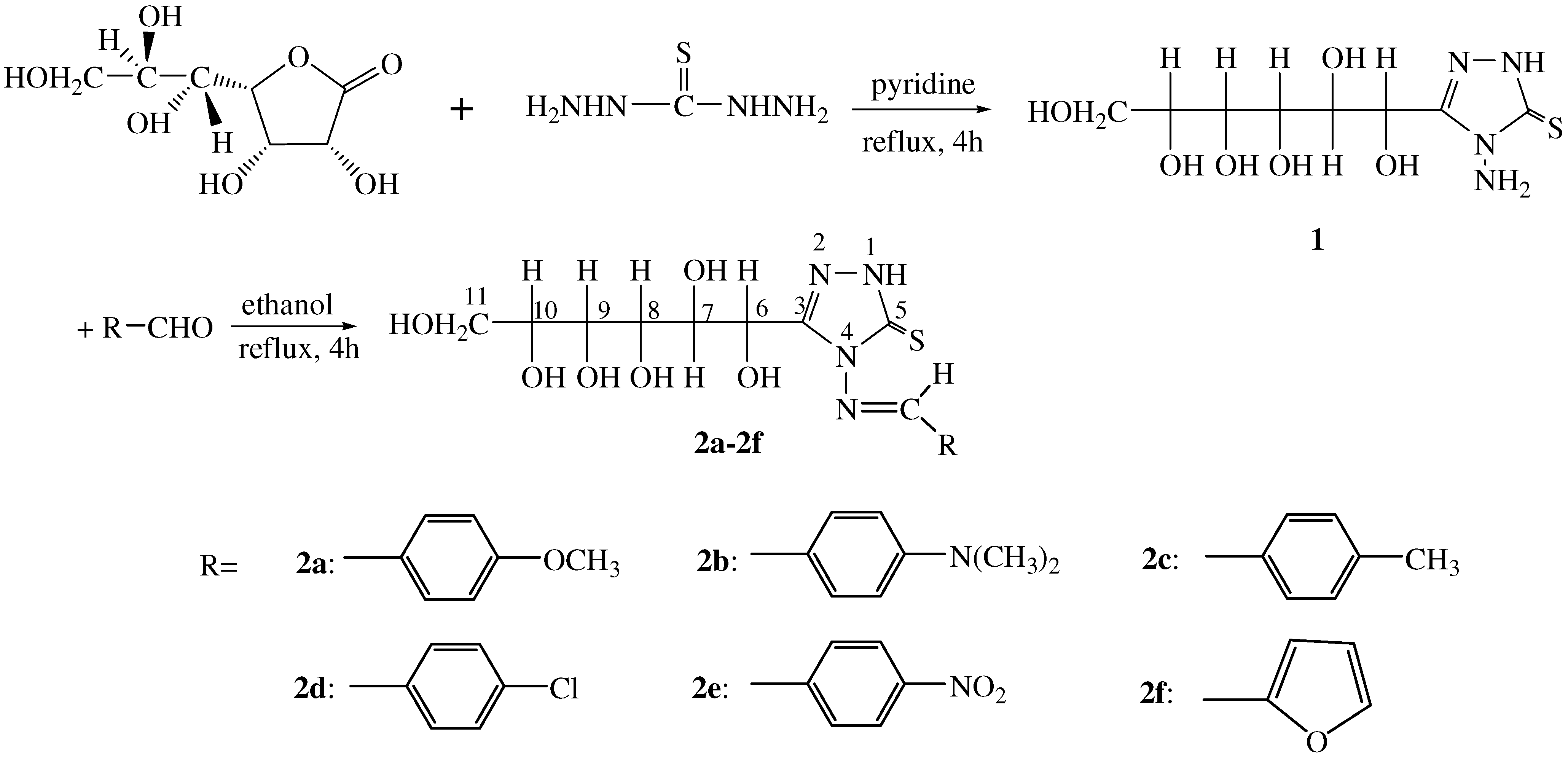Results and Discussion
The synthesis route is outlined in
Scheme 1. In our previous research [
10], we attempted to prepare 3,6-disubstituted-5,6-dihydrogen-1,2,4-triazolo[3,4-
b][1,3,4]thiadiazoles according to a reported procedure [
11], so we now treated 3-substituted-4-amino-1,2,4-triazole-5-thione with the appropriate substituted benzaldehyde. Unfortunately, the desired intramolecular Mannich reaction did not take place and we only obtained open-chain hydrazones [
10]. On the one hand, these compounds exhibit a good inhibitory effects on the growth of wheat and radish radicles; on the other hand, their water-solubilities are poor. Prompted by these results, we tried to attach hydrophilic D-glucoheptonic residues at the 3-position in order to improve their bioactivities by increasing water-solubility.
