(-)-Catechin in Cocoa and Chocolate: Occurence and Analysis of an Atypical Flavan-3-ol Enantiomer
Abstract
:Introduction
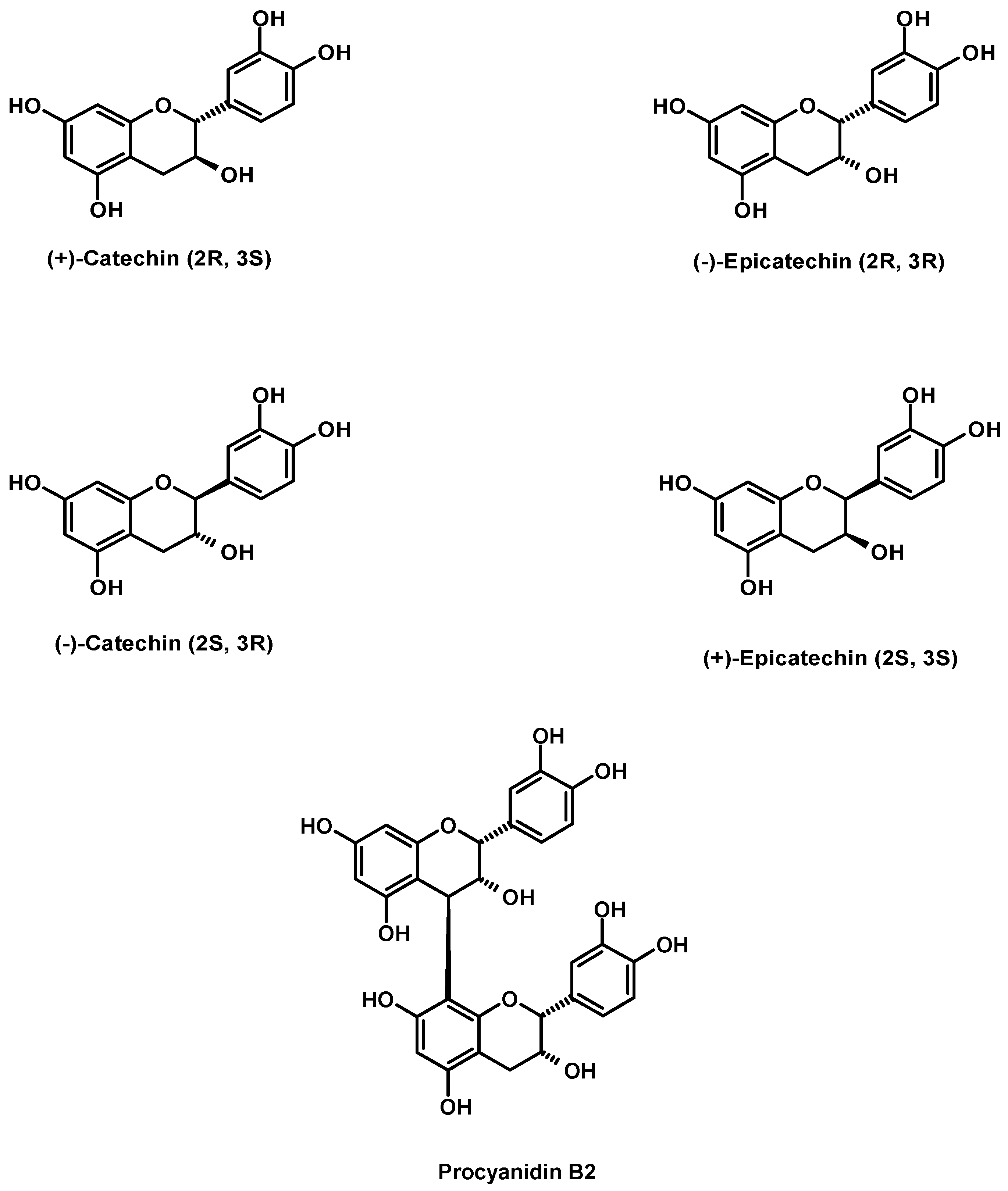
Results and Discussion
Analysis of cocoa and cocoa products by CCE
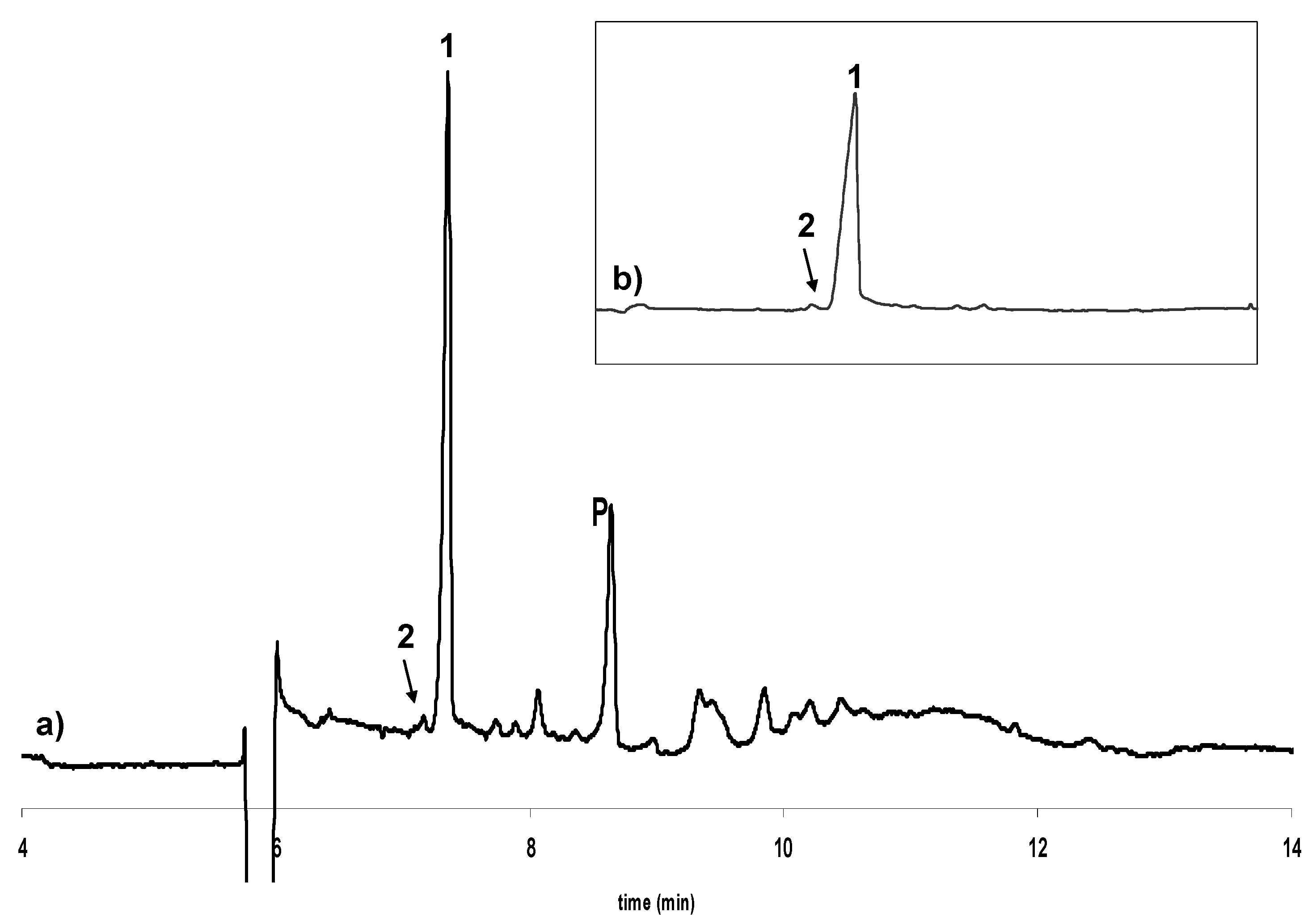
Influence of temperature
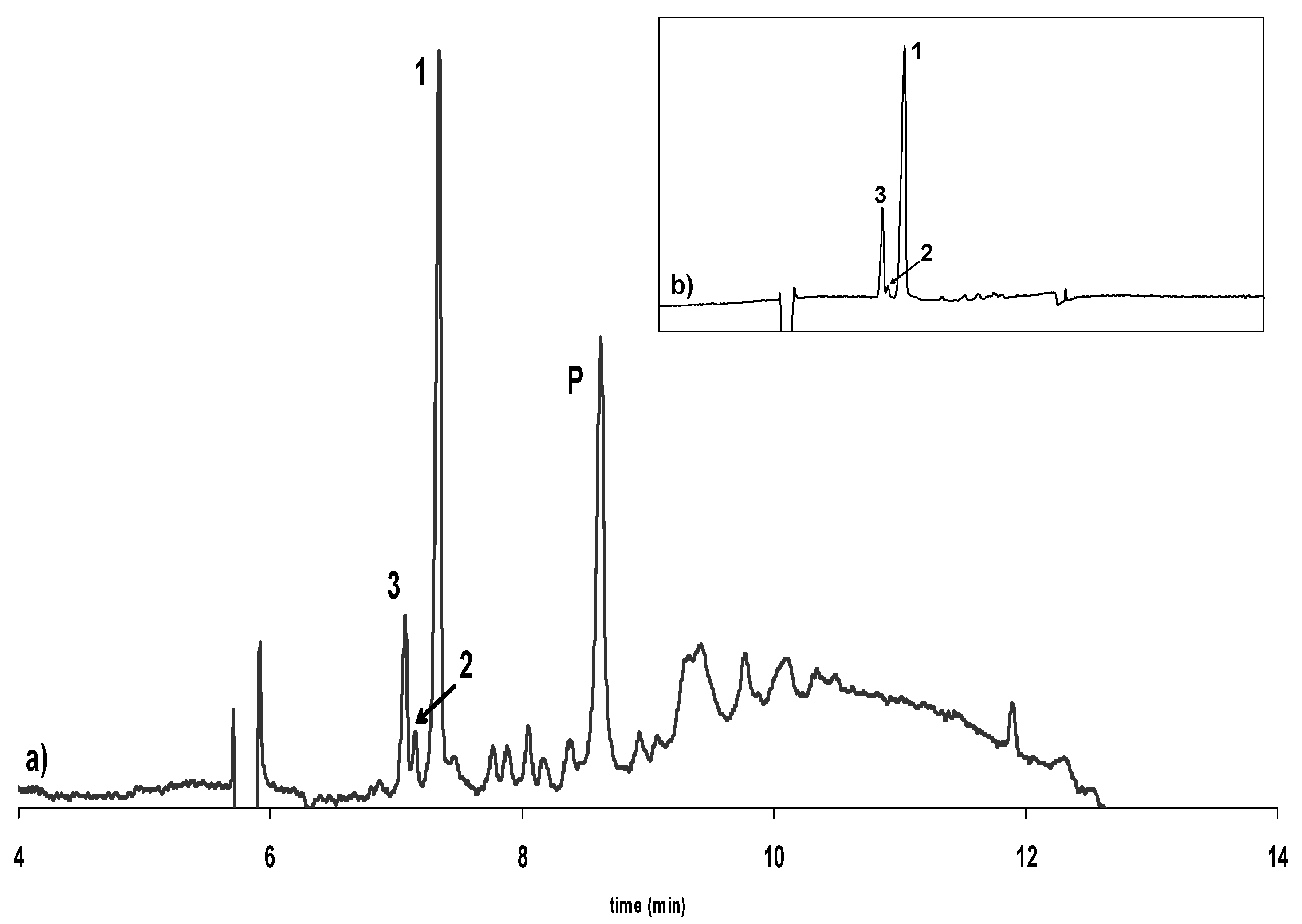
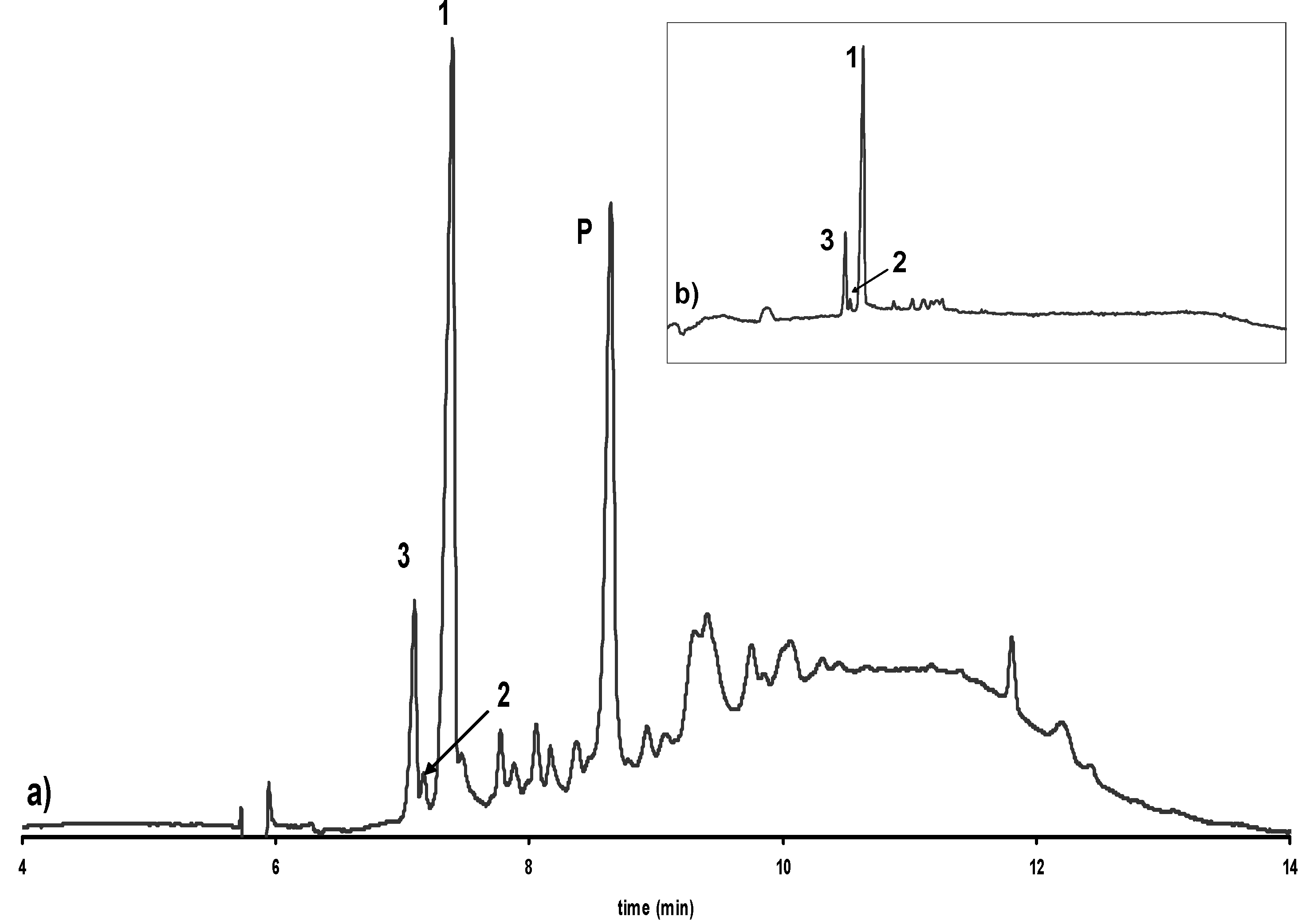
Influence of alkalization
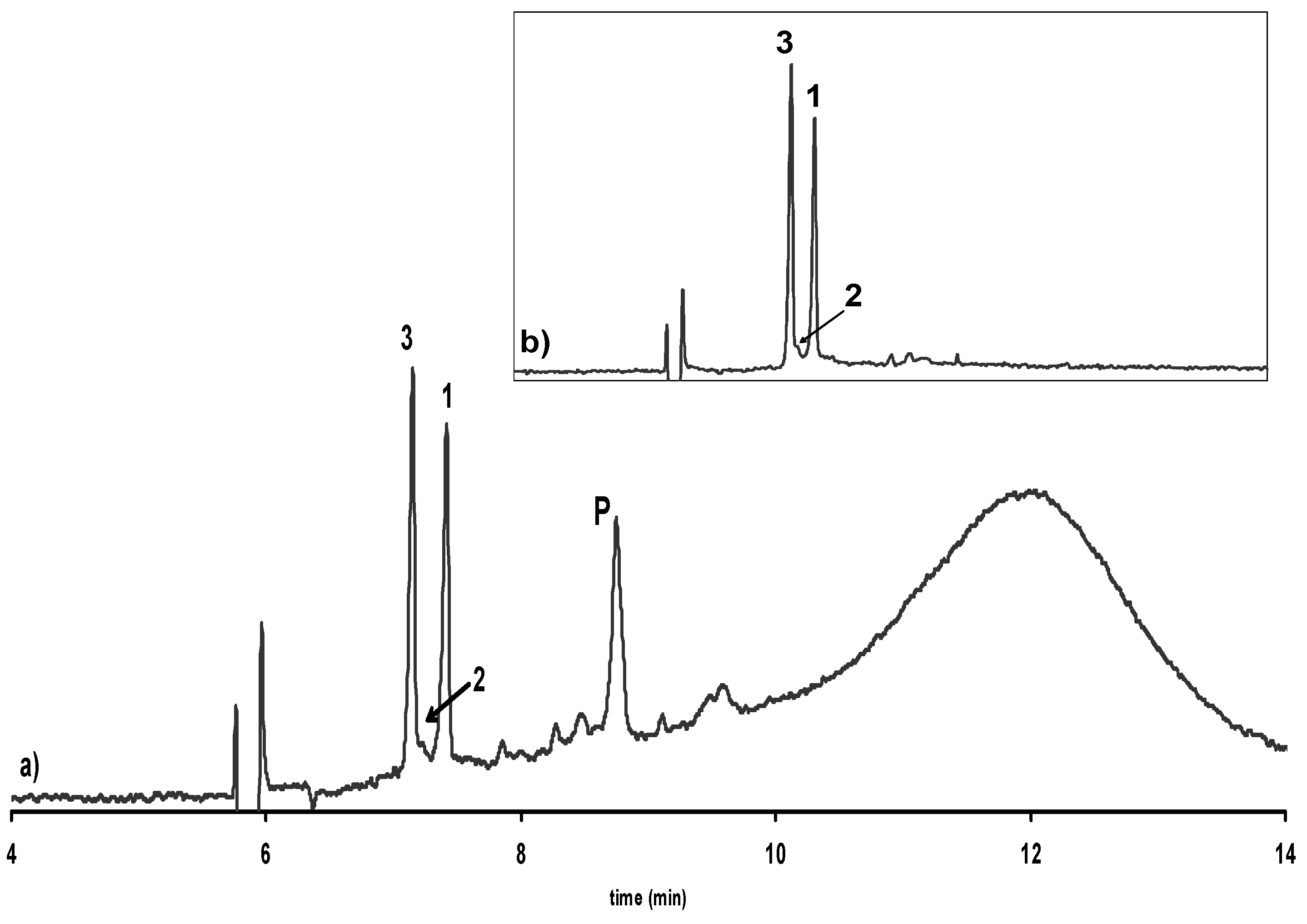
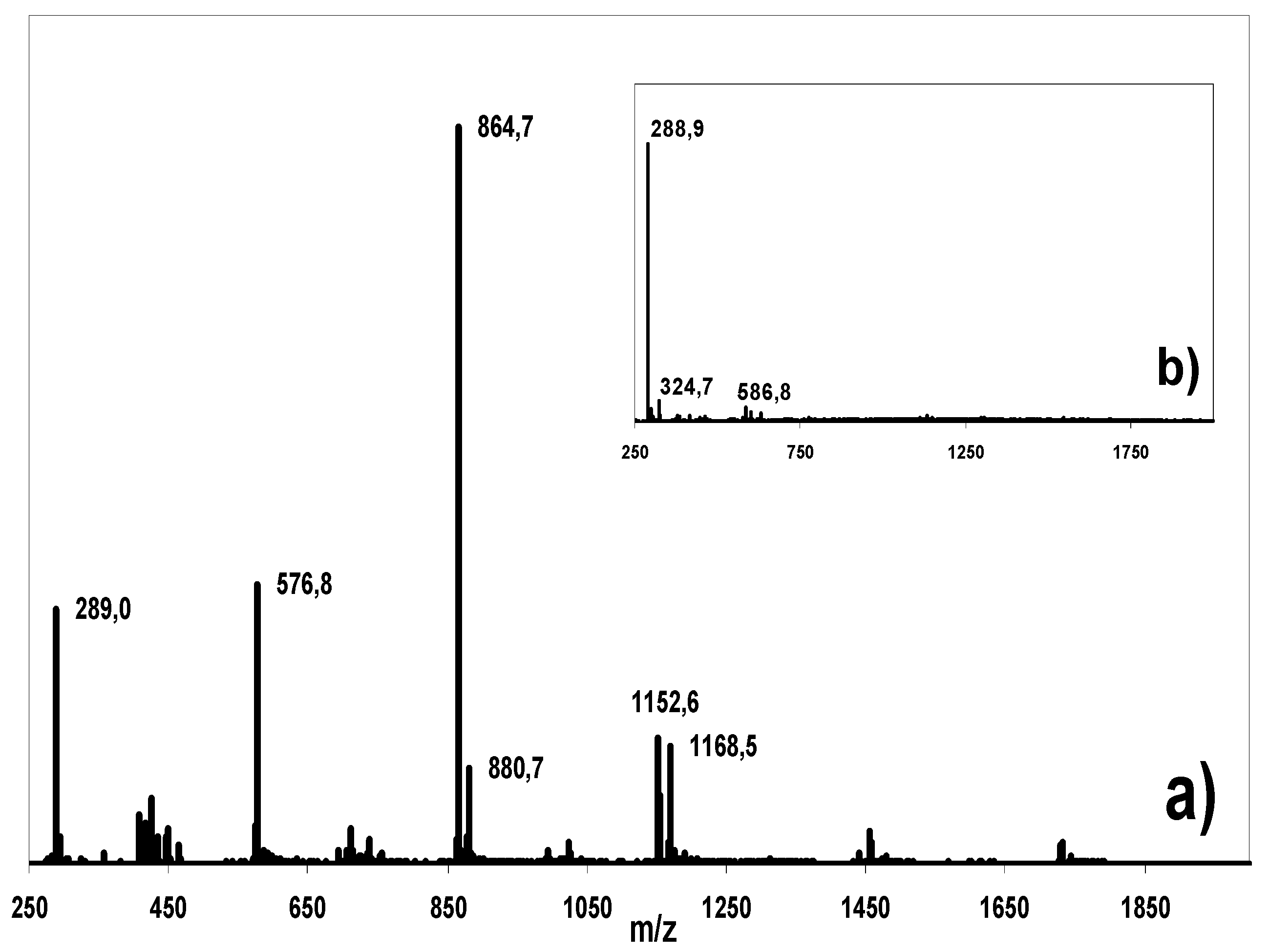
Isolation, purification and LC-MS analysis
Comparison of the flavan-3-ol peak ratios
| Raw, unfermented cocoa beans | - * |
| Roasted cocoa beans | 18 : 82 |
| Roasted cocoa beans (nibs) | 19 : 81 |
| Cocoa liquor (non-alkalized) | 18 : 82 |
| Chocolate (85% cocoa solids) | 18 : 82 |
| Cocoa Powder (non-alkalized) | 24 : 76 |
| Cocoa Powder (non- alkalized) | 35 : 65 |
| Cocoa Powder (alkalized)** | 42 : 58 |
| Cocoa Powder (alkalized) ** | 65 : 35 |
Conclusions
Outlook
Experimental
General
Roasting, alkalization, temperature and UV experiments
Isolation and purification of monomeric flavan-3-ols from extracts
HPLC- MS analysis
Chiral capillary electrophoretic analysis
Analytical characteristics (CCE)
