General procedure for the synthesis of 4H-imidazoles 5:
Dinitrile
4 (1 mmol) was dissolved or suspended in dry THF (25 mL). After the addition of 1.0M Li-HMDS solution in THF or
n-hexane (5 mL, 5 mmol), the reaction mixture was stirred at room temperature for 4 days and then evaporated to dryness
in vacuo. The residue was dissolved in dry toluene (20 mL) and of chlorotrimethylsilane (0.75 mL, 6.0 mmol) was added. The reaction mixture was heated to 110 °C for 12 h and then the solvent was removed
in vacuo. The silylated amidine was dissolved in dry THF (20 mL) and cyclized by addition of KF (0.35 g, 6.0 mmol), 18-crown-6-ether (1.58 g, 6.0 mmol) and the appropriate
bis-imidoylchloride (1.5 mmol; Ar = 4-tol, 4-
n-C
4H
9-C
6H
4-, 4-
t-C
4H
9-C
6H
4- [
11]), whereby the colour changed from yellow to deep red. The mixture was stirred for 3-4 hours at about 40 to 50 °C to ensure completion of the reaction. The 4
H-imidazoles
5 were obtained by filtration and column chromatography of the crude product (SiO
2, toluene/acetone 50:1).
9-{5-p-Tolylamino-4-[p-tolylimino]-4H-imidazol-2-yl}-benzo[b]triphenylene-14-carbonitrile (5a, Ar = 4-tolyl): Yield 21%, red solid, practically insoluble in all organic solvents tested; IR cm-1: 3283 (NH), 2210 (CN); MS (DEI): m/z (%): 577 (M+, 100), 486 (28), 459 (88), 326 (20), 215 (12), 106 (6), 91 (9); UV/vis (THF) λ max (log ε): 408 nm (4.2), 453 (4.2), 482 (4.3), 519 (4.1); CV: ERED1 = -0.42 V (CN), ERED2 = -0.72 V, ERED3 = -1.05 V.
9-{5-p-n-Butylphenylamino-4-[p-n-butylphenylimino]-4H-imidazol-2-yl}-benzo[b]triphenylene-14-carbonitrile (5a, Ar = 4-n-butylphenyl): Yield 28%, red solid; IR cm-1: 3480 (NH), 2210 (CN); MS (DEI): m/z (%): 661 (M+, 20), 351 (75), 175 (68), 106 (100); UV/vis (THF) λ max (log ε): 423 nm (4.1), 455 (4.1), 485 (4.2), 524 (4.0); 1H-NMR (250 MHz, THF-d8) δ (ppm): 0.89 (t, 6H), 1.55 (m, 4H), 2.56 (t, 4H), 7.31 (t, 2H), 7.52 (t, 2H), 7.71 (m, 6H), 7.88 (d, 3J = 8.8 Hz, 4H), 8.52 (d, 3J = 8.8 Hz, 2H), 8.61 (d, 3J = 8.8 Hz, 2H), 10.07 (s, 1H); CV: ERED1 = -0.47 V (CN), ERED2 = -0.85 V, ERED3 = -1.16 V.
9-{5-p-t-Butylphenylamino-4-[p-t-butylphenylimino]-4H-imidazol-2-yl}-benzo[b]triphenylene-14-carbonitrile (5a, Ar = 4-t-butylphenyl): Yield 30%, red solid; IR cm-1: 3338 (NH), 2212 (CN); MS (EI): m/z (%): 661 (M+, 10), 352 (60), 337 (95), 328 (12), 175 (46), 160 (100), 134 (92); UV/vis (THF) λ max (log ε): 211 nm (4.8), 298 (4.7), 386 (4.0), 481 (4.2), 521 (4.1); 1H-NMR (250 MHz, THF-d8) δ (ppm): 1.31 (s, 18H), 7.37 (d, 3J = 8.8 Hz, 4H), 7.3-7.4 (m, 6H), 7.76 (d, 3J = 8.8 Hz, 4H), 7.91 (d, 3J = 8.8 Hz, 2H), 8.52 (t, 2H), 8.60 (t, 2H), 10.12 (s, 1H); CV: ERED1 = -0.51 V (CN), ERED2 = -0.87 V, ERED3 = -1.17 V.
