171–175. [Google Scholar]
- Nagahashi, G.; Douds, D. D. Partial separation of root exudate components and their effects upon the growth of germinated spores of AM fungi. Mycol. Res. 2000, 104, 1453–1464. [Google Scholar] [CrossRef]
- Siame, B. A.; Weerasuriya, Y.; Wood, K.; Ejeta, G.; Butler, L. G. Isolation of strigol, a germination stimulant for Striga asiatica, from host plants. J. Agric. Food Chem. 1993, 41, 1486–1491. [Google Scholar] [CrossRef]
- Awad, A. A.; Sato, D.; Kusumoto, D.; Kamioka, H.; Takeuchi, Y.; Yoneyama, K. Characterization of strigolactones, germination stimulants for the root parasitic plants Striga and Orobanche, produced by maize, millet and sorghum. Plant Growth Regul. 2006, 48, 221–227. [Google Scholar]
- Hauck, C.; Muller, S.; Schildknecht, H. A germination stimulant for parasitic flowering plants from Sorghum bicolor, a genuine host plant. J. Plant Physiol. 1992, 139, 474–478. [Google Scholar] [CrossRef]
- Lendzemo, V. W.; Kuyper, T. W.; Matusova, R.; Bouwmeester, H. J.; Van Ast, A. Colonization by arbuscular mycorrhizal fungi of sorghum leads to reduced germination and subsequent attachment and emergence of Striga hermonthica. Plant Signal. Behav. 2007, 2, 58–62. [Google Scholar] [CrossRef]
- Besserer, A.; Puech-Pàges, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J. C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biology 2006, 4, 1239–1247. [Google Scholar]
- Reda, F. Studies on striga host range and sorghum genotype screening for resistance. In Proceedings of the 5th International Symposium on Parasitic Weeds, Nairobi, Kenya, 24-30 June, 1991; Ransom, J. K., Musselman, L. J., Worsham, A. D., Eds.; CIMMYT: Nairobi, Kenya, 1991; pp. 545–550. [Google Scholar]
- Matusova, R.; Rani, K.; Verstappen, F. W. A.; Franssen, M. C. R.; Beale, M. H.; Bouwmeester, H. J. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 2005, 139, 920–934. [Google Scholar] [CrossRef]
- Rubiales, D.; Pérez-de-Luque, A.; Cubero, J. I.; Sillero, J. C. Crenate broomrape (Orobanche crenata) infection in field pea cultivars. Crop Prot. 2003, 22, 865–872. [Google Scholar] [CrossRef]
- Rodriguez-Conde, M. F.; Moreno, M. T.; Cubero, J. I.; Rubiales, D. Characterization of the Orobanche-Medicago truncatula for studying early stages of the host-parasite interaction. Weed Res. 2004, 44, 218–223. [Google Scholar] [CrossRef]
- Yokota, T.; Sakal, H.; Okuno, K.; Yoneyama, K.; Takeuchi, Y. Alectrol and Orobanchol, germination stimulants for Orobanche minor, from its host red clover. Phytochemistry 1998, 49, 1967–1973. [Google Scholar] [CrossRef]
- Sato, D.; Awad, A. A.; Takeuchi, Y.; Yoneyama, K. Confirmation and quantification of strigolactones, germination stimulants for root parasitic plants Striga and Orobanche, produced by cotton. Biosci. Biotechn. Biochem. 2005, 69, 98–102. [Google Scholar]
- Müller, S.; Hauck, C.; Schildknecht, H. Germination stimulants produced by Vigna unguiculata Walp cv. Saunders Upright. J. Plant Growth Regul. 1992, 11, 77–84. [Google Scholar] [CrossRef]
- Berner, D. K.; Williams, O. A. Germination Stimulation of Striga gesnerioides seeds by hosts and nonhosts. Plant Dis. 1998, 82, 1242–1247. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef]