Acknowledgments
References
- Arts, I.C.W.; van de Putte, B.; Hollman, P.C.H. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food Chem. 2000, 48, 1746–1751. [Google Scholar]
- Harnley, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid content of U.S. fruits, vegetables, and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar]
- Hooper, L.; Cassidy, A. A review of the health potential of bioactive compounds. J. Sci. Food Agric. 2006, 86, 1805–1813. [Google Scholar] [CrossRef]
- Treutter, D. Significane of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147–157. [Google Scholar] [CrossRef]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2006, 51, 116–134. [Google Scholar] [CrossRef]
- Kermez, Z.; Chetrit, D.; Shoseyov, O.; Regev-Shosani, G. Protection of lipids from oxidation by epicatechin, trans-resveratrol, and gallic and caffeic acids in intestinal model systems. J. Agric. Food Chem. 2006, 54, 10288–10293. [Google Scholar] [CrossRef]
- Murphy, K.J.; Chronopoulos, A.K.; Singh, I.; Francis, M.A.; Moriarty, H.; Pike, M.J.; Turner, A.H.; Mann, N.J.; Sinclair, A.J. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cocoa) inhibit platelet function. Am. J. Clin. Nutr. 2003, 77, 1466–1473. [Google Scholar]
- Hurell, R.F.; Reddy, M.; Cook, J.D. Inhibition of non-haem iron aborption in man by polyphenolic-containing beverages. Brit. J. Nutr. 1999, 81, 289–295. [Google Scholar] [PubMed]
- Vinson, J.A.; Proch, J.; Bose, P.; Muchler, S.; Taffera, P.; Shuta, D.; Samman, N.; Agbor, G.A. Chocolate is a powerfull ex vivo and in vivo antioxidant, an antiathereosclerotic agent in animal model, and a significant contributor to antioxidants in european and american diets. J. Agric. Food Chem. 2006, 54, 8071–8076. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Crespy, V.; Oliveira, M.; Cooper, K.A.; Gibson, B.B.; Williamson, G. (+)-Catechin is more bioavailable than (-)-catechin: Relevance to the bioavailability of catechin from cocoa. Free Radical Res. 2006, 40, 1029–1034. [Google Scholar] [CrossRef]
- Nyfeler, F.; Moser, U.K.; Walter, P. Stereospecific effects of (+)- and (-)-catechin on glycogen metabolism in isolated rat hepatocytes. Biochim. Biophys. Acta 1983, 763, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liang, R.; Yang, B.; Wang, L.; Yang, Q.; Wang, Y. Protection of (-)-catechin gallate and (+)-epicatechin on xanthine-xanthine oxidase system injury in cultered cardiomyocytes. Zhongguo Shiyan Fangjixue Zazhi 2006, 12, 36–38. [Google Scholar]