Scheme 1.
Synthesis of the title compounds.
Scheme 1.
Synthesis of the title compounds.
The key precursor for the synthesis of the title systems is compound
1. Nevertheless, our initial attempts gave a disappointing result. After work up, we found it to be syrupy, which was inconvenient to continue into the following step, but dissolved in ethanol at room temperature, compound
1 precipitated from the solvent slowly as a white powder. Next, as we used to do in our previous experiment [
10]; we mixed compound
1 with more than ten differently substituted benzaldehydes, maintaining pH values during the reaction at 5-6 because the acidity of the reaction medium is crucial. Results were again discouraging. Less than 10% yield of the title compounds was achieved after work up and
13C-NMR showed that the products were impure. They could not be purified using column chromatography on silica gel because the polarity of the title compounds are so high that they stayed at the original position on TLC when eluted with methanol. To improvement this, first of all we added activated molecular sieves (4Å). The result was encouraging, in that 50-70% yield of the crude title products was achieved, and the products could now be purified through recrystallization from ethanol. We understand that the products exist in the
E configuration form because of the lower energy (see
Scheme 1).
IR spectra of the title compounds
The absence of N-H and C=O absorption bands in the IR spectra confirmed that the title compounds 2 were obtained via condensation. The OH group stretching vibration bands are at 3200-3400 cm-1. The C-H stretching vibration bands of CH2 group are at 2920-2990 cm-1. The characteristic stretching vibrations of the products are at 1570-1620 cm-1 (C=N) and 1440-1590 cm-1 (N=C-S).
1H-NMR spectra of the title compounds
In the 1H-NMR spectrum of the key intermediate 4-amino-3-(D-glucoheptonic-hexitol-1-yl)-1H-1, 2,4-triazole-5-thione (1), we observed an absorption at δ 5.46 ppm (s, 2H, -NH2). In all the title compounds 2, the above absorption disappeared and additional resonances assigned to the -CH=N-(δ 9.21-10.18 ppm) were observed, which confirmed the condensation between the amino group and the carbonyl group. A downfield signal appearing at δ 13.69-13.99 ppm is attributed to the –NH-C=S moiety. The general chemical shift of the -CH=N- proton is less than 8.8 ppm, while that of the title compounds appears at δ 9.21-10.18 ppm because the proton is deshielded by the C=S group and it forms intramolecular hydrogen bonding to O-C6. Meanwhile, we noted that in all title compounds, there are broad and single absorption peaks or sharp double peaks at δ 5.79-5.91 ppm assigned to the C6-O-H. The differently substituted aromatic rings result in the protons appearing at δ 8.39-6.81 ppm.
13 C-NMR spectra of the title compounds
The intermediate 1 exhibited absorption peaks at δ 165.32, 152.79 ppm due to N-C=N and N-C=S, respectively, and at δ 74.10, 74.05, 71.07, 67.21, 65.00, 63.36 ppm due to the six D-glucoheptonic acid residue carbon atoms. The title compounds showed absorptions at δ 161-166, 151-163, 152-162 ppm due to N-C=N, N-C=S and Ph-CH=N groups, respectively. The δ values differ among the title compounds as a result of the resulting of the different substituents in the phenyl group.
