Introduction
1,5-Dihydro-4
H-pyrazolo[3,4-
d]pyrimidine = 8-aza-7-deazahypoxanthine (allopurinol,
1b,
Figure 1; IUPAC numbering is used throughout the manuscript) [
1,
2] is a progressive inhibitor of xanthine oxidase with alloxanthine – a 6-oxo derivative of
1b – being the actual inhibitor [
3]. This has led to a clinical application in the treatment of gout and related metabolic disorders [
4]. The value of allopurinol as well of its 4-amino analogue is augmented by their effects on pyrimidine and purine biosynthesis [
5,
6].
We have previously reported on the inhibitor properties of pyrrolo[2,3-
d]pyrimidines (
i.e. 7-deazapurines) towards xanthine oxidase (from cow’s milk) [
7]. In this context we now disclose that 2-methylthio-3,7-dihydro-4
H-pyrrolo[2,3-d]pyrimidin-4-one (
2a) [
8,
9] is also a moderately active competitive inhibitor of xanthine oxidase, with an inhibition constant (
Ki) of 120 µM. A corresponding dimeric inhibitor was obtained when two 4-methoxy-2-methylthio-7
H-pyrrolo[2,3-
d]pyrimidine molecules were linked by a methylene bridge
via liquid-liquid phase-transfer-catalyzed alkylation of the latter using dichloromethane, followed by hydrolysis of the methoxy groups with dilute hydrochloric acid (→
2b) [
10]. The methylenebis(heterocycle)
2b was also tested as a xanthine oxidase inhibitor and found to exhibit a
Ki value of 6 µM, which is 20-fold more potent than the corresponding
Ki of the monomeric heterocycle
2a.
In this paper we now report the synthesis of methylenebis(allopurinols) 3b–5b applying nucleophilic substitution reactions (SNAr) on the corresponding 4-methoxy precursors 3a–5a, as well as the influence of dimethylsulfoxide on the reaction kinetics.
Figure 1.
Chemical structures of novel methylenebis(heterocycles).
Figure 1.
Chemical structures of novel methylenebis(heterocycles).
Results and Discussion
4-Methoxy-1
H-pyrazolo[3,4-
d]pyrimidine (
1a) [
1,
11,
12] has been proven as a versatile heterocycle for the synthesis of alkylated [
13] and glycosylated [
14] pyrazolo[3,4-
d]pyrimidines, while allopurinol has not [
15]. In phase-transfer-catalyzed reactions this lactam-protected chromophore can be alkylated exclusively at N(1) and N(2), while reactions on the pyrimidine ring do not take place.
The methoxy group can subsequently undergo S
NAr nucleophilic displacement reactions, which are particularly advantageous for the synthesis of N(1)- and N(2)-glycosylated allopurinol nucleosides [
13,
16,
17]. Cleavage of the methoxy function with acid always bears the risk of concomitant N-glycosylic bond cleavage. In a number of methylation and glycosylation reactions of pyrrolo[2,3-
d]pyrimidines under liquid-liquid phase-transfer catalysis conditions with dichloromethane as solvent we observed a regioselective bridging of two chromophore moieties via N(7) by a methylene bridge [
10]. This reaction is then always favored when the alkyl halide is almost inert.
In contrast to this, the reaction of the heterocycle 1a with dichloromethane proceeded very slowly with formation of three reaction products. However, when dibromomethane was added to the reaction mixture compound 1a had disappeared completely with 60 min. Silica gel chromatography separated three methylenebis(4-methoxy-pyrazolo[3,4-d]pyrimidines) in a 8:16:1 ratio. Comparison of their 1H- and 13C-NMR spectra with those of 1a, as well as with those of its N(1)- and N(2)-methylated derivatives, revealed that the fastest migrating product is the N(1)-N(1) bridged compound 3a, as only one set of NMR signals could be observed. The second zone contained the unsymmetrically bridged compound 4a. In this case, the NMR spectra showed two sets of signals – one for the N(1)- and a second one for the N(2)-alkylated chromophore. The slowest migrating zone contained the N(2)-N(2) bridged compound 5a in low yield. This may be due to the low reactivity of the 2-nitrogen compared to N(1).
Table 1.
[1H-13C] Coupling constants of compounds 3a–5a a.
Table 1.
[1H-13C] Coupling constants of compounds 3a–5a a.
| Compound | | 3a | 4a b | 5a |
|---|
| C-6 | H-C(6) | 205.5 | 204.1/205.6 | 204.1 |
| C-3 | H-C(3) | 195.9 | 195.9/196.2 | 197.6 |
| C-3 | CH2 | - | 3.0 | 3.0 |
| C-3a | H-C(3) | 10.1 | 7.2/10.4 | 8.0 |
| C-7a | CH2 | n. r. | - | - |
| C-7a | H-C(6) | n. r. | - | 12.8 |
| C-7a | H-C(3) | n. r. | - | 6.8 |
| CH2 | CH2 | 155.4 | 157.3 | 156.0 |
| CH2 | H-C(3) | - | - | ~ 2 |
For unequivocal structural proof proton-coupled
13C-NMR spectra of the three regioisomers
3a–
5a were obtained (
Table 1). While C(7a) of compound
3a appears as a complex multiplet, due to three
3J(C,H) couplings with H-C(6), H-C(3) and CH
2, the corresponding C(7a) of compound
5a shows only two
3J(C,H) couplings with H-C(6) and H-C(3) (12.8 and 6.8 Hz, respectively). Correspondingly, the CH
2 groups of both isomers exhibit different coupling patterns: the multiplicity of CH
2 of
3a is a triplet (
1J(C,H) = 155.4 Hz), while that of
5a shows a triplet of triplets due to an additional
3J(C,H) coupling with both H-C(3) atoms. In addition, the C(3) signal of the isomer
5a exhibits an additional fine splitting compared with that of
3a, due to a long range coupling (
3J(C,H)) with its methylene group. All these data confirmed the proposed structures of compounds
3a and
5a [
18].
