Comparative Study of Regioselective Synthesis of β-Aminoalcohols under Solventless Conditions Catalyzed by Sulfated Zirconia and SZ/MCM-41
Abstract
:Introduction
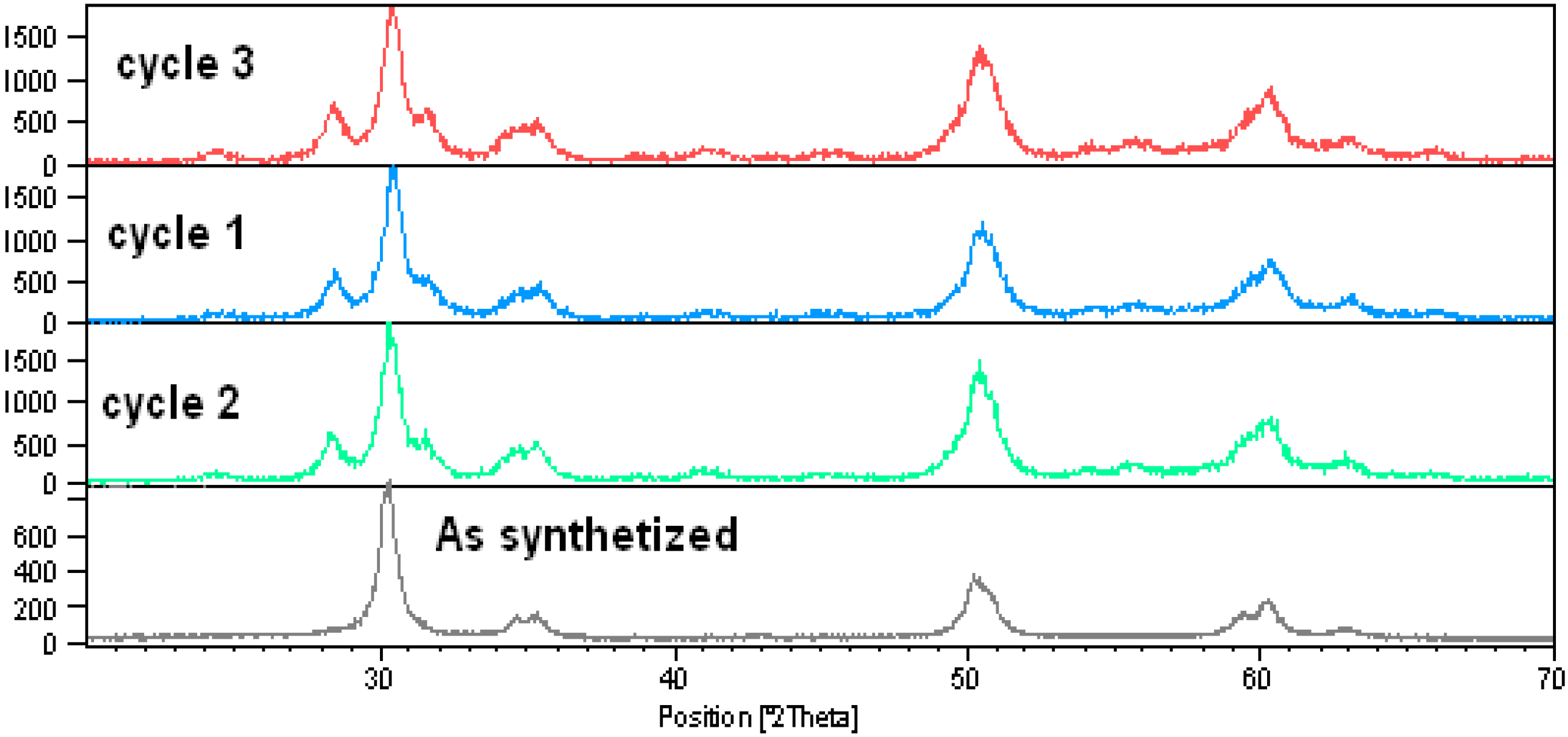
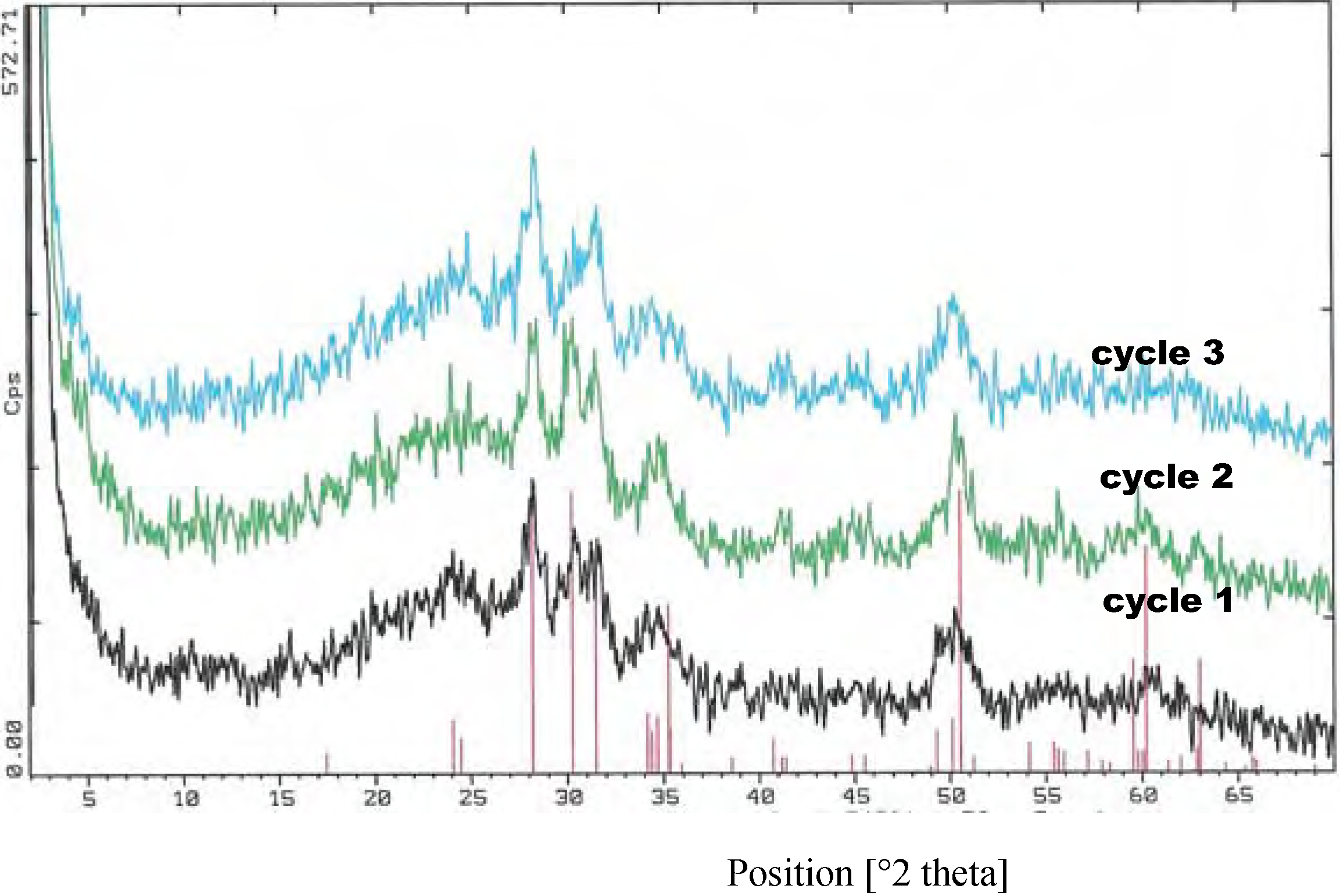
Results and Discussion
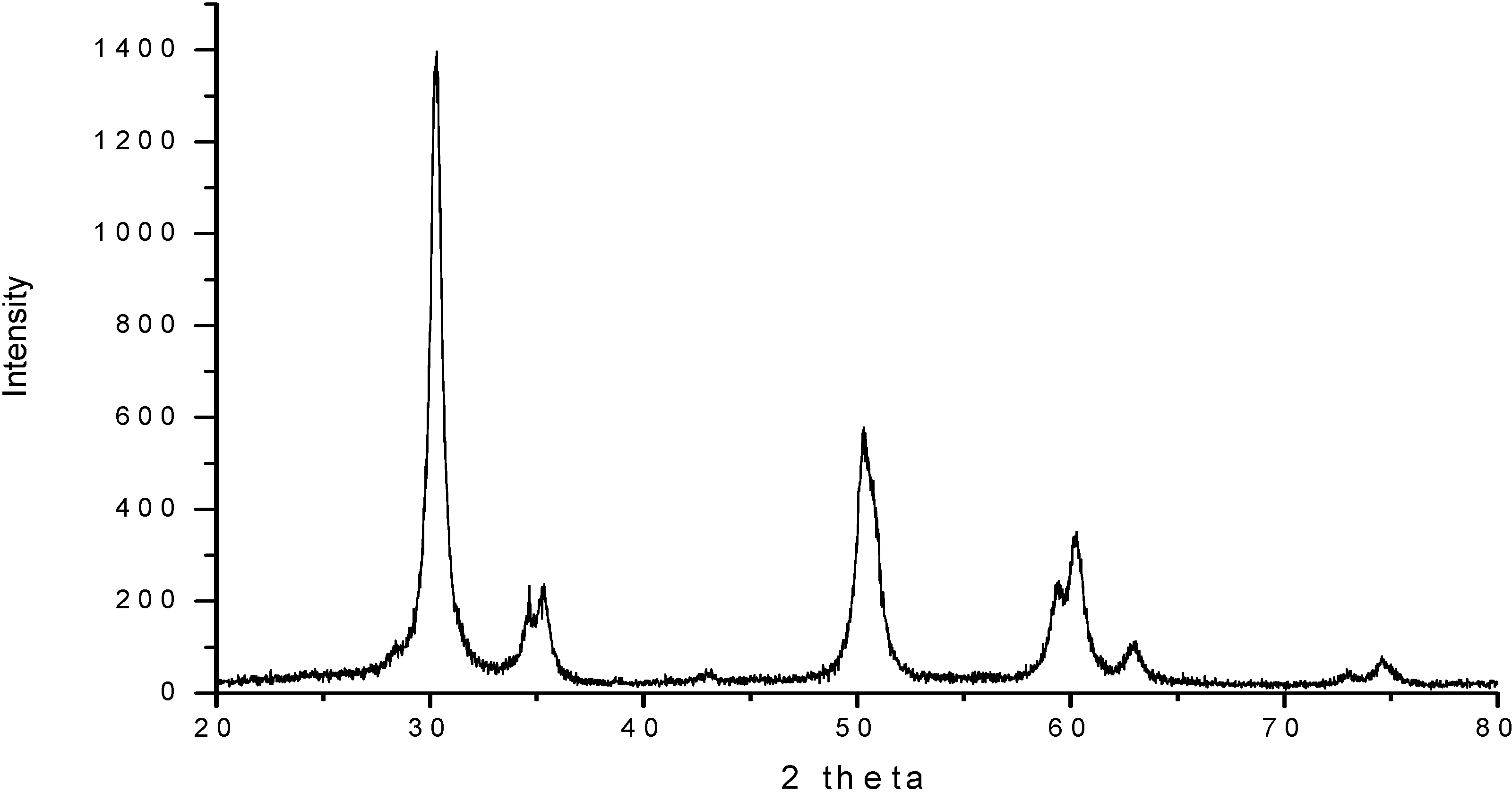
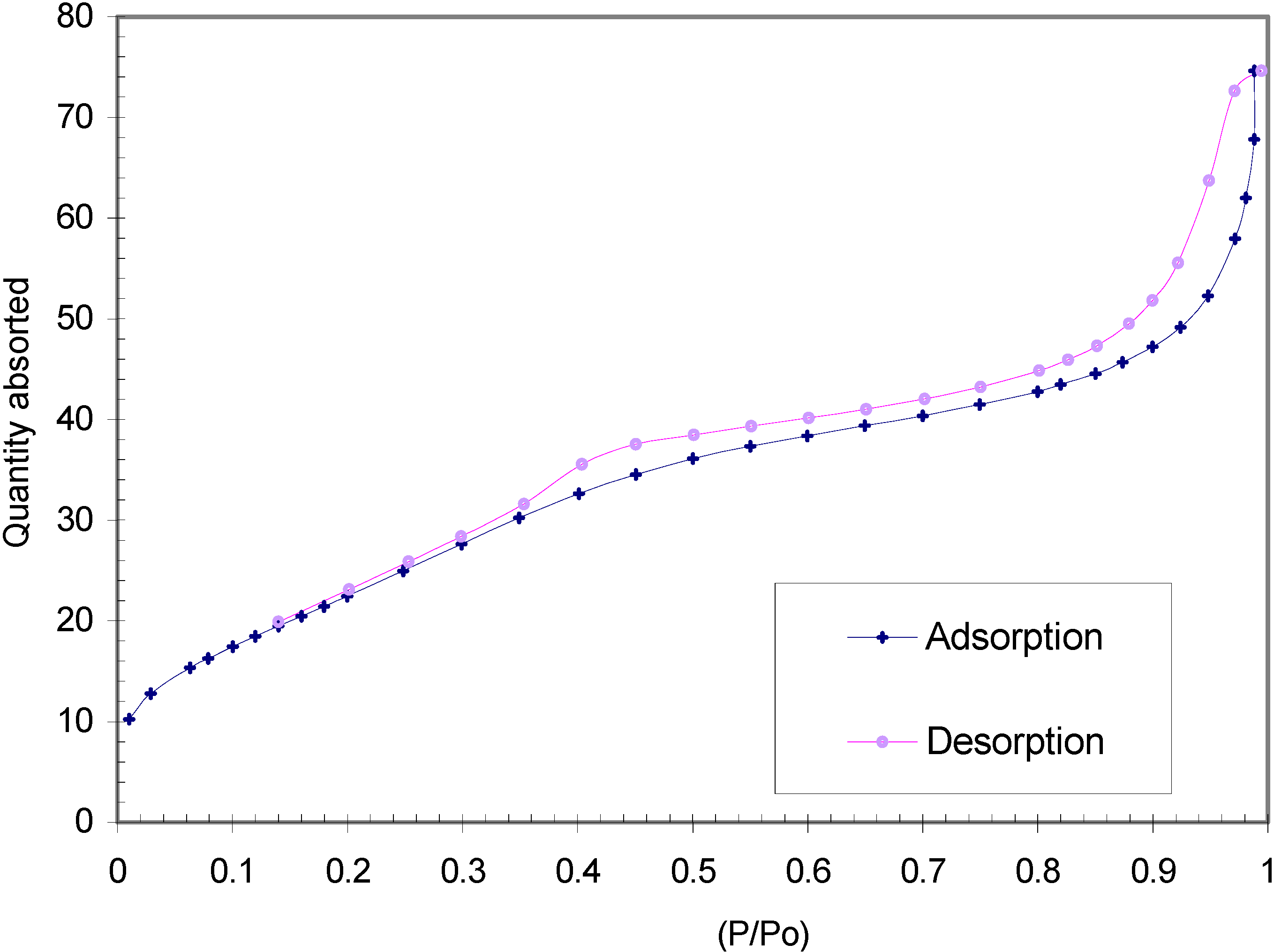
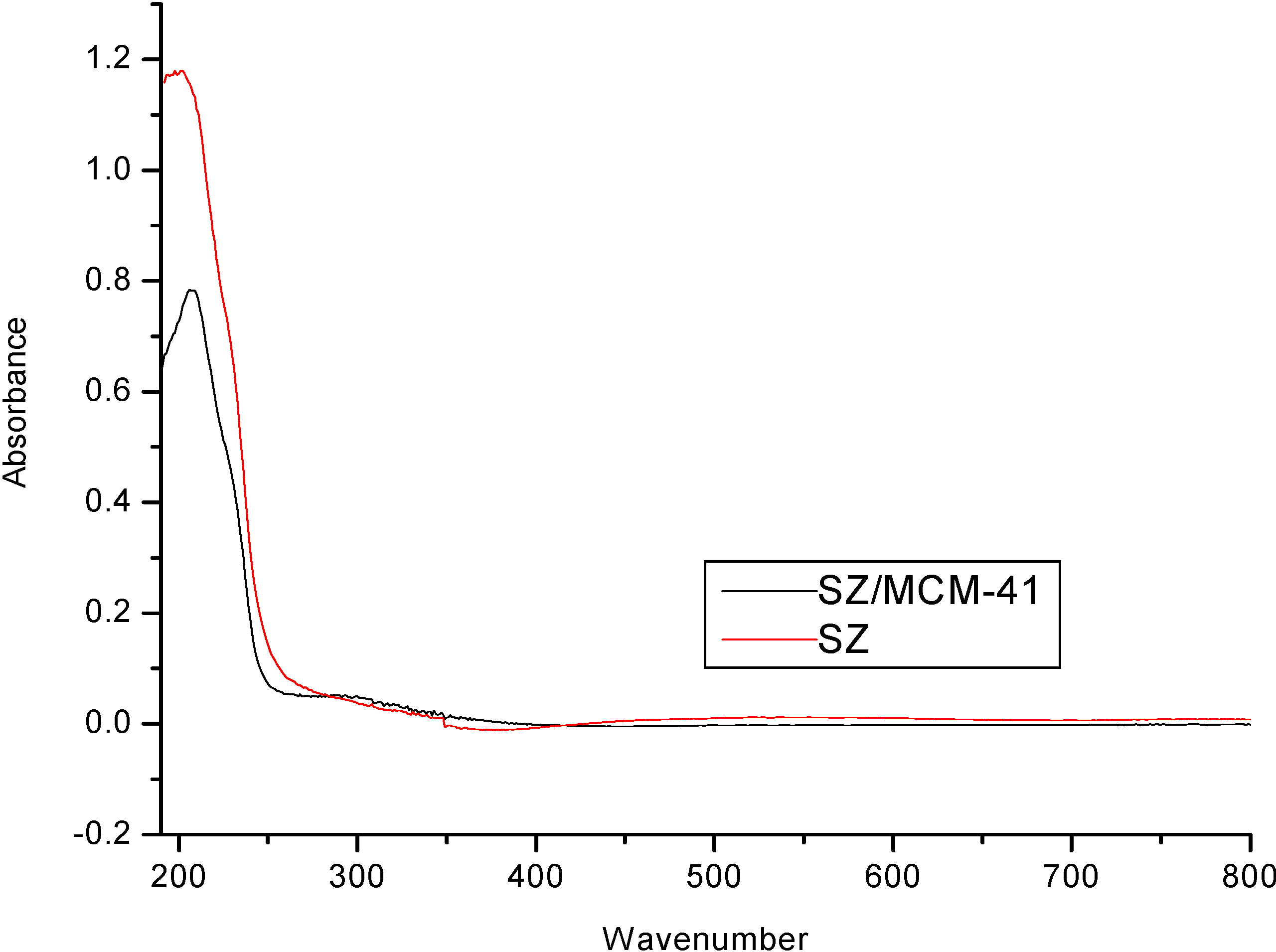
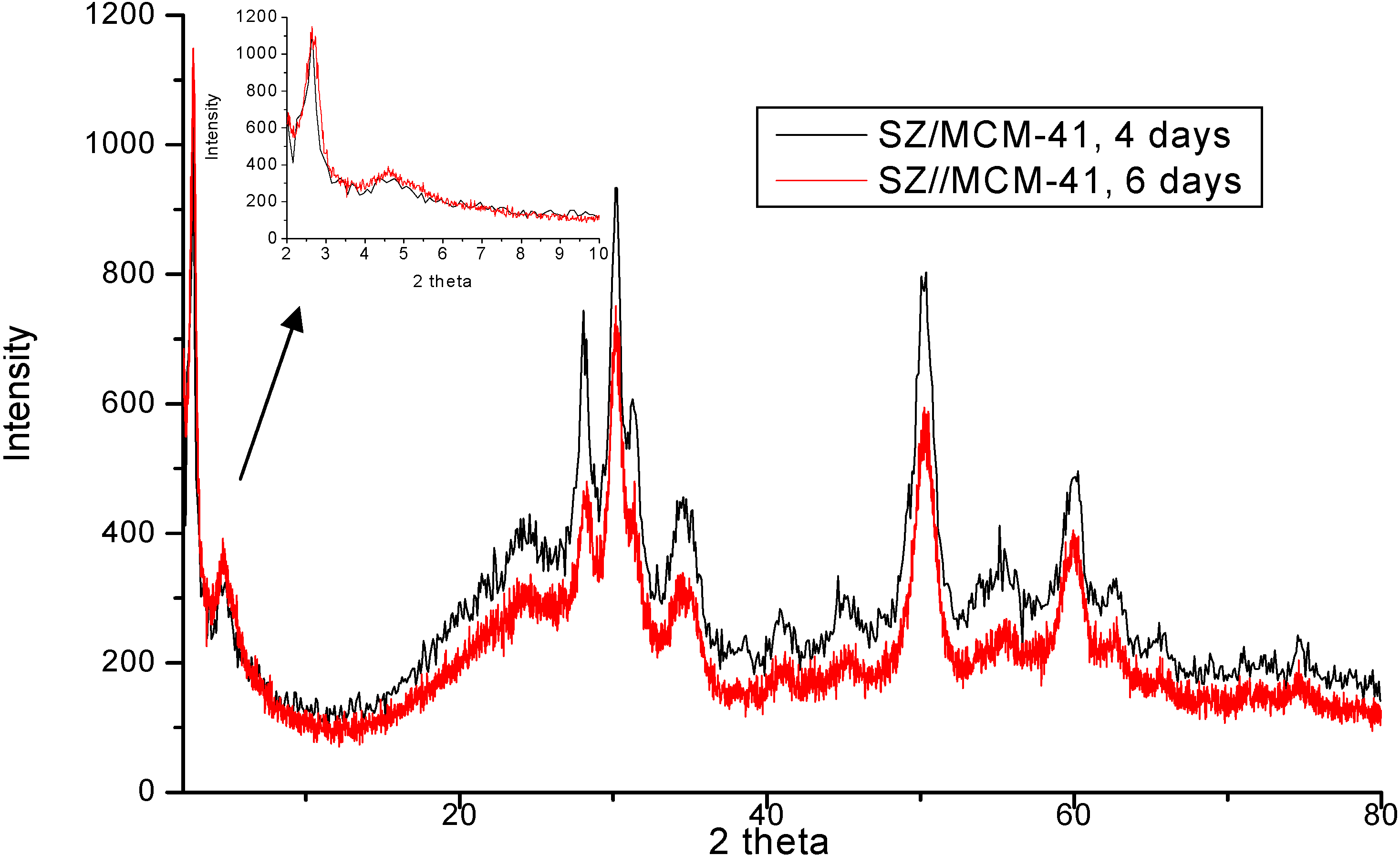
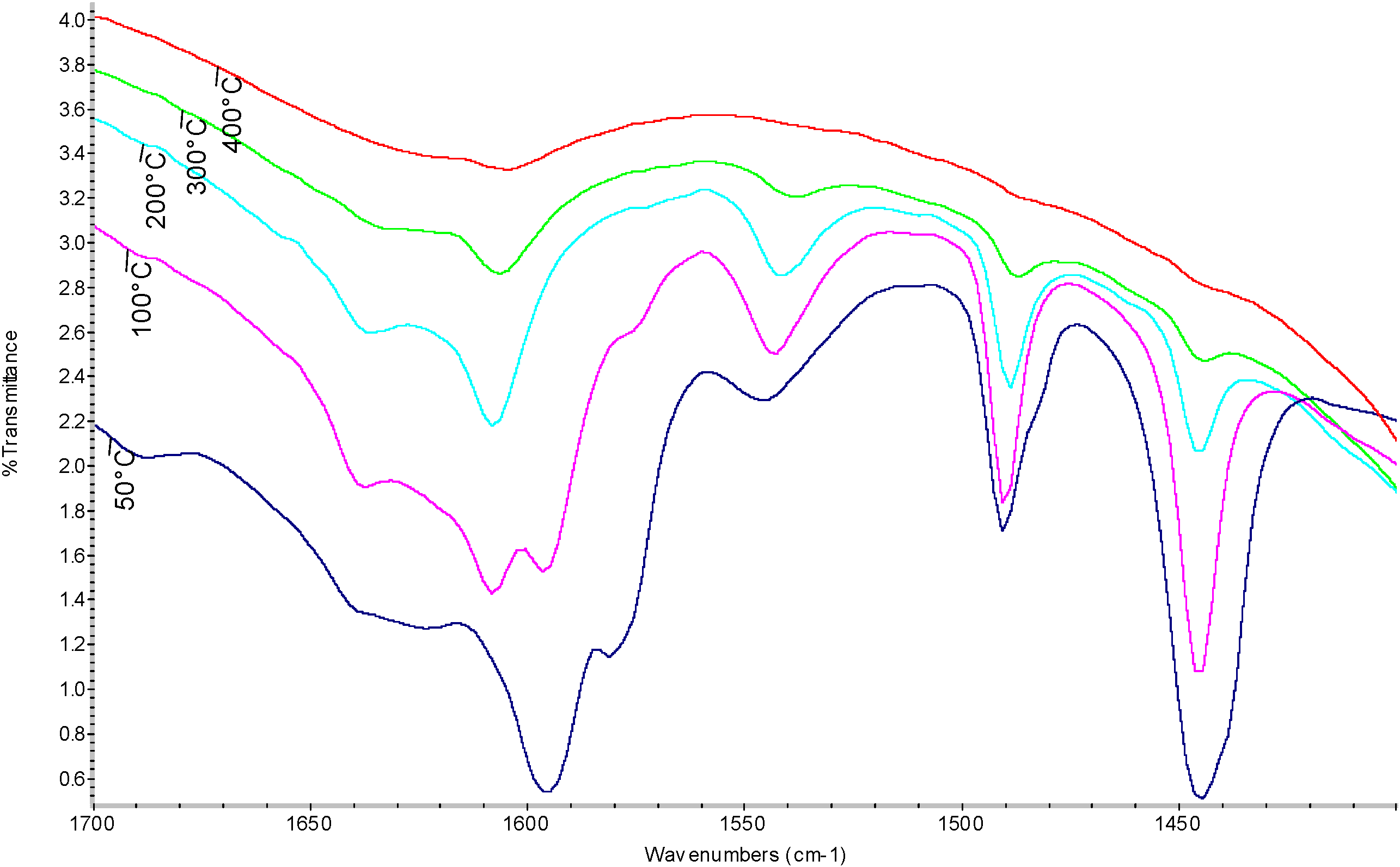
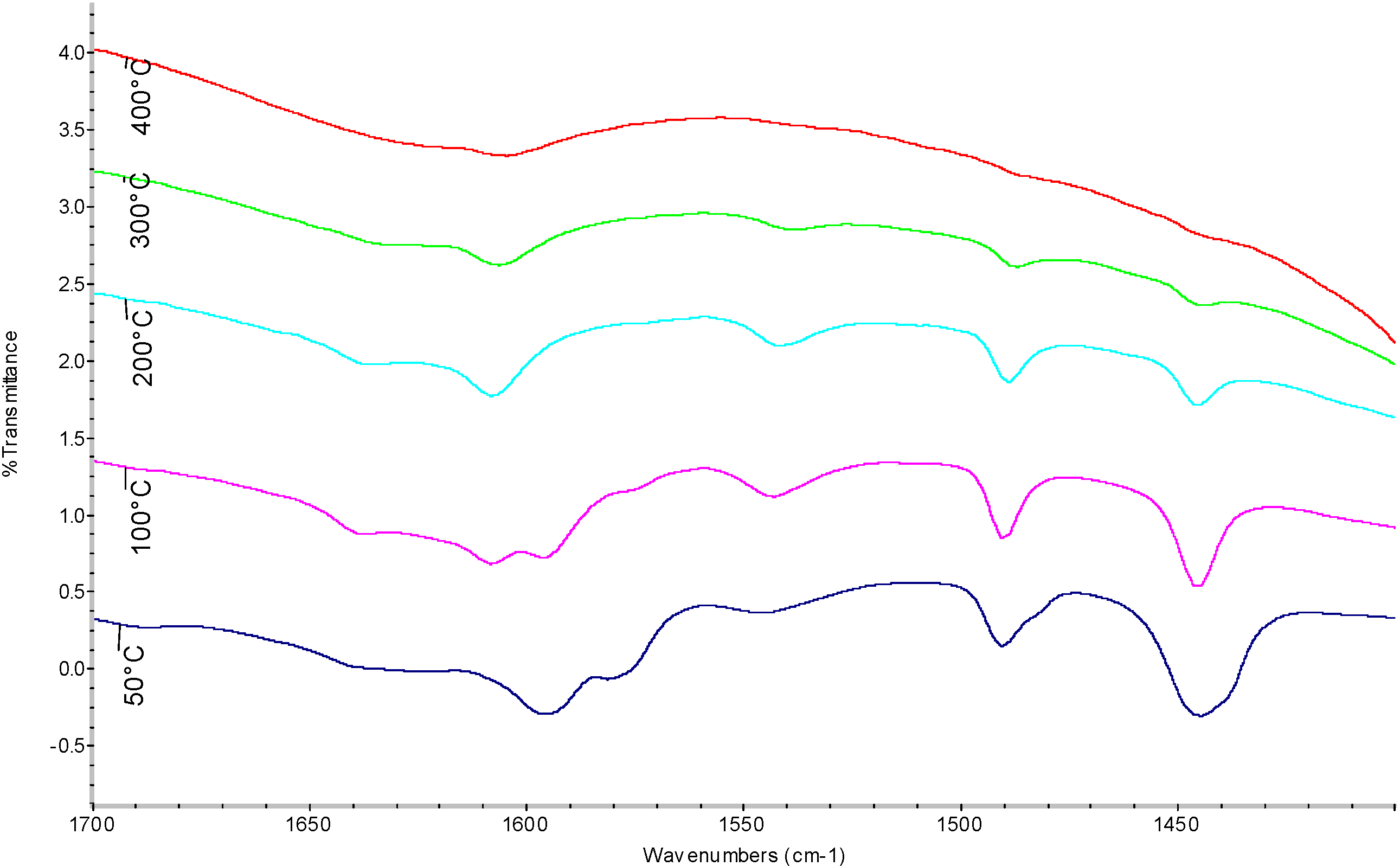
| Temperature (°C) | Sulfated Zirconia (µmol pyg-1) | SZ/MCM-41 (µmol py g-1) | ||||
|---|---|---|---|---|---|---|
| Brønsted | Lewis | Total | Brønsted | Lewis | Total | |
| 50 | 101 | 179 | 280 | 18 | 224 | 242 |
| 100 | 69 | 74 | 143 | 30 | 71 | 101 |
| 200 | 33 | 52 | 85 | 18 | 17 | 35 |
| 300 | 14 | 27 | 41 | 5 | 5 | 10 |


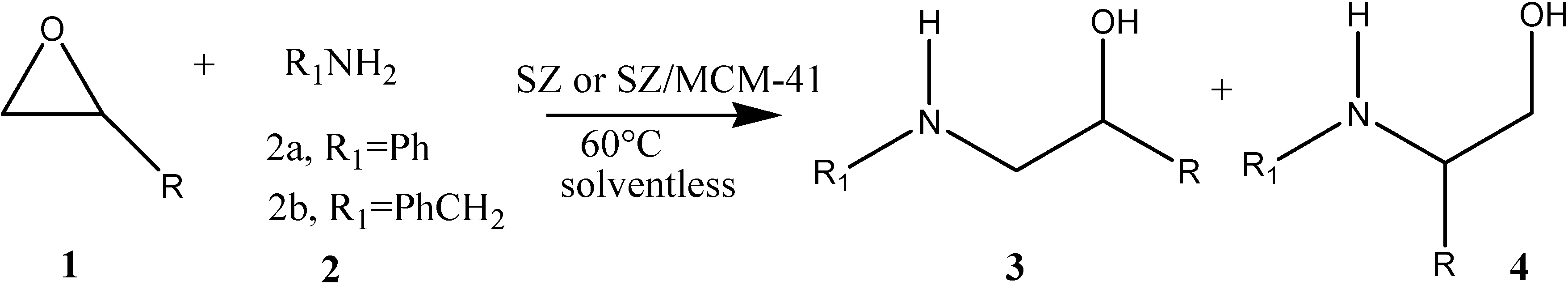
| Entry | 1 | 2 | Time (h) | 3/4 Yield (%) Cat. = SZ | 3/4 Yield (%) Cat. = SZ/MCM-41 |