Water-solubility of the title compounds
The solubility of the newly synthesized compounds,
2a-2f, was much greater than that of the compounds which we prepared in our previous research [
10] (see
Table 1), which was so poor that we had to add some ethanol to help them dissolve in water when we prepared the culture solutions for bioactivity testing.
Table 1.
The water-solubility of the title compounds (mg/100mL at 20°C ).
Table 1.
The water-solubility of the title compounds (mg/100mL at 20°C ).
| Compound | 2a | 2b | 2c | 2d | 2e | 2f |
|---|
| Solubility in H2O | | | | | | |
31 |
17 |
26 |
28 |
14 |
37 |
Biological evaluation
The title compounds have been investigated for their biological activities regulating the growth of wheat and radish with reference to sterilized distilled water. After treating with culture solution of 10 μg/mL and 100 μg/mL of the title compounds
2a-f for 5 days, the growth regulating percentage has been calculated. The biological activity test data are presented in
Table 2. The results indicated that among the tested compounds, almost all the newly prepared compounds showed weak to moderate promoting effects on the growth of the stalks and radicles of radish (a dicotyledon) at a concentration of 10 μg /mL and 100 μg /mL, and nearly all products also showed weak to moderate inhibition of the growth of the stalk and the radicle of wheat (a monocotyledon) at these concentrations. Therefore we conclude that the structure-activity relationships of these compounds is worth studying further.
Table 2.
Effect of compounds 1, 2a-f on the plant growth-regulation of wheat and radish a.
Table 2.
Effect of compounds 1, 2a-f on the plant growth-regulation of wheat and radish a.
| Compound | Concentrations (μg/mL ) | Radish | Wheat |
|---|
| Stalk | Radicle | Stalk | Radicle |
|---|
| 1 | 100 | ** | ** | ++ | ++++++ |
| 10 | **** | **** | *** | * |
| 2a | 100 | + | + | *** | *** |
| 10 | + | * | ** | *** |
| 2b | 100 | * | + | *** | *** |
| 10 | ++ | ++++ | ** | ** |
| 2c | 100 | ++ | +++ | *** | *** |
| 10 | ++ | +++ | * | + |
| 2d | 100 | + | ** | ** | ** |
| 10 | ++ | ++++ | * | * |
| 2e | 100 | ** | ++ | ** | **** |
| 10 | +++ | ++ | * | *** |
| 2f | 100 | + | ** | *** | *** |
| 10 | + | * | ** | *** |
| a: “ + ” represents promotion rate. + : <10%; ++ : 10-30%; +++ : 30-50%; ++++ : 50-70%; +++++ : 70-90%; ++++++: >90%. “ * ” represents inhibition rate. * : <10%; ** : 10-30%; *** : 30-50%; **** : 50-70%; ***** : 70-90%; ******: >90%. |
Experimental Section
General
Mps (uncorrected) were taken on an XT-4 melting point apparatus. IR spectra were determined on a Nicolet 670FT-IR using the Smart OMNI-Sampler in the range 4000─400 cm-1. 1H-NMR and 13C- NMR spectra were recorded in DMSO-d6 solution using TMS as an internal reference on a Bruker Avance-300 NMR spectrometer at 300 and 75 MHz, respectively. MS spectra were recorded on an Agilent 1100 LC/MS. The contents of carbon, hydrogen and nitrogen were determined on a Flash-1112 series elemental analyzer. All reagents used were analytical reagents.