- Cho, S.Y.; Park, P.J.; Shin, H.J.; Kim, Y.K.; Shin, D.W.; Shin, E.S.; Lee, H.H.; Lee, B.G.; Baik, J.H.; Lee, T.R. (-)-Catechin suppresses expression of Kruppel-like factor 7 and increases expression and secretion of adiponectin protein in 3T3-L1 cells. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1166–E1172. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, J.; Kühnel, C.; Brandt, J.; Duy, D.; Punyasiri, P.A.N.; Forkmann, G.; Fischer, T.C. Biosynthesis of flavan-3-ols by leucoanthocyanidin 4-reductase and anthocyanidin reductase in leaves of grape (Vitis vinifera L.), apple (Malus x domestica Borkh.) and other crops. Plant Physiol. Bioch. 2006, 44, 323–334. [Google Scholar]
- Ellis, C.J.; Yeap Foo, L.; Porter, L.J. Enantiomerism: A characteristic of the proanthocyanidin chemistry of the Monocotyledonae. Phytochem. 1983, 22, 483–487. [Google Scholar] [CrossRef]
- Blair, A.C.; Nissen, S.J.; Brunk, G.R.; Hufbauer, R.A. A lack of evidence for an ecological role of the putative allelochemical (+/-)-catechin in spotted knapweed invasion success. J. Chem. Ecol. 2006, 32, 2327–2331. [Google Scholar]