|---|---|---|---|---|---|
| 1 | 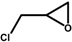 | 2a | 0.5 | 74/0 | 23/0 |
| 2 [41,42]* | 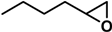 | 2a | 5 | 61/20 | 63/0 |
| 3 [43] |  | 2b | 6 | 82/0 | 35/0 |
| 4* | 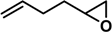 | 2a | 5 | 63/13 | 55/0 |
| 5 |  | 2b | 5 | 81/0 | 54/0 |
| 6 [44,45(b,c)] |  | 2a | 5 | 97/0 | 84/0 |
| 7 |  | 2b | 5 | 71/0 | 14/0 |
| 8 [45] | 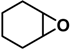 | 2a | 5 | 93/0 | 99/0 |
| 9 |  | 2b | 5 | 83/0 | 45/0 |
| 10 [46](d), 45(c), 42]* | 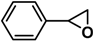 | 2a | 1 | 0/96 | 5/91 |
| 11 [41,47] |  | 2b | 6 | 53/31 | 55/25 |
| 12 [41] |  | 2a | 5 | 81/0 | 83/0 |
| 13 |  | 2b | 5 | 87/0 | 81/0 |
| 14 [41, 46] | 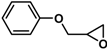 | 2a | 0.5 | 86/0 | 81/0 |
| 15 [41] |  | 2b | 0.5 | 87/0 | 52/0 |
| 16 | 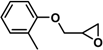 | 2a | 1 | 76/0 | 57/0 |
| 17 |  | 2b | 1 | 77/0 | 51/0 |
| 18 |  | 2a | 1 | 76/0 | 59/0 |