12-{5-p-n-Butylphenylamino-4-[p-n-butylphenylimino]-4H-imidazol-2-yl}-benzo[k]fluoranthene-7-carbonitrile (5b, Ar = 4-n-butylphenyl): Yield 15%, red solid; IR cm-1: 3298 (NH), 2224 (CN); MS (DEI): m/z (%): 635 (M+, 8), 578 (10), 508 (40), 352 (25), 306 (50), 263 (83), 175 (59), 132 (100), 106 (66); UV/vis (THF) λ max (log ε): 315 nm (4.2), 403 (4.3), 487 (4.4), 523 (4.3); 1H-NMR (250 MHz, THF-d8) δ (ppm): 0.95 (t, 6H), 1.35 (m, 4H), 1.58 (m, 4H), 2.62 (m, 4H), 6.98-7.33 (m, 14H), 7.73 (d, 3J = 8.3 Hz, 4H); 13C-NMR (100 MHz, THF-d8) δ (ppm): 13.8, 21.8, 31.5, 31.9, 34.8, 35.0, 101.4, 116.8, 117.6, 120.9, 121.2, 122.5, 125.5, 126.1, 126.3, 126.4, 126.8, 127.2 128.1, 128.2, 128.6, 129.3, 131.2, 132.0, 132.5, 132.8, 132.9, 133.1, 134.0, 135.4, 136.2, 138.4, 141.0, 148.0, 158.9; CV: ERED1 = -1.15 V, ERED2 = -1.48 V.
12-{5-p-t-Butylphenylamino-4-[p-t-butylphenylimino]-4H-imidazol-2-yl}-benzo[k]fluoranthene-7-carbonitrile (5b, Ar = 4-t-butylphenyl): Yield 18%, red solid; IR cm-1: 3315 (NH), 2221 (CN); MS (DEI): m/z (%): 635 (M+, 30), 578 (35), 477 (58), 352 (100), 337 (94), 302 (26), 160 (39), 132 (28); UV/vis (THF) λ max (log ε): 286 nm (4.2), 406 (4.2), 490 (4.4), 517 (4.3); 1H-NMR (250 MHz, THF-d8) δ (ppm): 1.28 (s, 18H), 7.07-7.18 (m, 6H), 7.36-7.40 (m, 8H), 7.74 (d, 3J = 8.3 Hz, 4H); 13C-NMR (100 MHz, THF-d8) δ (ppm): 31.4, 31.6, 34.8, 35.0, 101.4, 116.8, 