In the gated-decoupled 13C-NMR spectrum of the unsymmetrically bridged isomer 4a the coupling patterns of both chromophore moieties behave additively. A positive indicator for the correct structure is the fact that only the C(3) signal of the N(2)-alkylated moiety shows an additional fine splitting due to a 3J(C,H) coupling with CH2 (3 Hz) while the corresponding C(3) signal of the N(1)-alkylated chromophore moiety exhibits only a doublet (1J(C,H) = 196.2 Hz).
Nucleophilic displacement of the 4-methoxy groups of the methylenebis(4-methoxy-pyrazolo[3,4-
d]pyrimidines)
3a–
5a by 1N NaOH/MeOH (1:1, v/v) gave the corresponding methylenebis-(allopurinols)
3b–
5b (
Figure 1). Their structures were confirmed by UV- and
1H-NMR spectroscopy as well as by elemental analyses. The different UV spectra of educts and products allow a simple quantitative spectrophotometric monitoring and the determination of pseudo first-order kinetic constants (half life τ and k) of the S
NAr reactions (
Table 2). As can be seen from
Table 2 the reaction rates of S
NAr reactions of the methylenebis(4-methoxy-pyrazolo[3,4-
d]pyrimidines)
3a–
5a are significantly faster than those of the N(1)- and N(2)-methylated compounds. In case of the N(2)-N(2)-bridged derivative the half life is one order of magnitude higher than for the N(2)-methylated chromophore.
