New Diterpenoid Alkaloids from the Roots of Delphinium tiantaishanense
Abstract
:Introduction

Results and Discussion
 ) and HMBC (
) and HMBC (  ) correlations of 1 and 2.
) correlations of 1 and 2.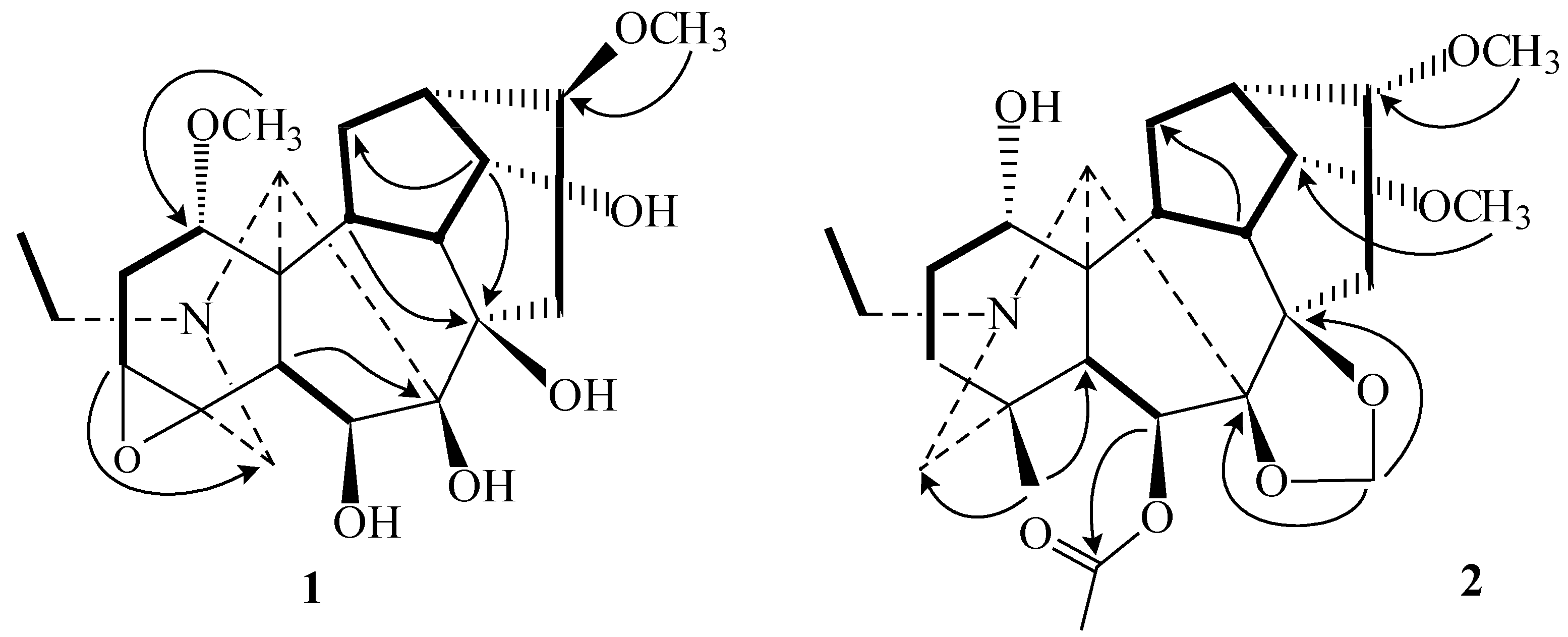
 ) and HMBC (
) and HMBC (  ) correlations of 3 and 4.
) correlations of 3 and 4.