Synthesis of 4-amino-3-(D-glucoheptonic-hexitol-1-yl)-1H-1, 2, 4-triazole-5-thione (1)
To a solution of
α-
D-glucoheptonic
γ-lactone (4.16 g, 20 mmol) dissolved in pyridine was added thiocarbohydrazide (2.12 g, 20 mmol), which was prepared according to the literature method [
12]. The mixture was refluxed for 4 h under stirring. After concentration under reduced pressure, the crude product was recrystallized from alcohol to afford compound
1 as a white powder (yield%: 80%); m.p.: 153─155 °C (from ethanol); IR (cm
-1): 3470 (OH, NH), 2925 (CH
2), 1620 (C=N), 1502 (N=C-S);
1H- NMR: 13.54 (s, 1H, N-H), 5.46 (s, 2H, -NH
2), 4.05-3.99 (m, 6H, O-H), 3.42-3.38 (m, 7H, -OCH);
13C-NMR: 165.32, 152.79, 74.10, 74.05, 71.70, 67.21, 65.00, 63.36; MS-ESI (
m/e, relative intensity, %): 297 (M
++1, 100), 279 (7), 278, 241, 205, 181, 159; Elemental anal. calcd. (%) for C
8H
16N
4O
6S: C, 32.43; H, 5.44; N, 18.91. Found (%): C, 32.28; H, 5.48; N, 18.67.
General procedure for the preparation of the title compounds 2a-f
Aromatic aldehydes (1.1 mmol, distilled under reduced pressure before use) were added to a solution of 4-amino-3-(D-glucoheptonic-hexitol-1-yl)-1H-1,2,4-triazole-5-thione (1, 1.0 mmol,296 mg) in absolute ethanol (20 mL). The pH value then was adjusted to 5-6 with dilute HCl and activated molecular sieves (4Å) were added. The mixture was stirred and refluxed for 6 h under a nitrogen atmosphere. The mixture was filtered through Celite and the Celite was washed with ethanol. The filtrate was evaporated in vacuo and the crude product was dissolved in as little ethanol as possible, kept at room temperature and recrystallized from ethanol to afford the pure products 2a-f.
3-(D-glucoheptonic-hexitol-1-yl)-4-(4-methoxybenzylidene)amino-1H-1,2,4-triazole-5-thione (2a)
Yield 70%; white powder; m.p. 190-191˚C. IR (cm-1): 3325 (OH, NH), 3057 (ArH), 2954 (CH2), 1604 (C=N), 1519 (N─C=S), 1485, 1446 (aromatic ring skeleton vibration), 1260 (C=S); 1H-NMR: 13.79 (s, 1H, NH-C=S), 9.53 (s, 1H, CH=N), 7.87 (d, 2H, J=8.64Hz, Ar-H), 7.12 (d, 2H, J=8.61 Hz, Ar-H), 5.79 (d, 1H, J=8.61 Hz, O-H), 4.80 (m, 1H, -OCH), 4.69 (br, 1H, O-H), 4.52 (br, 2H, O-H), 4.27 (br, 2H, O-H), 4.07-3.99 (m, 2H, -OCH), 3.86 (s, 3H, OCH3 ), 3.42-3.33 (m, 4H, -OCH); 13C-NMR: 164.74 (N-C=N), 162.97 (N-C=S), 161.53 (Ph-CH=N), 152.10, 130.80, 124.82, 114.76 (Ar-C), 74.23, 73.83, 71.71, 67.14, 65.08, 63.34, 55.69 (C-O); MS-ESI (m/e, relative intensity, %): 415 (M++1, 100), 383 (5), 282 (3), 269 (10); Elemental anal. calcd. (%) for C16H22N4O7S: C, 46.37; H, 5.35; N, 13.52. Found (%): C, 46.18; H, 5.26; N, 13.75.
3-(D-glucoheptonic-hexitol-1-yl)-4-(4-N,N-dimethylbenzylidene)amino-1H-1,2,4-triazole-5-thione (2b)
Yield 62%; light yellow powder; m.p. 238-240˚C. IR (cm-1): 3294 (OH, NH), 3170 (ArH), 2986 (CH2), 1612 (C=N), 1583 (N-C=S), 1533 (aromatic ring skeleton vibration), 1287 (C=S); 1H-NMR: 13.69 (s, 1H, NH—C=S), 9.21 (s, 1H, CH=N), 7.71 (d, 2H, J=8.67Hz, Ar-H), 6.81 (d, 2H, J=8.67 Hz, Ar-H), 5.70 (br, 1H, O-H), 4.76 (d, 1H, J=9.27 Hz, -OCH), 4.30 (br, 5H, O-H), 4.06-3.99 (m, 2H, -OCH), 3.59-3.37 (m, 4H, -OCH), 3.03 (s, 6H, NCH3); 13C-NMR: 165.92(N-C=N), 161.55 (N=C), 153.29 (Ph-CH=N), 151.97, 130.57, 119.00, 111.66 (Ar-C), 74.30, 73.90, 71.67, 67.05, 65.00, 63.34 (C-O), 39.79 (NCH3); MS-ESI (m/e, relative intensity, %): 428 (M++1, 100), 415 (3), 297 (5), 279 (15), 269 (2); Elemental anal. calcd. (%) for C17H25N5O6S: C, 47.76; H, 5.89; N, 16.38. Found (%): C, 47.89; H, 5.92; N, 16.10.