Table 2.
Pseudo first order kinetic data of nucleophilic OCH3 group displacement by hydroxyl of 4-methoxy-pyrazolo[3,4-d]pyrimidine derivatives.
Table 2.
Pseudo first order kinetic data of nucleophilic OCH3 group displacement by hydroxyl of 4-methoxy-pyrazolo[3,4-d]pyrimidine derivatives.
| Reaction conditions | 1N NaOH/MeOH (1:1, v/v) | 0.5 N NaOH | | |
|---|
| | half life τ [min] | k [min-1 x 103] | half life τ [min] | k [min-1x103] | λ a) [nm] | m b) |
|---|
| N(1)-methylated 1a | 305 | 2.28 | 95 | 7.3 | 273 | 8.8 |
| N(2)-methylated 1a | 200 | 3.57 | 134 | 5.2 | 283 | 7.9 |
| 3a | 145 | 4.81 | 90 | 7.7 | 273 | 8.8 |
| 4a | 48 | 14.4 | - | - | 273 | 7.3 |
| 5a | 20 | 34.6 | - | - | 283 | 7.9 |
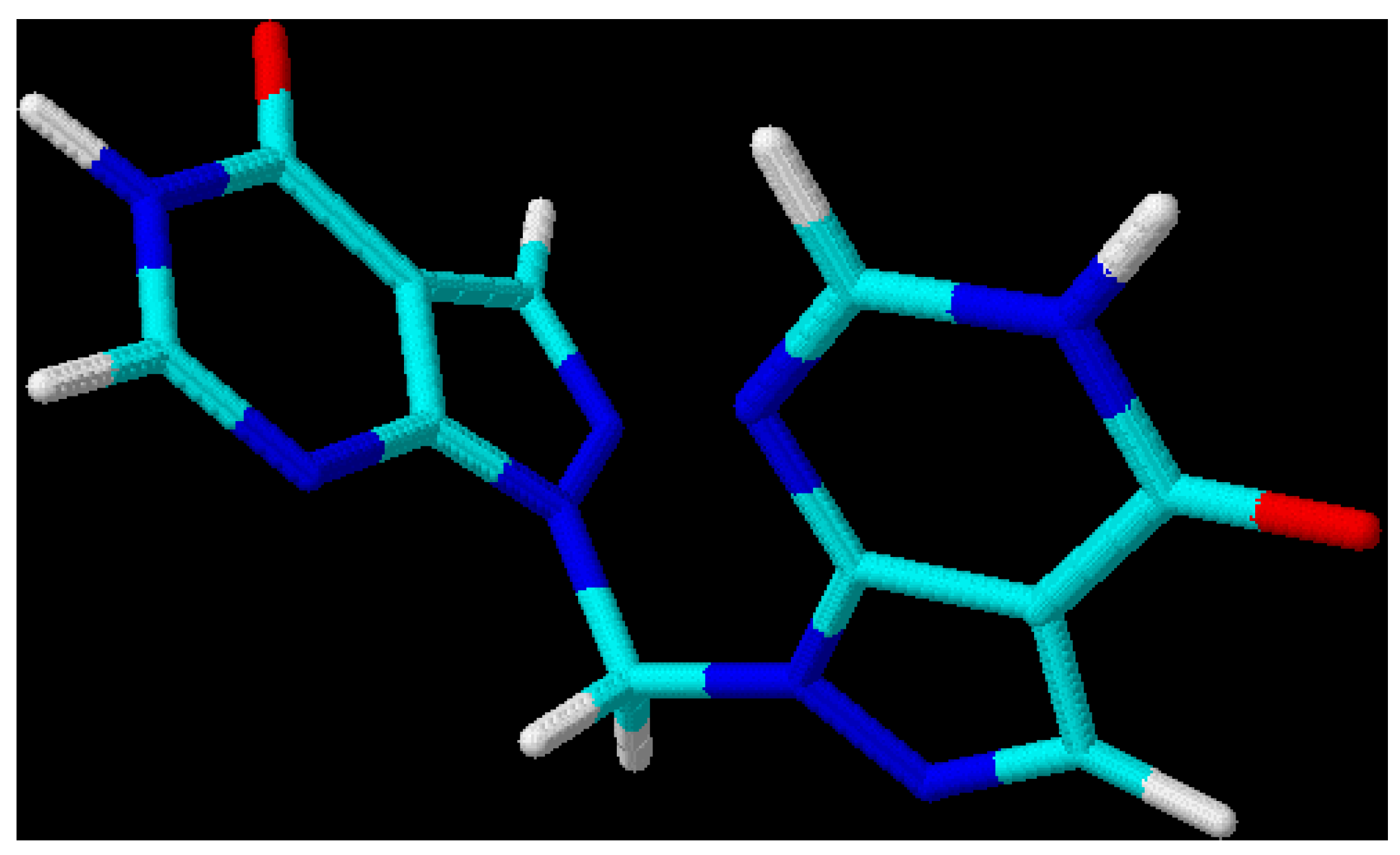
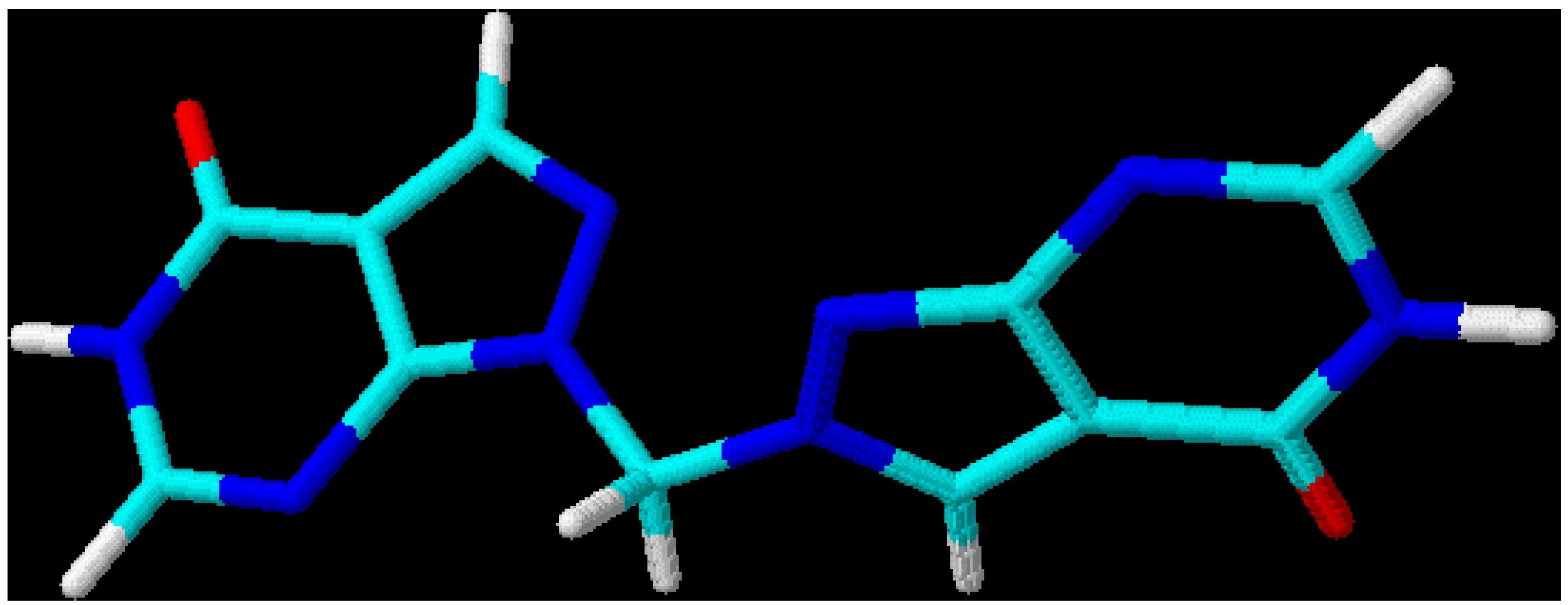
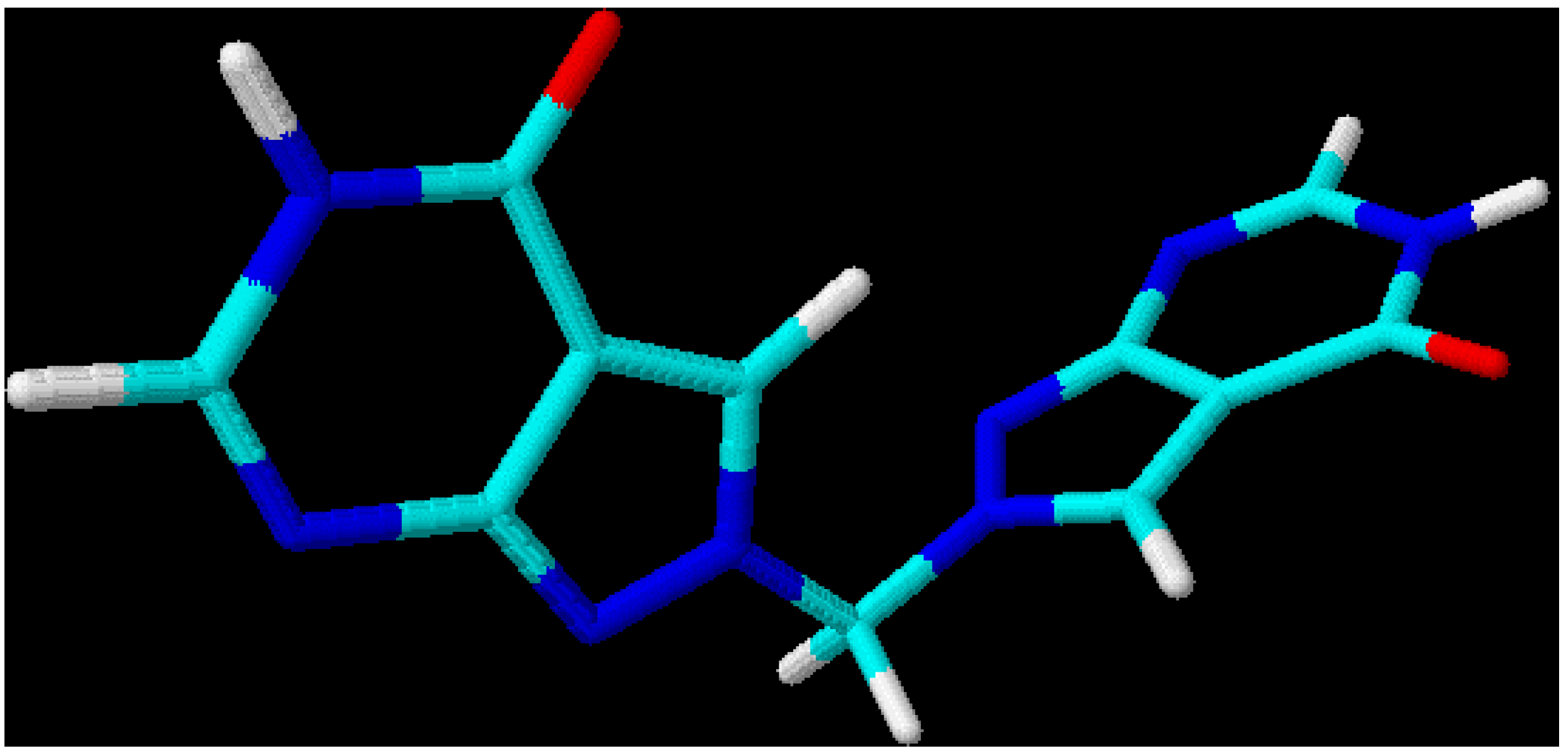
This leads to the assumption that a strong interaction exists between both chromophore moieties, probably caused by an orbital overlap of the two heterocyclic π-electron systems separated by the non-conjugating methylene group (homoconjugation). In this context it is interesting to see that for all three regioisomeric methylenebis(allopurinols) 3D-optimized molecular models [
19] display structures with heterocyclic planes which are perpendicular to each other (
Figure 2,
Figure 3 and
Figure 4,
Table 3); the heterocycles are not stacked one upon the other, as it has been shown for ethylenebis- or higher alkyl analogues [
20,
21]. A mutual (-M) – effect of one chromophore on the other leads to an increase in electrophilicity at C(4), compared with the methylated heterocycles and, therefore, to a higher reaction rate for displacement reactions by hydroxyl. Even a weaker nucleophile such as ammonia (25 % aq. NH
3) is able to substitute the 4-methoxy group of compound
3a, yielding compound
3c. If the nucleophilic displacement reaction of the methoxy group by hydroxyl on compound
5a was extended for more than 1 h, an opening of the pyrimidine rings at C(6) was observed, and small amounts of compound
6 were formed [
22]. If the nucleophilic displacement reactions of
3a–
5a are performed in a methanol-free medium (0.5 N NaOH), all reaction rates are enhanced (
Table 3) which points to a weaker solvation of the reaction intermediates by methanol-water mixtures compared to pure water.
Figure 2.
3D-Optimized structure of compound
3b [
19].
Figure 2.
3D-Optimized structure of compound
3b [
19].
Figure 3.