| 19 [48] |  | 2b | 1 | 85/0 | 82/0 |
Conclusions
Experimental
General
Sulfated zirconia synthesis
MCM-41 synthesis
Synthesis of sulfated zirconia supported on MCM-41
β-Aminoalcohol synthesis
Acknowledgments
References and Notes
- Mejri, I.; Younes, M. K.; Ghorbel, A. Comparative study of the textural and structural properties of the aerogel and xerogel sulphated zirconia. J Sol-Gel Sci. Techn. 2006, 40, 3–8. [Google Scholar] Pârvulescu, V.; Coman, S.; Grange, P.; Pârvulescu, V. I. Preparation and characterization of sulfated zirconia catalysts obtained via various procedures. Appl. Catal. A. 1999, 17, 27–43. [Google Scholar]
- Arata, K.; Matsuhashi, H.; Hino, M.; Nakamura, H. Synthesis of solid superacids and their activities for reactions of alkanes. Catal. Today 2003, 81, 17–30. [Google Scholar] [CrossRef]
- Chen, W. H.; Ko, H. H.; Sakthivel, A.; Huang, S. J.; Liu, S. H.; Lo, A. Y.; Tsai, T. C.; Liu, S. B. A solid-state NMR, FT-IR and TPD study on acid properties of sulfated and metal-promoted zirconia: Influence of promoter and sulfation treatment. Catal. Today 2006, 116, 111–120. [Google Scholar] [CrossRef]