- Gbehounou, G.; Adango, E. Trap crops of Striga hermonthica: in vitro identification and effectiveness in situ. Crop Prot. 2003, 22, 395–404. [Google Scholar] [CrossRef]
- Okpo, E. S.; Lagoke, S. T. O.; Ndahi, W. B.; Olufajo, O. O.; Tabo, R. Germination of witchweed (Striga hermonthica (Del.) Benth.) seeds in response to stimulation by root exudates of soybean (Glycine max L.). Global J. Agr. Sci. 2003, 2, 25–32. [Google Scholar]
- Cook, C. E.; Whichard, L. P.; Wall, M. E.; Egley, G. H.; Coggon, P.; Luhan, P. A.; McPhail, A. T. Germination stimulants. 2. The structure of strigol - a potent seed germination stimulant for witchweed (Striga lutea Lour.). J. Am. Chem. Soc. 1972, 94, 6198–6199. [Google Scholar] [CrossRef]
- Gonzalez-Verdejo, C. I.; Barandiaran, X.; Moreno, M. T.; Cubero, J. I.; di Pietro, A. An improved axenic system for studying pre-infection development of the parasitic plant Orobanche ramosa. Ann. Bot. 2005, 96, 1121–1127. [Google Scholar] [CrossRef]
- Yoneyama, K.; Takeuchi, Y.; Sato, D.; Sekimoto, H.; Yokota, T. Determination and quantification of strigolactones. In Proceedings of the 8th International Parasitic Weed Symposium; Joel, D. M., Ed.; International Parasitic Plant Society: Amsterdam, 2004; p. 9. [Google Scholar]
- Jain, R.; Foy, C. L. Nutrient effects on parasitism and germination of Egyptian broomrape (Orobanche aegyptiaca). Weed Techn. 1991, 6, 269–275. [Google Scholar]
- Mosse, B.; Hepper, C. M. Vesicular-arbuscular mycorrhizal infections in root organ cultures. Physiol. Plant. Pathol. 1975, 5, 215–223. [Google Scholar] [CrossRef]
- Powell, C. L. Development of mycorrhizal infection from Endogene spores and infected root fragments. Trans. Br. Mycol. Soc. 1976, 66, 439–445. [Google Scholar] [CrossRef]
- Mosse, B. Some studies relating to “independent“ growth of vesicular-arbuscular endophytes. Can. J. Bot. 1988, 66, 2533–2540. [Google Scholar] [CrossRef]
- Giovannetti, M.; Sbrana, C.; Avio, L.; Citernesi, A. S.; Logi, C. Differential hyphal morphogenesis in arbuscular mycorrhizal fungi during pre-infection stages. New Phytol. 1993, 125, 587–594. [Google Scholar] [CrossRef]
- Giovannetti, M.; Sbrana, C.; Logi, C. Early processes involved in host recognition by arbuscular mycorrhizal fungi. New Phytol. 1994, 127, 703–709. [Google Scholar] [CrossRef]
- Giovannetti, M.; Sbrana, C. Meeting a non-host: the behaviour of AM fungi. Mycorrhiza 1998, 8, 123–130. [Google Scholar] [CrossRef]
- Tamasloukht, M. B.; Séjalon-Delmas, N.; Kluever, A.; Jauneau, A.; Roux, C.; Bécard, G.; Franken, P. Root factors induce mitochondrial-related gene expression and fungal respiration during the developmental switch from asymbiosis to presymbiosis in the arbuscular mycorrhizal fungus Gigaspora rosea. Plant Physiol. 2003, 131, 1468–1478. [Google Scholar] [CrossRef]
- Tsai, S. M.; Phillips, D. A. Flavonoids released naturally from alfalfa promote development of symbiotic Glomus spores in vitro. Appl. Environ. Microbiol. 1991, 57, 1485–1488. [Google Scholar]
- Phillips, D. A.; Tsai, S. M. Flavonoids as plant signals to the rhizosphere microbes. Mycorrhiza 1992, 1, 55–58. [Google Scholar] [CrossRef]