- Prithiviraj, B.; Perry, L.G.; Badri, D.V.; Vivanco, J. M. Chemical facilitation and induced pathogen resistance mediated by root-secreted phytotoxin. New Phytol. 2007, 173, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Kofink, M.; Papagiannopoulos, M.; Galensa, R. Enantioseparation of catechin and epicatechin in plant food by chiral capillary electrophoresis. Eur. Food Res. Technol. 2007, 225, 569–577. [Google Scholar] [CrossRef]
- Haslam, E. The flavonoids; Harborne, J.B., Ed.; Chapman and Hall: London, 1975; Chapter 10; pp. 550–553. [Google Scholar]
- Kennedy, J.A.; Munro, M.H.G.; Powell, H. K. J.; Porter, L.J.; Yeap Foo, L. The protonation reactions of catechin and epicatechin and related compounds. Aust. J. Chem. 1984, 37, 885–892. [Google Scholar] [CrossRef]
- Kodama, S.; Yamamoto, A.; Akinobu, M.; Yanai, H. Direct enatioseparation of catechin and epicatechin in tea drinks by 6-O-α-D-glucosyl-β-cyclodextrin-modified micellar electrokinetic chromatography. Electrophoresis 2004, 25, 2892–2898. [Google Scholar] [CrossRef] [PubMed]