117.6, 120.9, 121.2, 122.5, 125.5, 126.1, 126.3, 126.4, 126.8, 127.2 128.1, 128.2, 128.6, 129.3, 131.2, 132.0, 132.5, 132.8, 132.9, 133.1, 134.0, 135.4, 136.2, 138.4, 141.0, 148.0, 158.9; CV: ERED1 = -1.02 V, ERED2 = -1.50 V.
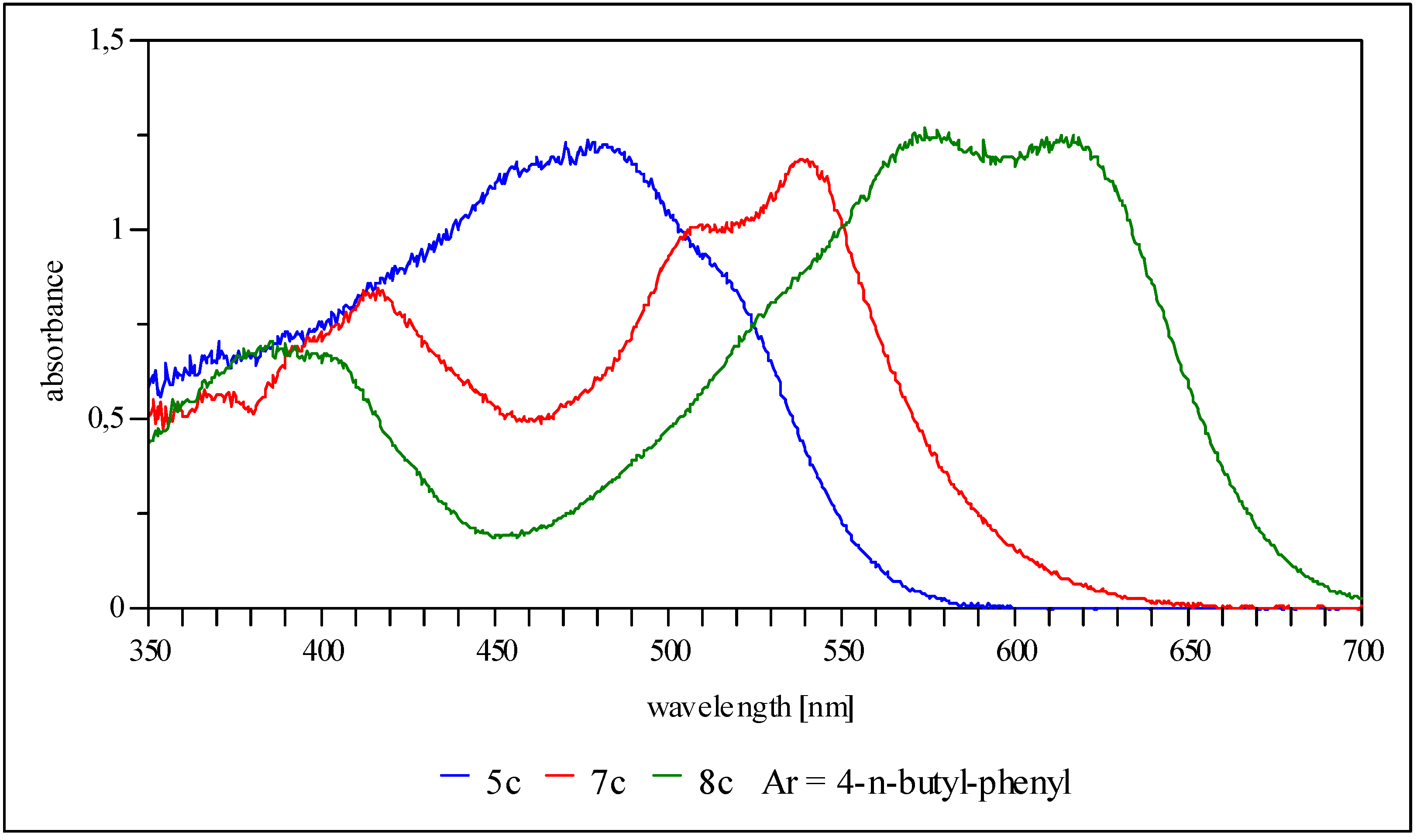
10-{5-p-Tolylamino-4-[p-tolylimino]-4H-imidazol-2-yl}-anthracene-9-carbonitrile (5c, Ar = 4-tolyl): Yield 19%, red solid; IR cm-1: 3545 (NH), 2222 (CN); MS (DEI): m/z (%): 477 (M+, 100), 462 (10), 359 (4), 228 (10), 221 (2), 106 (2); UV/vis (THF) λmax (log ε): 390 nm (4.1), 410 (4.1), 452 (4.2), 480 (4.3), 520 (4.2); 1H-NMR could not be recorded (almost insoluble in organic solvents); CV: ERED1 = -0.44 V (CN), ERED2 = -0.84 V, ERED3 = -1.12 V.
10-{5-p-n-Butylphenylamino-4-[p-n-butylphenylimino]-4H-imidazol-2-yl}-anthracene-9-carbonitrile (5c, Ar = 4-n-butylphenyl): Yield 27%, red solid; IR cm-1: 3257 (NH), 2221 (CN); MS (DEI): m/z (%): 561 (M+, 100), 504 (68), 402 (20), 228 (10); UV/vis (THF) λmax (log ε): 393 nm (4.1), 411 (4.2), 455 (4.2), 482 (4.3), 525 (4.2); 1H-NMR (250 MHz, CD2Cl2) δ (ppm): 0.82 (t, 3J = 7.5 Hz, 6H), 1.24 (m, 4H), 1.47 (m, 4H), 2.48 (q, 3J = 7.5 Hz, 4H), 7.12 (d, 3J = 8.3 Hz, 4H), 7.52 (d, 3J = 7.8 Hz, 2H), 7.68 (d, 3J = 7.8 Hz, 2H), 7.77 (d, 3J = 8.3 Hz, 4H), 8.42 (t, 3J = 7.3 Hz, 2H), 8.52 (t, 3J = 7.3 Hz, 2H); CV: ERED1 = -0.44 V (CN), ERED2 = -0.75 V, ERED3 = -1.12 V.