3D-Optimized structure of compound
4b [
19].
Figure 3.
3D-Optimized structure of compound
4b [
19].
Figure 4.
3D-Optimized structure of compound
5b [
19].
Figure 4.
3D-Optimized structure of compound
5b [
19].
Table 3.
Selected torsion and bond angles as well as inter-moiety N-N distances (Å) of compounds 3b–5b.
Table 3.
Selected torsion and bond angles as well as inter-moiety N-N distances (Å) of compounds 3b–5b.
| Compound | torsion and bond angles [deg]; inter-moiety N-N distances (Å) |
|---|
| 3b [1,1’-methylenebis(allopurinol)] | N(2)-N(1)-C(8)-N(1’), | -79.3 |
| | N(2’)-N(1’)-C(8)-N(1), | -22.2 |
| | C(7a)-N(1)-C(8)-N(1’), | -90.4 |
| | C(7a’)-N(1’)-C(8)-N(1’), | -41.4 |
| | N(1)-C(8)-N(1’). | 114.2 |
| | N(2) - N(2’), | 4.1 |
| 4b [1,2’-methylenebis(allopurinol)] | N(2)-N(1)-C(8)-N(2’), | -14.8 |
| | N(1’)-N(2’)-C(8)-N(1), | -59.2 |
| | C(7a)-N(1)-C(8)-N(2’), | 165.8 |
| | C(3’)-N(2’)-C(8)-N(1), | 122.0 |
| | N(1)-C(8)-N(1’), | 115.9 |
| | N(2) – N(1’), | 3.2 |
| 5b [2,2’-methylenebis(allopurinol)] | N(1)-N(2)-C(8)-N(2’), | 177.8 |
| | N(1’)-N(2’)-C(8)-N(2), | 94.1 |
| | C(3)-N(2)-C(8)-N(2’), | -2.1 |
| | C(3’)-N(2’)-C(8)-N(2), | -86.4 |
| | N(2)-C(8)-N(2), | 112.4 |
| | N(1) – N(1’), | 4.5 |
It has been postulated that any bimolecular reaction of a small anion passing through a larger polarizable transition state will be accelerated considerably upon changing from protic to aprotic solvents [
23]. Based on this hypothesis, the rate of an ester saponification has been reported to be drastically effected by the solvent composition, e.g. when aqueous alcohol is substituted by aqueous dimethylsulfoxide [
24,
25,
26,
27,
28,
29].
Figure 5.
Pseudo first-order rate constant k as well as ln k versus DMSO concentration for the reaction of 3a → 3b.
Figure 5.
Pseudo first-order rate constant k as well as ln k versus DMSO concentration for the reaction of 3a → 3b.
This prompted us to measure the influence of the DMSO concentration on the reaction rates of nucleophilic displacements on compounds
3a–
5a in 1N NaOH/DMSO mixtures (
Figure 5). This figure using
3a as an example how the reaction rate (rate constant k for pseudo first-order reactions) increases with the DMSO concentration. Analogous results were obtained for
4a and
5a (data not shown). Two reasons have been reported [
23] for such hydrolysis rate enhancements in water - DMSO - sodium hydroxide mixtures: (i) with increasing DMSO concentrations the activity of the OH
- ion is enhanced; at a mole fraction x
DMSO of 0.35 the thermodynamic excess functions are maximal, and with increasing DMSO content the solvation of OH
- is reduced. (ii) At lower DMSO concentrations the solvent lowers the energy of the reaction intermediate by specific solvation. This leads consequently to a rate enhancement. Because in our case the mole fraction of DMSO ranges between 0 and 0.3 (0 – 60 vol-%), the second effect should be predominant. For all reactions measured under pseudo first-order conditions linear ln k
versus x
DMSO plots were obtained following the relation:
where m = sensitivity of the substrates to the dimethylsulfoxide content in the varying solvent compositions; ln k = natural logarithm of the pseudo first-order rate constant; ln k
0 = natural logarithm of the pseudo first-order rate constant in a DMSO-free medium and x
DMSO = mole fraction of dimethylsulfoxide).