- Nagahashi, G.; Douds, D. D. Rapid and sensitive bioassay to study signals between root exudates and arbuscular mycorrhizal fungi. Biotechnol. Techniques 1999, 13, 893–897. [Google Scholar] [CrossRef]
- Nagahashi, G.; Douds, D. D. Action spectrum for the induction of hyphal branches of an arbuscular mycorrhizal fungus: exposure sites versus branching sites. Mycol. Res. 2003, 107, 1075–1082. [Google Scholar] [CrossRef]
- Akiyama, K.; Hayashi, H. Strigolactones: Chemical signals in fungal symbionts and parasitic weeds in plant roots. Ann. Bot. 2006, 97, 925–931. [Google Scholar] [CrossRef]
- Vierheilig, H.; Alt-Hug, M.; Engel-Streitwolf, R.; Mäder, P.; Wiemken, A. Studies on the attractional effect of root exudates on hyphal growth of an arbuscular mycorrhizal fungus in a soil compartment-membrane system. Plant Soil 1998, 203, 137–144. [Google Scholar] [CrossRef]
- Sbrana, C.M.; Giovannetti, M. Chemotropism in the arbuscular mycorrhizal fungus Glomus mosseae. Mycorrhiza 2005, 15, 539–545. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Roux, C.; Girard, D.; Bécard, G.; Puech, V. Strigolactones: Promising plant signals. Plant Signal. Behav. 2007, in press. [Google Scholar]
- Vierheilig, H.; Garcia-Garrido, J. M.; Wyss, U.; Piché, Y. Systemic suppression of mycorrhizal colonization of barley roots already colonized by AM fungi. Soil Biol. Biochem. 2000, 32, 589–595. [Google Scholar] [CrossRef]
- Vierheilig, H.; Maier, W.; Wyss, U.; Samson, J.; Strack, D.; Piché, Y. Cyclohexenone derivative- and phosphate-levels in split-root systems and their role in the systemic suppression of mycorrhization in precolonized barley plants. J. Plant Physiol. 2000, 157, 593–599. [Google Scholar] [CrossRef]
- Catford, J. G.; Staehelin, C.; Lerat, S.; Piché, Y.; Vierheilig, H. Suppression of arbuscular mycorrhizal colonization and nodulation in split-root systems of alfalfa after pre-inoculation and treatment with Nod factors. J. Exp. Bot. 2003, 54, 1481–1487. [Google Scholar] [CrossRef]
- Vierheilig, H. Further root colonization by arbuscular mycorrhizal fungi in already mycorrhizal plants is suppressed after a critical level of root colonization. J. Plant Physiol. 2004, 161, 339–341. [Google Scholar] [CrossRef]
- Meixner, C.; Ludwig-Müller, J.; Miersch, O.; Gresshoff, P.; Staehelin, C.; Vierheilig, H. Lack of mycorrhizal autoregulation and phytohormonal changes in the supernodulating soybean mutant nts1007. Planta 2005, 222, 709–715. [Google Scholar] [CrossRef]
- Meixner, C.; Vegvari, G.; Ludwig-Müller, J.; Gagnon, H.; Steinkellner, S.; Staehelin, C.; Gresshoff, P.; Vierheilig, H. Two defined alleles of the LRR receptor kinase GmNARK in supernodulating soybean govern differing autoregulation of mycorrhization. Physiol Plantarum 2007, 130, 261–270. [Google Scholar] [CrossRef]
- Lendzemo, V. W.; Kuyper, T. W. Effects of arbuscular mycorrhizal fungi on damage by Striga hermonthica on two contrasting cultivars of sorghum, Sorghum bicolor. Agr. Ecosyst. Environ. 2001, 87, 29–35. [Google Scholar] [CrossRef]