- Li, X.; Nagaoka, K.; Lercher, J. A. Labile sulfates as key components in active sulfated zirconia for n-butane isomerization at low temperatures. J. Catal. 2004, 227, 130–137. [Google Scholar] [CrossRef]
- Marcus, R.; Diebold, U.; Gonzalez, R. D. The locus of sulfate sites on sulfated zirconia. Catal. Letts. 2003, 86, 151–156. [Google Scholar] [CrossRef]
- Biró, K.; Figueras, F.; Békássy, S. Acylation of B15C5 crown ether by acetic anhydride in the absence of solvent, on sulfated zirconias prepared in different conditions. Appl. Catal. A. 2002, 229, 235–243. [Google Scholar] [CrossRef]
- Deutsch, J.; Prescott, H. A.; Müller, D.; Kemnitz, E.; Lieskea, H. Acylation of naphthalenes and anthracene on sulfated zirconia. J. Catal. 2005, 231, 269–278. [Google Scholar] [CrossRef]
- Reddy, B. M.; Sreekanth, P. M.; Lakshmanan, P. Sulfated zirconia as an efficient catalyst for organic synthesis and transformation reactions. J. Mol. Catal. 2005, 237, 93–100. [Google Scholar] [CrossRef]
- Zhuang, Q.; Miller, J. M. One-pot sol-gel synthesis of sulfated ZrO2-SiO2 catalysts for alcohol dehydration. Can. J. Chem. 2001, 79(8), 1220–1223. [Google Scholar] [CrossRef]
- Escalona-Platero, E.; Penarroya-Mentruit, M. Acetylene polymerization on sulfated zirconia: detection of intermediates by infrared spectroscopy. Catal. Letts. 1997, 45(1,2), 59–63. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, L.; Lu, H.; Wang, R.; Lin, S.; Jiang, D.; Xiao, F. S. Sulfated zirconia supported in mesoporous materials. Appl. Catal. A. 2002, 237, 21–31. [Google Scholar] [CrossRef] Yurdakoc, M.; Akcay, M.; Tonbul, K.; Yurdakoc, K. Acidity of silica-alumina Catalysis by amine titration using Hammett indicators and FT-IR Study. Turk J. Chem. 1999, 23, 319–327. [Google Scholar] Morterra, C.; Garronet, E.; Bolis, V.; Fubini, B. An infrared spectroscopic characterization of the coordinative adsorption of carbon monoxide on TiO2. Spectrochim. Acta, Part A. 1987, 43, 1577–1581. [Google Scholar]