| NO. | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH Mult (J = Hz) | δC | δH Mult (J = Hz) | |
| 1 | 77.7 d | 3.90 s | 71.6 d | 3.81 br s |
| 2 | 31.6 t | 1.26 m (α) | 29.2 t | 1.26 br s (α) |
| 2.18 m (β) | 1.56 br s (β) | |||
| 3 | 57.8 d | 3.05 m | 30.9 t | 1.54 m (β) |
| - | 1.58 m (α) | |||
| 4 | 58.3 s | - | 32.8 s | - |
| 5 | 52.2 d | 1.56 s | 51.1 d | 1.39 m |
| 6 | 79.8 d | 4.82 s | 78.2 d | 5.44 s |
| 7 | 91.3 s | - | 90.9 s | - |
| 8 | 84.4 s | - | 84.4 s | - |
| 9 | 43.0 d | 3.12 t (5.6) | 39.5 d | 3.44 m |
| 10 | 43.5 d | 2.12 m | 45.2 d | 2.14 m |
| 11 | 54.0 s | - | 51.3 s | - |
| 12 | 29.5 t | 1.58 m (β) | 30.3 t | 1.87 m (β) |
| 2.12 m (α) | 2.18 m (α) | |||
| 13 | 40.4 d | 2.26 m | 37.2 d | 2.44 m |
| 14 | 74.3 d | 4.03 t (4.4) | 83.5 d | 3.73 t (4.0) |
| 15 | 26.7 t | 1.97 m (α) | 34.2 t | 1.88 m (α) |
| 2.59 m (β) | 2.51 m (β) | |||
| 16 | 82.3 d | 3.39 s | 82.2 d | 3.28 m |
| 17 | 67.2 d | 2.91 d (1.5) | 65.3 d | 3.02 s |
| 18 | - | - | 26.7 q | 0.99 s |
| 19 | 54.3 t | 2.49 ABq | 61.1 t | 2.44 m |
| - | 3.36 ABq hidden | 2.51 m | ||
| 21 | 49.9 t | 2.98 m | 49.6 t | 2.74 m |
| 3.39 m | 2.87 m | |||
| 22 | 13.9 q | 1.07 t (7.2) | 13.3 q | 1.11 t (7.2) |
| 1-OCH3 | 51.7 q | 3.51 s | - | - |
| 14-OCH3 | - | 57.4 q | 3.43 s | |
| 16-OCH3 | 56.4 q | 3.41 s | 56.1 q | 3.36 s |
| -OCH2O- | 93.9 t | 4.92 s | ||
| 4.96 s | ||||
| OAc | 169.7 s | |||
| 21.5 q | 2.07 s | |||
| NO. | 3 | 4 | ||
|---|---|---|---|---|
| δC | δH Mult (J = Hz) | δC | δH Mult (J = Hz) | |
| 1 | 80.4 d | 3.23 m | 29.0 t | 1.55 m (β) |
| - | 1.81 m (α) | |||
| 2 | 25.6 t | 1.53 m (α) | 19.9 t | 0.87 br s (β) |
| 1.92 m (β) | 1.61 m (α) | |||
| 3 | 31.9 t | 1.31 m (β) | 33.1 t | 1.25 m |
| 1.72 m (α) | 1.47 m | |||
| 4 | 44.4 s | - | 37.5 s | - |
| 5 | 52.5 d | 1.41 br s | 60.0 d | 1.67 s |
| 6 | 79.9 d | 5.25 s | 68.0 d | 3.33 s |
| 7 | 90.9 s | - | 70.4 d | 5.44 d (2.8) |
| 8 | 82.9 s | - | 52.5 s | - |
| 9 | 39.8 d | 3.40 m | 47.1 d | 2.43 dd (6.6, 1.5) |
| 10 | 47.9 d | 2.20 m | 50.9 s | - |
| 11 | 49.8 s | - | 75.4 d | 5.28 d (6.4) |
| 12 | 28.7 t | 2.13 m (β) | 40.1 d | 2.28 br s |
| 1.88 dd (α) (10, 5.2) | - | |||
| 13 | 38.7 d | 2.40 m | 28.0 t | 1.43 m |
| 2.34 m (hidden) | ||||
| 14 | 83.4 d | 3.73 t (3.2) | 39.2 d | 2.34 t (9.6) |
| 15 | 33.4 t | 1.85 m (α) | 66.0 d | 4.06 s |
| 2.71 m (β) | - | |||
| 16 | 81.3 d | 3.28 t (5.2) | 150.8 s | - |
| 17 | 63.5 d | 4.16 br s | 112.2 t | 5.04 d (0.9) |
| 18 | 22.1 q | 1.17 s | 28.9 q | 0.97 s |
| 19 | 169.9 d | 7.46 br s | 62.7 t | 2.49 s |
| 20 | - | - | 74.1 d | 2.75 s |
| 1-OCH3 | 55.4 q | 3.22 s | - | - |
| 14-OCH3 | 57.7 q | 3.45 s | - | - |
| 16-OCH3 | 56.4 q | 3.34 s | - | - |
| -OCH2O- | 93.9 t | 4.93 s | - | - |
| 4.97 s | - | |||
| OAc | 169.5 s | - | 170.3 s | - |
| 21.5 q | 2.06 s | 21.4 q | 2.06 s | |
| ArCO | 167.1 s | - | ||
| 1’ | 129.7 s | - | ||
| 2’, 6’ | 130.1 d | 8.14 d (6.8) | ||
| 3’ ,5’ | 128.4 d | 7.45 m | ||
| 4’ | 133.4 d | 7.58 m | ||

Experimental
General
Plant Material
Extraction and Isolation
 +35.4° (c=0.84, CHCl3); IR (KBr) cm-1: 3425, 2943, 2879, 1707, 1638, 1460; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3): see Table 1; HR-ESI-MS: m/z [M+H]+ 424.2351, calcd for C22H34NO7, 424.2330.