10-{5-p-t-Butylphenylamino-4-[p-t-butylphenylimino]-4H-imidazol-2-yl}-anthracene-9-carbonitrile (5c, 4-t-butylphenyl): Yield 29%, red solid; IR cm-1: ▯3456 (NH), 2220 (CN); MS (DEI): m/z (%): 561 (M+, 8), 504 (1), 436 (3), 379 (4), 351 (100), 336 (19), 296 (16), 175 (21), 160 (21), 134 (17), 91 (23); UV/vis (THF) λmax (log ε): 453 nm (4.3), 484 (4.3), 526 (4.1); 1H-NMR (250 MHz, THF-d8) δ (ppm): 1.30 (s, 18H), 7.36 (d, 3J = 8.5 Hz, 2H), 7.41 (d, 3J = 8.5 Hz, 4H), 7.61 (t, 2H), 7.69 (d, 3J = 8.5 Hz, 2H), 7.80 (t, 2H), 7.99 (d, 3J = 8.5 Hz, 4H), 10.01 (s, 1H); CV: ERED1 = -0.47 V (CN), ERED2 = -0.79 V, ERED3 = -1.13 V.
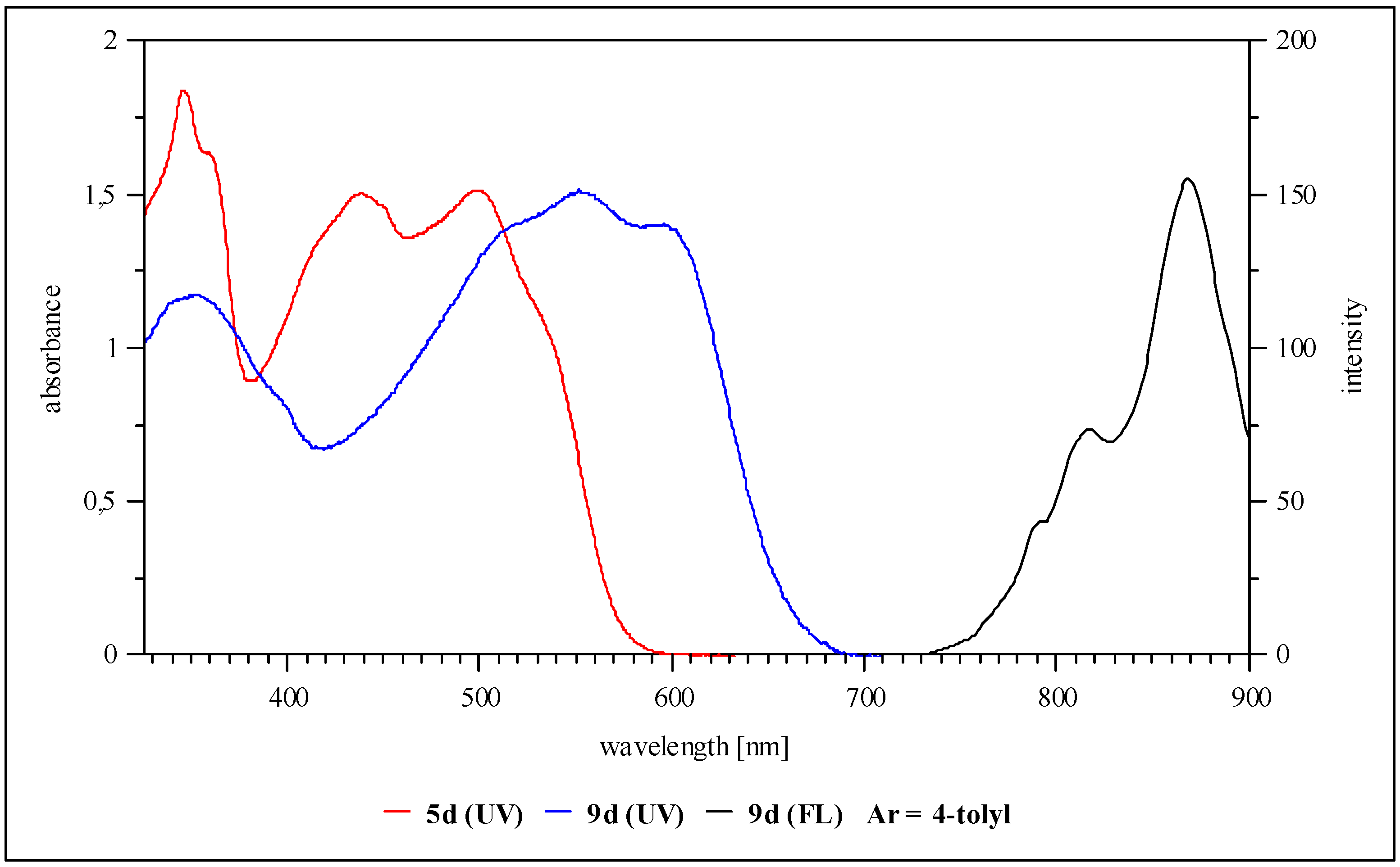
1,5-Di-n-hexyloxy-6-{5-p-tolylamino-4-[p-tolylimino]-4H-imidazol-2-yl}-naphthalene-2-carbonitrile (5d, Ar = 4-tolyl): Yield 20%, deep red solid; IR cm-1: 3278 (NH), 2227 (CN); MS (DEI): m/z (%): 627 (M+, 16), 268 (100), 205 (20), 177 (18), 133 (25), 106 (72); UV/vis (THF) λmax (log ε): 345 nm (4.2), 440 (4.4), 500 (4.3), 536 (4.1); 1H-NMR (250 MHz, THF-d8) δ (ppm): 0.89 (t, 6H), 1.29 (m, 8H), 1.40 (m, 4H), 1.96 (m, 4H), 2.37 (s, 6H), 4.46 (t, 4H), 7.24 (d, 3J = 8.8 Hz, 4H), 7.60 (d, 3J = 8.8 Hz, 1H), 7.70 (d, 3J = 8.8 Hz, 2H), 7.98 (d, 3J = 8.8 Hz, 4H), 8.70 (d, 3J = 8.8 Hz, 1H); CV: ERED1 = -0.90 V, ERED2 = -1.31 V.