- Gotti, R.; Furlanetto, S.; Pinzauti, S.; Cavrini, V. Analysis of catechins in Theobroma cacoa beans by cyclodextrin-modified micellar electrokinetic chromatography. J. Chromatogr. A 2006, 1112, 345–352. [Google Scholar] [CrossRef]
- Gel-Moreto, N. Enantiomerentrennung von Polyphenolen in Citrus mittels Kapillarelektrophorese (CE) und Hochleistungsflüssigkeitschromatographie (HPLC). PhD Thesis, University of Bonn, 2003. [Google Scholar]
- Hammerstone, J.F., JR.; Acquarone, V. Process for controlling the isomerization of (-)-epicatechin and (+)-catechin in edible products. U.S. Pat. Appl. 2007/0003640, 2007. [Google Scholar]
- Woefel, K.A.; Robbins, R.J. Products containing polyphenols. WO 2007/002883, 2007. [Google Scholar]
- Hammerstone, J.F., JR.; Acquarone, V. Heat-processed products having altered monomer profiles and processes for controlling the epimerization of (-)-epicatechin and (+)-catechin in the products. WO 2007/002852, 2007. [Google Scholar]
- Beckett, S.T. The science of chocolate; Royal Society of Chemistry: Cambridge, 2000; pp. 31–47. [Google Scholar]
- Komatsu, Y.; Suematsu, S.; Hisanobu, Y.; Saigo, H.; Matsuda, R.; Hara, K. Effects of pH and temperature on reaction kinetics of catechins in green tea infusion. Biosci. Biotech. Biochem. 1993, 57(6), 907–910. [Google Scholar]