+35.4° (c=0.84, CHCl3); IR (KBr) cm-1: 3425, 2943, 2879, 1707, 1638, 1460; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3): see Table 1; HR-ESI-MS: m/z [M+H]+ 424.2351, calcd for C22H34NO7, 424.2330. -23.9° (c=0.73, CHCl3); IR (KBr) cm-1: 3396, 2932, 1738, 1457, 1367, 1084; 1H-NMR (400 MHz, CDCl3) and 13C- NMR (100 MHz, CDCl3): see Table 1; HR-ESI-MS: m/z 478.2801 [M+H]+, calcd for C26H40NO7, 478.2799.
-23.9° (c=0.73, CHCl3); IR (KBr) cm-1: 3396, 2932, 1738, 1457, 1367, 1084; 1H-NMR (400 MHz, CDCl3) and 13C- NMR (100 MHz, CDCl3): see Table 1; HR-ESI-MS: m/z 478.2801 [M+H]+, calcd for C26H40NO7, 478.2799. +24.2° (c=0.26, CHCl3); IR (KBr) cm-1: 3396, 2926, 1741, 1637, 1461, 1365; 1H-NMR (600 MHz, CDCl3) and 13C-NMR (150 MHz, CDCl3): see Table 2; HR-ESI-MS: m/z 462.2473 [M+H]+, calcd for C25H36NO7, 462.2486.
+24.2° (c=0.26, CHCl3); IR (KBr) cm-1: 3396, 2926, 1741, 1637, 1461, 1365; 1H-NMR (600 MHz, CDCl3) and 13C-NMR (150 MHz, CDCl3): see Table 2; HR-ESI-MS: m/z 462.2473 [M+H]+, calcd for C25H36NO7, 462.2486. +35.6° (c=0.85, CHCl3); IR (KBr) cm-1: 3446, 2943, 1736, 1714, 1243, 1108; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3): see Table 2; HR-ESI-MS: m/z 476.2439 [M+H]+, calcd for C29H34NO5, 476.2431.
+35.6° (c=0.85, CHCl3); IR (KBr) cm-1: 3446, 2943, 1736, 1714, 1243, 1108; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3): see Table 2; HR-ESI-MS: m/z 476.2439 [M+H]+, calcd for C29H34NO5, 476.2431.Acknowledgments
References
- Zhang, W. J.; Chen, G. H. A new species of Delphinium L. from Sichuan, China. West China J. Pharm Sci 2006, 21, 560–561. [Google Scholar]
- Pelletier, S. W.; Mody, N. V.; Joshi, B. S.; Schramn, L. C. Alkaloids: Chemical and Biological Perspectives; Pelletier, S. W., Ed.; John Wiley. & Sons Press: New York, 1984; Vol. 2, pp. 206–210. [Google Scholar]
- Pelletier, S. W.; Joshi, B. S. Alkaloids: Chemical and Biological Perspectives; Pelletier, S. W., Ed.; John Wiley & Sons Press: New York, 1991; Vol. 7, p. 297. [Google Scholar]
- Boido, V.; Edwards, O. E.; Handa, K. L.; Kolt, R. J.; Purushothaman, K. K. Alkaloids of Acontitum columbianum Nutt. Can. J. Chem 1984, 62, 778–784. [Google Scholar]
- Wang, F. P.; Liang, X. T. Alkaloids: Chemistry and Biology; Cordell, G. A., Ed.; Elsevier Press: New York, 2002; Vol. 59, pp. 1–280. [Google Scholar]
- Sample Availability: Available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Li, J.; Chen, D.-L.; Jian, X.-X.; Wang, F.-P. New Diterpenoid Alkaloids from the Roots of Delphinium tiantaishanense. Molecules 2007, 12, 353-360. https://doi.org/10.3390/12030353
Li J, Chen D-L, Jian X-X, Wang F-P. New Diterpenoid Alkaloids from the Roots of Delphinium tiantaishanense. Molecules. 2007; 12(3):353-360. https://doi.org/10.3390/12030353
Chicago/Turabian StyleLi, Jie, Dong-Lin Chen, Xi-Xian Jian, and Feng-Peng Wang. 2007. "New Diterpenoid Alkaloids from the Roots of Delphinium tiantaishanense" Molecules 12, no. 3: 353-360. https://doi.org/10.3390/12030353