3-(D-glucoheptonic-hexitol-1-yl)-4-(4-methylbenzylidene)amino-1H-1,2,4-triazole-5-thione (2c)
Yield 58%; white powder; m.p. 226-229˚C. IR (cm-1): 3379 (OH, NH), 3020 (ArH), 2927 (CH2), 1603 (C=N), 1465 (N-C=S), 1450 (aromatic ring skeleton vibration), 1275 (C=S); 1H-NMR: 13.84 (br, 1H, NH-C=S), 9.65 (s, 1H, CH=N), 7.82 (d, 2H, J=7.35 Hz, Ar-H), 7.38 (d, 2H, J=7.26 Hz, Ar-H), 5.83 (d, 1H, J=5.49 Hz, O-H), 4.83-4.78 (m, 1H, -OCH), 4.75 (m, 1H, O-H), 4.55 (br, 2H, O-H), 4.27-4.08 (m, 2H, O-H), 4.05-3.98 (m, 2H, -OCH), 3.54-3.38 (m, 4H, -OCH), 2.40 (s, 3H, Ar-CH3); 13C-NMR: 165.86 (N-C=N), 161.36 (N-C=S), 152.05 (Ph-CH=N), 142.93, 129.70, 129.56, 128.71 (Ar-C), 77.56, 74.10, 72.71, 66.94, 64.93, 63.19 (C—O), 21.26 (Ar-CH3); MS-ESI (m/e, relative intensity, %): 399 (M++1, 100), 367 (3), 282 (8), 279 (4); Elemental anal. calcd. (%) for C16H22N4O6S: C, 48.23; H, 5.57; N, 14.06. Found (%): C, 48.06; H, 5.68; N, 14.29.
3-(D-glucoheptonic-hexitol-1-yl)-4-(4-chlorobenzylidene)amino-1H-1,2,4-triazole-5-thione (2d)
Yield 55%; white powder; m.p. 215-217˚C. IR (cm-1): 3323 (OH, NH), 3050 (ArH), 2935 (CH2), 1590 (C=N), 1461 (N-C=S), 1273 (C=S); 1H-NMR: 13.90 (s, 1H, NH-C=S), 9.84 (d, 1H, J=1.65 Hz, CH=N), 7.96 (d, 2H, J=8.40 Hz, Ar-H), 7.65 (d, 2H, J=8.40 Hz, Ar-H), 5.87-5.84 (m, 1H, O-H), 4.82-4.81 (m, 1H, -OCH), 4.75-4.72 (m, 1H, O-H), 4.55 (br, 2H, O-H), 4.37-4.28 (m, 2H, O-H), 4.06-3.97 (m, 2H, -OCH), 3.55-3.32 (m, 4H, -OCH); 13C-NMR: 163.09 (N-C=N), 161.84 (N-C=S), 152.59 (Ph-CH=N), 137.67, 131.64, 130.75, 129.74 (Ar-C), 74.46, 74.04, 72.05, 67.51, 65.44, 63.63 (C-O); MS-ESI (m/e, relative intensity, %): 421 (M++2, 35), 419 (M+, 100), 282 (4); Elemental anal. calcd. (%) for C15H19ClN4O6S: C, 43.01; H, 4.57; N, 13.38. Found (%): C, 43.26; H, 4.62; N, 13.12.