- Gworgwor, N. A.; Weber, H. C. Arbuscular mycorrhizal fungi-parasite-host interaction for the control of Striga hermonthica (Del.) Benth. in sorghum [Sorghum bicolor (L.) Moench]. Mycorrhiza 2003, 13, 277–281. [Google Scholar] [CrossRef]
- Lendzemo, V. W.; Kuyper, T. W.; Kropff, M. J.; van Ast, A. Field inoculation with arbuscular mycorrhizal fungi reduces Striga hermonthica performance on cereal crops and has the potential to contribute to integrated Striga management. Field Crops Res. 2005, 91, 51–61. [Google Scholar] [CrossRef]
- Lendzemo, V. W. The tripartite interaction between sorghum, Striga hermonthica, and arbuscular mycorrhizal fungi. PhD Thesis, Wageningen University, Wageningen, The Netherlands, 2004. [Google Scholar]
- Pinior, A.; Wyss, U.; Piché, Y.; Vierheilig, H. Plants colonized by AM fungi regulate further root colonization by AM fungi through altered root exudation. Can. J. Bot. 1999, 77, 891–897. [Google Scholar]
- Pinior, A. Wurzelexsudate mykorrhizierter Pflanzen und deren regulierender Einfluss auf arbuskuläre Mykorrhizapilze. Master Thesis, Christian-Albrechts-Universität Kiel, Kiel, Germany, 1999. [Google Scholar]
- Yoneyama, K.; Yoneyama, K.; Takeuchi, Y.; Sekimoto, H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasite. Planta 2007, in press. [Google Scholar]
- Yoneyama, K.; Takeuchi, Y.; Yokota, T. Natural germination stimulants for Orobanche minor Sm. In Proceedings of the 7th International Parasitic Weed Symposium; Fer, A., Thalouarn, P., Joel, D. M., Musselman, L. J., Parker, C., Verkleij, J. A. C., Eds.; Faculté des Sciences, Université de Nantes: Nantes, France, 2001; p. 123. [Google Scholar]
- Nagahashi, G.; Douds, D. D.; Abney, G. D. Phosphorus amendment inhibits hyphal branching of the VAM fungus Gigaspora margarita directly and indirectly through its effect on root exudation. Mycorrhiza 1996, 6, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.; Read, D. Mycorrhizal symbiosis; Academic Press: London, 1997. [Google Scholar]
- Landa, B. B.; Navas-Cortés, J. A.; Castillo, P.; Vovlas, N.; Pujadas-Salvà, A. J.; Jiménez- Díaz, R. M. First report of broomrape (Orobanche crenata) infecting lettuce in southern Spain. Plant Disease 2006, 90, 1112. [Google Scholar]
- Samples Availability: Available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Steinkellner, S.; Lendzemo, V.; Langer, I.; Schweiger, P.; Khaosaad, T.; Toussaint, J.-P.; Vierheilig, H. Flavonoids and Strigolactones in Root Exudates as Signals in Symbiotic and Pathogenic Plant-Fungus Interactions. Molecules 2007, 12, 1290-1306. https://doi.org/10.3390/12071290
Steinkellner S, Lendzemo V, Langer I, Schweiger P, Khaosaad T, Toussaint J-P, Vierheilig H. Flavonoids and Strigolactones in Root Exudates as Signals in Symbiotic and Pathogenic Plant-Fungus Interactions. Molecules. 2007; 12(7):1290-1306. https://doi.org/10.3390/12071290
Chicago/Turabian StyleSteinkellner, Siegrid, Venasius Lendzemo, Ingrid Langer, Peter Schweiger, Thanasan Khaosaad, Jean-Patrick Toussaint, and Horst Vierheilig. 2007. "Flavonoids and Strigolactones in Root Exudates as Signals in Symbiotic and Pathogenic Plant-Fungus Interactions" Molecules 12, no. 7: 1290-1306. https://doi.org/10.3390/12071290