1,5-Di-n-hexyloxy-6-{5-p-n-butylphenylamino-4-[p-n-butylphenylimino]-4H-imidazol-2-yl}-naphtha-lene-2-carbonitrile (5d, Ar = 4-n-butylphenyl): Yield 27%, red solid; IR cm-1: 3296 (NH), 2227 (CN); MS (DEI): m/z (%): 711 (M+, 20), 483 (24), 378 (28), 352 (20), 210 (100), 149 (10), 106 (21); UV/vis (THF) λmax (log ε): 345 nm (4.2), 415 (4.3), 503 (4.3), 540 (4.2); 1H-NMR (250 MHz, THF-d8) δ (ppm): 0.93 (t, 3J = 7.0 Hz, 12H), 1.29 (m, 4H), 1.40 (m, 8H), 1.60 (m, 8H), 1.95 (m, 4H), 2.59 (t, 3J = 7.5 Hz, 4H), 4.46 (t, 3J = 6.5 Hz, 4H), 7.15 (d, 3J = 8.5 Hz, 2H), 7.69 (m, 6H), 8.02 (d, 3J = 8.5 Hz, 4H), 10.08 (s, broad, 1H); CV: ERED1 = -0.94 V, ERED2 = -1.39 V.
Boratetraazapentalenes
9 were prepared according to literature [
2].
9-[2-Difluoro-1,3-bis-(4-t-butylphenyl)-1,2-dihydro-1,3,4,6-tetraaza-2-borapentalen-5-yl]-benzo[b]-triphenylene-14-carbonitrile (9a, Ar = 4-t-butylphenyl): Yield 52%, dark violet solid; IR cm-1: 2212 (CN); MS (DEI): m/z (%): 709 (M+, 18), 661 (6), 488 (24), 462 (26), 328 (100), 253 (14); UV/vis (THF) λmax (log ε): 372 nm (4.1), 529 (4.2), 572 (4.2), 614 (4.2); 1H-NMR (400 MHz, THF-d8) δ (ppm): 1.33 (s, 18H), 7.42 (d, 3J = 8.0 Hz, 4H), 7.40-7.65 (m, 6H), 7.85 (d, 3J = 8.0 Hz, 4H), 8.02 (d, 3J = 8.0 Hz, 2H), 8.62 (t, 2H), 8.67 (t, 2H); Fluorescence (THF, 475 nm); λmax,em: 681 nm; CV: ERED1 = -0.48 V, ERED2 = -1.16 V, KSEM = 3.4*1011.