The sensitivity values, m, are listed in
Table 2. As can be seen, all S
NAr reactions performed show almost the same sensitivity toward the dimethylsulfoxide concentration in the solvent composition (7.9 –8.8). There is no significant difference between the N(1)- and N(2)-alkylated heterocycles. This becomes understandable under the assumption that in all cases similar reaction intermediates of the
Meisenheimer complex type are formed. This is likely because the OCH
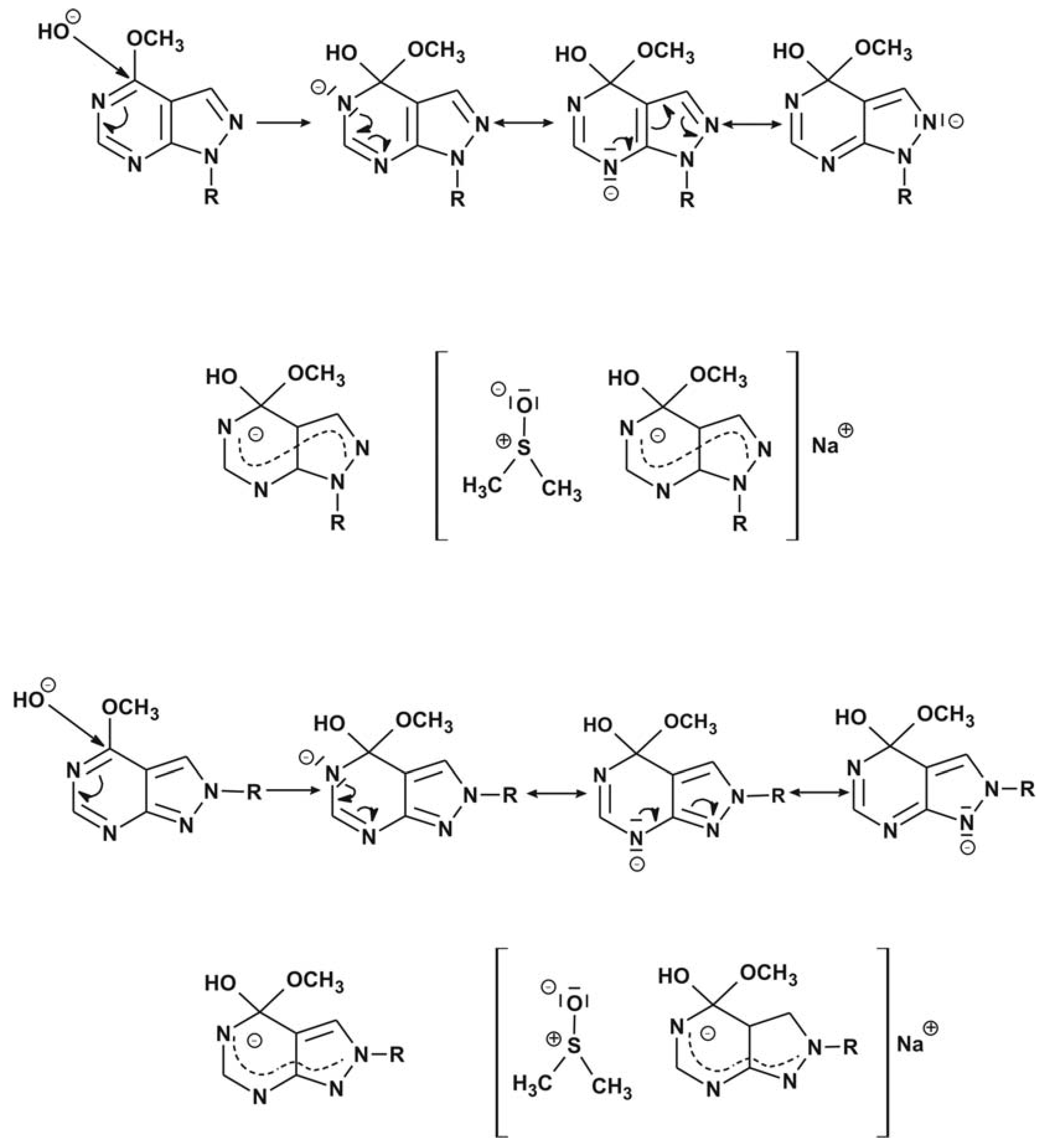
3 group is a rather weak leaving group. Both intermediates can be formulated by three mesomeric
Lewis structures which are probably energetically equivalent as their negative charges are located on the nitrogen atoms (
Scheme 1).
Scheme 1.
Mesomeric structures of the Meisenheimer complexes formed as intermediates during the nucleophilic displacement reactions of 3a and 5a with OH- and its solvation by dimethylsulfoxide.
Scheme 1.
Mesomeric structures of the Meisenheimer complexes formed as intermediates during the nucleophilic displacement reactions of 3a and 5a with OH- and its solvation by dimethylsulfoxide.
This implies that for the nucleophilic displacement reactions of the methoxy groups of the N(1)- as well as for the N(2)-alkylated 4-methoxy-pyrazolo[3,4-
d]pyrimidines the intermediates with a tetrahedral C(4) are energetically lowered by the same amount through solvation by dimethylsulfoxide (
Scheme 1).
Experimental Section
General
All chemicals were purchased from Aldrich, Sigma or Fluka (Sigma-Aldrich Chemie GmbH, Deisenhofen, Germany). Solvents were of laboratory grade and were distilled before use. Thin layer chromatography (TL): glass sheets, silica gel 60 F
254, 0.2 mm layer (Macherey-Nagel & Co, Düren, Germany). Column flash chromatography (FC): silica gel 60 (Merck, Germany) at 0.5 bar (4 x 10
4 Pa); sample collection with an UltroRac II fraction collector (LKB Instrumnets, Bromma, Sweden). Melting points (uncorrected): Linström apparatus (Wagner & Munz), Germany). UV spectra and reaction kinetics were measured in MT-4 cuvettes (1 mL) on a SuperScan 3 (Varian, Australia) or a Shimadzu U-210 (Shimadzu, Japan) spectrophotometers. The temperature was controlled with a Lauda R20-K thermostat, connected to a R40/2 digital thermometer (MGW Lauda, Germany). NMR Spectra were measured on AC-250 and AMX-500 spectrometers (Bruker, Karlsruhe, Germany) operating at 250.13/500.14 MHz (
1H) and 62.896/125.700 MHz (
13C), respectively. Chemical shifts (δ values) are in parts per million relative to tetramethylsilane used as internal standard. Elemental analyses were performed by Mikroanalytisches Laboratorium Beller (Göttingen, Germany). Inhibition constants,
Ki, were taken from Dixon plots, measured according to [
7].