- Zimmermann, B.F.; Galensa, R. One for all-all for one: Proof of authenticity and tracing of foods with flavonoids. Analysis of proanthocyanidins in barley and malt. Eur. Food Res. Technol. 2006, 24, 385–393. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, E. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000, 33, 423–447. [Google Scholar] [CrossRef]
- Kiatgrajai, P.; Wellons, J.D.; Gollob, L.; White, J.D. Kinetics of epimerization of (+)-catechin and its rearrangement to catechinic acid. J. Org. Chem. 1982, 47, 2910–2912. [Google Scholar] [CrossRef]
- Wätzig, H.; Degenhardt, M.; Kunkel, A. Strategies for capillary electrophoresis: Method development and validation for pharmaceutical and biological applications. Electrophoresis 1998, 19, 2695–2752. [Google Scholar]
- Papagiannopoulos, M.; Wollseifen, H.R.; Mellenthin, A.; Haber, B.; Galensa, R. Identification and quantification of polyphenols in Carob Fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MSn. J. Agric. Food Chem. 2004, 52, 3784–3791. [Google Scholar]
- Friedrich, W.; Eberhardt, A.; Galensa, R. Investigations of proanthocyanidins by HPLC with electrospray ionization mass spectrometry. Eur. Food Res. Technol. 2000, 211, 56–64. [Google Scholar] [CrossRef]
- Papagiannopoulos, M.; Zimmermann, B.; Mellenthin, A.; Krappe, M.; Maio, G.; Galensa, R. On-line coupling of pressurized liquid extraction, solid-phase-extraction and high-performance liquid chromatography for automated analysis of proanthocyanidins in malt. J. Chromatogr. A 2002, 958, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the corresponding author.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Kofink, M.; Papagiannopoulos, M.; Galensa, R. (-)-Catechin in Cocoa and Chocolate: Occurence and Analysis of an Atypical Flavan-3-ol Enantiomer. Molecules 2007, 12, 1274-1288. https://doi.org/10.3390/12071274
Kofink M, Papagiannopoulos M, Galensa R. (-)-Catechin in Cocoa and Chocolate: Occurence and Analysis of an Atypical Flavan-3-ol Enantiomer. Molecules. 2007; 12(7):1274-1288. https://doi.org/10.3390/12071274
Chicago/Turabian StyleKofink, Michael, Menelaos Papagiannopoulos, and Rudolf Galensa. 2007. "(-)-Catechin in Cocoa and Chocolate: Occurence and Analysis of an Atypical Flavan-3-ol Enantiomer" Molecules 12, no. 7: 1274-1288. https://doi.org/10.3390/12071274