3-(D-glucoheptonic-hexitol-1-yl)-4-(4-nitrobenzylidene)amino-1H-1,2,4-triazole-5-thione (2e)
Yield 50%; yellow powder; m.p. 219-221˚C. IR (cm-1): 3343 (OH, NH), 3185 (ArH), 2925 (CH2), 1574 (C=N), 1453 (N─C=S), 1261 (C=S); 1H-NMR: 13.99 (br, 1H, NH-C=S), 10.18 (s, 1H, CH=N), 8.39 (d, 2H, J=8.22 Hz, Ar-H), 8.20 (d, 2H, J=8.28 Hz, Ar-H), 5.91 (d, 1H, J=5.76 Hz, O-H), 4.89-4.84 (m, 1H, -OCH), 4.84-4.77 (m, 1H, O-H), 4.58 (br, 2H, O-H), 4.39-4.33 (m, 2H, O—H), 4.11-3.99 (m, 2H, -OCH), 3.41-3.36 (m, 4H, -OCH); 13C-NMR: 161.41 (N-C=N), 160.34 (N-C=S), 152.31 (Ph-CH=N), 149.44, 138.25, 129.67, 124.47 (Ar-C), 72.91, 73.52, 71.62, 67.10, 65.02, 63.15 (C-O); MS-ESI (m/e, relative intensity, %): 430 (M++1, 100), 371 (4), 341 (2), 297 (6), 282 (22), 279(3); Elemental anal. calcd. (%) for C15H19N5O8S: C, 41.96; H, 4.46; N, 16.31. Found (%): C, 41.72; H, 4.58; N, 16.52.
3-(D-glucoheptonic-hexitol-1-yl)-4-(2-furanylidene)amino-1H-1,2,4-triazole-5-thione (2d)
Yield 70%; light grey powder; m.p. 235-237˚C. IR (cm-1): 3327 (OH, NH), 3149 (ArH), 2950 (CH2), 1610 (C=N), 1480 (N-C=S), 1280 (C=S); 1H-NMR: 13.85 (s, 1H, NH-C=S), 9.60 (s, 1H, CH=N), 8.07 ( s, 1H, furan-H), 7.35 (s, 1H, furan-H), 6.78 (s, 1H, furan-H ), 5.79 (m, 1H, O-H), 4.79 (d, 1H, J=8.97 Hz, -OCH), 4.50-4.38 (m, 5H, O-H), 4.04-4.00 (m, 2H, -OCH), 3.59-3.38 (m, 4H, -OCH); 13C-NMR: 161.37(N-C=N), 152.73 (N-C=S), 152.29 (Ph-CH=N), 148.02, 147.49, 120.15, 113.07 (Ar-C), 74.25, 74.01, 71.67, 66.98, 64.76, 63.34 (C-O); MS-ESI (m/e, relative intensity, %): 375 (M++1), 357 (4), 341 (2), 279 (4), 265, 235; Elemental anal. calcd. (%) for C13H18N4O7S: C, 41.71; H, 4.85; N, 14.97. Found (%): C, 41.95; H, 4.62; N, 14.69.
Biological Evaluation: Effect of compounds 2a-2j on the vegetative growth of wheat and radish plants
Seeds were rinsed with sterilized distilled water for four times. The following steps were all carried out under a horizontal laminar flow hood. Twenty seeds of each species were chosen and individually placed in culture dishes of 9 cm diameter containing two pieces of filter paper and a solution of the tested compounds 2a-2f (5 mL, 10μg/mL and 100μg/mL, respectively), and were incubated in a growth chamber at 25˚C, with a 16h/8h photoperiod. A set of controls with sterilized distilled water were prepared concomitantly. Plant lengths were recorded on the 5th day, both for the treated plants and for the controls. Experiments were run in duplicate. The equations of the growth regulating percentage (for wheat and radish stalks and radicles) are: the average of sample length (cm) – the average of the controls (cm)/ the average of the controls (cm) *100%.