Methylenebis(4-methoxy-1H-pyrazolo[3,4-d]pyrimidines) 3a-c – General Procedure
Caution! Use a well ventilated hood. 4-Methoxy-1H-pyrazolo[3,4-d]pyrimidine (500 mg, 3.33 mmol), suspended in dichloromethane/dibromomethane (3:1, v/v, 20 mL), was added to a solution of tetrabutylammonium hydrogen sulfate (700 mg, 2.5 mmol) in 50 % aq. sodium hydroxide (20 mL) and agitated with a vibromixer for 1 h at room temperature. The biphasic mixture was then poured into chloroform (200 mL) containing glacial acetic acid (30 mL). After filtration of the precipitated sodium acetate the organic layer was washed twice with water, dried (calcium chloride) and evaporated to dryness. Crystallization from methanol gave a mixture of 3a–5a (305 mg, 61% yield). For isolation of the three regioisomers the mixture (500 mg) was dissolved in chloroform/methanol (9:1, v/v, 10 mL), applied to a silica gel column (silica gel 60H, 6 x 30 cm) and chromatographed with the same solvent mixture as eluent. Three zones were separated.
1,1’-Methylenebis(4-methoxy-1H-pyrazolo[3,4-d]pyrimidine (3a). The fastest migrating zone afforded compound 3a as colorless needles (155 mg, 32 % yield) with a melting point of 202-204 °C upon evaporation of the solvent and crystallization from methanol. TLC (silica gel, chloroform/methanol, 9:1, v/v): Rf = 0.92. UV (chloroform/methanol, 1:1, v/v): λmax = 247 nm (ε = 16.600); 1H-NMR ((CD3)2SO) δ 8.68 (1H, s, H-C(6)); 8.03 (1H, s, H-C(3)); 6.99 (2H, s, CH2); 4.13 (3H, s, OCH3); 13C-NMR ((CD3)2SO) δ 164.1 (C-4); 156.2 (C-6); 155.9 (C-7a); 133.2 (C-3); 103.0 (C-3a); 55.9 (CH2); 54.2 (OCH3); EI MS (70 eV): m/e 312 (52 %, M+), 163 (100 %, M+ - chromophore); Anal. Calcd. for C13H12N8O2: C, 49.99; H, 3.87; N, 35.88. Found: C, 49.84; H, 4.01; N, 35.99.
4-Methoxy-1-[(4-methoxy-2H-pyrazolo[3,4-d]pyrimidin-2-yl)methyl]-1H-pyrazolo[3,4-d]pyrimidine (4a). The second zone afforded compound 4a as colorless needles (309 mg, 64 % yield) with a melting point of 211-214 °C upon evaporation of the solvent and crystallization from methanol. TLC (silica gel, chloroform/methanol, 9:1, v/v): Rf = 0.68; UV (chloroform/methanol, 1:1, v/v): λmax = 251, 262 nm (ε = 15.100, 14.300); 1H-NMR ((CD3)2SO) δ 8.63 (1H, s, H-C(6), N(1)-alkylated moiety); 8.27 (1H, s, H-C(6), N(2)-alkylated moiety); 8.59 (1H, s, H-C(3), N(2)-alkylated moiety); 8.07 (1H, s, H-C(3), N(1)-alkylated moiety); 6.89 (2H, s, CH2); 4.13 (3H, s, OCH3, N(1)-alkylated moiety); 4.08 (3H, s, OCH3, N(2)-alkylated moiety); 13C-NMR ((CD3)2SO) δ 165.6 (C-4, N(2)-alkylated moiety); 164.2 (C-4, N(1)-alkylated moiety); 161.8 (C-7a, N(2)-alkylated moiety); 156.4 (C-6, N(2)-alkylated moety); 156.2 (C-6, N(1)-alkylated moiety); 156.0 (C-7a, N(1)-alkylated moiety); 133.8 (C-3, N(1)-alkylated moiety); 123.7 (C-3, N(2)-alkylated moiety); 104.3 (C-3a, N(2)-alkylated moiety); 103.4 (C-3a, N(1)-alkylated moiety); 62.2 (CH2); 54.3 (OCH3, N(1)-alkylated moiety); 54.0 (OCH3, N(2)-alkylated moiety); EI MS (70 eV): m/e 312 (100 %, M+), 163 (57 %, M+ - chromophore); Anal. Calcd. for C13H12N8O2: C, 49.99; H, 3.87; N, 35.88. Found: C, 50.10; H, 4.01; N, 35.92.