9-[2-Difluoro-1,3-bis-(4-n-butylphenyl)-1,2-dihydro-1,3,4,6-tetraaza-2-borapentalen-5-yl]-benzo[b]-triphenylene-14-carbonitrile (9a, Ar = 4-n-butylphenyl): Yield 54%, dark violet solid; IR cm-1: 2209 (CN); MS (DEI): m/z (%): 709 (M+, 20), 661 (2), 451 (28), 369 (20), 328 (56), 221 (24), 111 (23), 97 (34), 85 (64), 71 (87), 57 (100); UV/vis (THF) λmax (log ε): 375 nm (4.1), 531 (4.1), 573 (4.3), 616 (4.2); 1H-NMR (400 MHz, THF-d8) δ (ppm): 0.97 (t, 6H), 1.38 (m, 4H), 1.62 (m, 4H), 2.66 (m, 4H), 7.02-7.39 (m, 14H), 7.79 (d, 3J = 8.0 Hz, 4H); Fluorescence (THF, 441 nm): λmax,em: 699 nm, (toluene, 440nm): λmax,em: 706 nm; CV: ERED1 = -0.45 V, ERED2 = -1.14 V, KSEM = 5.0*1011.
12-[2-Difluoro-1,3-bis-(4-t-butylphenyl)-1,2-dihydro-1,3,4,6-tetraaza-2-borapentalen-5-yl]-benzo[k]-fluoranthene-7-carbonitrile (9b, Ar = 4-t-butylphenyl): Yield 48%, blue-violet solid; IR cm-1: 2220 (CN); MS (DEI): m/z (%): 683 (M+, 18), 635 (36), 578 (64), 302 (100), 134 (20), 91 (25), 57 (48); UV/vis (THF) λmax (log ε): 407 nm (4.3), 524 (4.4), 571 (4.3), 608 (4.1); 1H-NMR (400 MHz, THF-d8) δ (ppm): 1.31 (s, 18H), 7.10-7.22 (m, 6H), 7.38-7.43 (m, 8H), 7.76 (d, J = 8.0Hz, 4H); Fluorescence (toluene, 405 nm): λmax,em: 885 nm; CV: ERED1 = -0.88 V, ERED2 = -1.50 V, KSEM = 3.2*1010.
10-[2-Difluoro-1,3-bis-(4-t-butylphenyl)-1,2-dihydro-1,3,4,6-tetraaza-2-borapentalen-5-yl]-anthra-cene-9-carbonitrile (9c, Ar = 4-t-butylphenyl): Yield 40%, dark violet solid; IR cm-1: 2219 (CN); MS (EI): m/z (%): 609 (M+, 20), 561 (84), 552 (100), 504 (78), 228 (69), 134 (71); UV/vis (THF) λmax (log ε): 388 nm (4.1), 403 (4.1), 528 (4.2), 567 (4.2), 602 (4.2); 1H-NMR (400 MHz, THF-d8) δ (ppm): 1.30 (s, 18H), 7.41 (t, 2H), 7.54 (d, 3J = 8.0 Hz, 4H), 7.76 (t, 2H), 7.80-7.95 (m, 4H), 8.08 (d, 3J = 8.0 Hz, 4H); Fluorescence (THF, 462 nm): λmax,em: 676 nm; CV: ERED1 = -0.42 V, ERED2 = -1.05 V, KSEM = 4.8*1010.
1,5-Di-n-hexyloxy-6-[2-Difluoro-1,3-bis-(4-tolyl)-1,2-dihydro-1,3,4,6-tetraaza-2-borapentalen-5-yl]-naphthalene-2-carbonitrile (9d, Ar = 4-tolyl): Yield 52%, dark violet solid; IR cm-1: 2227 (CN); MS (DEI): m/z (%): 678 (M+, 21), 627 (38), 268 (100), 205 (20), 177 (18), 133 (25), 106 (72); UV/vis (THF) λmax (log ε): 352 nm (4.1), 511 (4.2), 553 (4.3), 596 (4.2); 1H-NMR (400 MHz, THF-d8) δ (ppm): 0.91 (t, 6H), 1.32 (m, 8H), 1.44 (m, 4H), 1.99 (m, 4H), 2.42 (s, 6H), 4.50 (t, 4H), 7.36 (d, 3J = 8.0 Hz, 4H), 7.81 (d, 3J = 8.0 Hz, 1H), 7.93 (d, 3J = 8.0 Hz, 2H), 8.18 (d, 3J = 8.0 Hz, 4H), 8.90 (d, 3J = 8.0 Hz, 1H); Fluorescence (THF, 394 nm): λmax,em: 870 nm; CV: ERED1 = -0.82 V, ERED2 = -1.47 V, KSEM = 1.0*1011.