- Chen, C. L.; Cheng, S.; Lin, H. P.; Wong, S. T.; Mou, C. Y. Sulfated zirconia catalyst supported on MCM-41 mesoporous molecular sieve. Appl. Catal. A. 2001, 215, 21–30. [Google Scholar] [CrossRef]
- Wanga, W.; Wang, J. H.; Chena, C. L.; Xua, N. P.; Mou, C. Y. n-Pentane isomerization over promoted SZ/MCM-41 catalysts. Catal. Today 2004, 97, 307–313. [Google Scholar] [CrossRef]
- Breda, A.; Signoretto, M.; Ghedini, E.; Pinna, F.; Cruciani, G. Acylation of veratrole over promoted SZ/MCM-41 catalysts: Influence of metal promotion. Appl. Catal. A. 2006, 308, 216–222. [Google Scholar] [CrossRef]
- Scarpi, D.; Lo Galbo, F.; Occhiato, E. G.; Guarna, A. Enantioselective addition of diethylzinc to aldehydes using. 1,4-aminoalcohols as chiral ligands. Tetrahedron: Asymmetry 2004, 15, 1319–1324. [Google Scholar] [CrossRef]
- Mojtahedi, M. M.; Saidi, M. R.; Bolourtchian, M. Microwave-assisted Aminolysis of Epoxides Under Solvent-free Conditions Catalyzed by Montmorillonite Clay. J. Chem. Res. 1999, 128–129. [Google Scholar] [CrossRef]
- Sagawa, S.; Abe, H.; Hase, Y.; Inaba, T. Catalytic Asymmetric Aminolysis of 3,5,8-Trioxabicyclo[5.1.0]octane Providing an Optically Pure 2-Amino-1,3,4-butanetriol Equivalent. J. Org. Chem. 1999, 64, 4962–4965. [Google Scholar] [CrossRef]
- Van de Weghe, P.; Collin, J. Ring opening reactions of epoxides catalyzed by samarium iodides. Tetrahedron Lett. 1995, 36, 1649–1652. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Asao, N.; Meguro, M.; Tsukada, N.; Nemoto, H.; Sadayori, N.; Wilson, J. G.; Nakamura, H. Regio- and stereo-selective ring opening of epoxides with amide cuprate reagents. J. Chem. Soc., Chem. Commun. 1993, 1201–1203. [Google Scholar]
- Fu, X. L.; Wu, S. H. A regio-and stereoselective synthesis of β-amino alcohols. Synth. Commun. 1997, 27, 1677–1683. [Google Scholar] [CrossRef]