2,2'-Methylenebis(4-methoxy-2H-pyrazolo[3,4-d]pyrimidine) (5a). From the slowest migrating zone compound 5a (18.6 mg, 4 % yield) was obtained as colorless needles upon evaporation of the solvent and crystallization of the residue from 1,4-dioxane; melting point 202-204 °C. TLC (silica gel, chloroform/methanol, 9:1, v/v): Rf = 0.45; UV (chloroform/methanol, 9:1, v/v): λmax = 262 nm (ε = 18.500); 1H-NMR ((CD3)2SO) δ 8.45 (1H, s, H-C(6)); 8.59 (1H, s, H-C(3); 6.79 (2H, s, CH2); 4.08 (3H, s, OCH3); 13C-NMR ((CD3)2SO) δ 165.9 (C-4); 161.2 (C-7a); 156.3 (C-6); 125.8 (C-3); 104.1 (C-3a); 64.7 (CH2); 54.0 (OCH3); EI MS (70 eV) m/e 312 (45 %, M+), 163 (100 %, M+ - chromophore); Anal. Calcd. for C13H12N8O2: C, 49.99; H, 3.87; N, 35.88. Found: C, 49.74; H, 3.97; N, 35.61.
Methylenebis(allopurinols) 3b–5b – General Procedure
Compounds 3a– 5a (3a, 4a: 500 mg, 1.65 mmol, each; 5a; 70 mg, 0.26 mmol) were each dissolved in 2N sodium hydroxide/MeOH (1:1, v/v, 20 mL) and refluxed for 1 h. In the cases of compounds 3b and 4b acidification of the reaction mixtures with 6N HCl afforded the corresponding methylenebis(allopurinols) directly as colorless solids; in the case of 5b further chromatographic purification proved to be necessary.
1,1'-Methylenebis(1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one) (3b). Compound 3b (440 mg, 95 % yield); m.p. > 320 °C. TLC (silica gel, 0.25 M LiCl): Rf = 0.75; UV (1N sodium hydroxide): λmax = 272 nm (ε = 19.500); 1H-NMR (CD3OD/NaOD): δ 6.71 (s, CH2); 8.02 (s, H-C(3)); 8.25 (s, H-C(6)); Anal. Calcd. for C11H8N8O2: C, 46.48; H, 2.84; N, 39.42. Found: C, 46.31; H, 2.98; N, 39.49.
1-[(4-Oxo-4,5-dihydro-2H-pyrazolo[3,4-d]pyrimidin-2-yl)methyl]-1,5-dihydro-4H-pyrazolo[3,4-d]- pyrimidin-4-one (4b). Compound 4b (423 mg, 91 % yield); m.p. > 320 °C; TLC (silica gel, 0.25 M LiCl): Rf = 0.70; UV (1N sodium hydroxide): λmax = 273, 281 (sh) nm (ε = 20.500, 18.700); 1H-NMR (CD3OD/NaOD): δ 6.71 (2H, s, CH2); 8.08 (1H, s, H-C(3), N(1)-alkylated moiety); 8.14 (1H, s, H-C(3), N(2)-alkylated moiety); 8.26 (1H, s, H-C(6), N(1)-alkylated moiety); 8.43 (1H, s, H-C(6), N(2)-alkylated moiety); Anal. Calcd. for C11H8N8O2: C, 46.48; H, 2.84; N, 39.42. Found: 46.39; H, 2.90; N, 39.42.
2,2'-Methylenebis(2,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one) (5b). The reaction mixture was chromatographed on a Dowex® 1x2 ion exchange resin column (1.5 x 30 cm). After washing with water (1 L) compound 5b (62 mg, 96 % yield) was eluted with aq. acetic acid (10 %). M.p. > 320 °C; TLC (silica gel, 0.25 M LiCl): Rf = 0.77; UV (1N sodium hydroxide): λmax = 283 nm (ε = 19.500); 1H-NMR (CD3OD/NaOD): δ 8.06 (2H, br. S, H-C(6) and H-C(3); 5.91 (2H, CH2); Anal. Calcd. for C11H8N8O2: C, 46.48; H, 2.84; N, 39.42. Found: C, 46.33; H, 2.96; N, 39.31.
1,1'-Methylenebis(1H-pyrazolo[3,4-d]pyrimidin-4-amine) (3c)
1,1’-Methylenebis(4-methoxy-1H-pyrazolo[3,4-d]pyrimidine (3a, 100 mg, 0.33 mmol) was dissolved in concentrated aq. ammonia (20 mL) and stirred for 60 min at room temperature. After evaporation of the solvent the residue was taken up in water and chromatographed on Amberlite® XAD, type 4 (Serva, Germany). Methanol/water (1:4, v/v) eluted one main peak from which compound 3c (70 mg, 75 % yield) was isolated as colorless solid. M.p.: 88-89°C; TLC (silica gel, chloroform/methanol, 9:1, v/v): Rf = 0.4; UV (chloroform/methanol, 1:1, v/v): λmax = 260, 276 nm (ε = 16.800, 18.700); 1H-NMR ((CD3)2SO) δ 8.25 (1H, s, H-C(6)); 8.09 (1H, s, H-C(3)); 7.77 and 7.26 (2H, br, NH2); 6.69 (2H, s, CH2); 13C-NMR ((CD3)2SO) δ 54.8 (CH2); 99.9 (C-3a); 133.8 (C-3); 154.1 (C-7a); 156.5 (C-6); 158.0 (C-4); Anal. Calcd. for C11H10N10: C, 46.80; H, 3.57; N, 49.62. Found: C, 46.72, H, 3.65; N, 49.50.