- Mlynarski, J.; Jankowska, J.; Rakiel, B. Direct asymmetric aldol-Tishchenko reaction of aliphatic ketones catalyzed by syn-aminoalcohol-YB (III) complexes. Chem. Commun. 2005, 38, 4854–4856. [Google Scholar] [CrossRef]
- Mirkhania, V.; Tangestaninejad, S.; Yadollahi, B.; Alipanah, L. Ammonium decatungstocerate (IV): an efficient catalyst for ring opening of epoxides with aromatic amines. Catal. Letts. 2005, 101, 93–97. [Google Scholar] [CrossRef]
- De, S. K.; Gibbs, R. A. Ruthenium(III) Chloride–Catalyzed Ring Opening of Epoxides with Aromatic Amines. Synth. Commun. 2005, 35, 2675–2680. [Google Scholar] [CrossRef]
- Raghavendra, S. N.; Goud, T. V.; Reddy, S. M.; Krishnaiah, P.; Venkateswarlu, Y. Zirconium (IV) Chloride Catalyzed Ring Opening of Epoxides with Aromatic Amines. Synth. Commun. 2004, 34, 727–734. [Google Scholar] [CrossRef]
- Kamal, A.; Arifuddin, M.; Rao, M. V. Enantioselective ring opening of epoxides with trimethylsilyl azide (TMSN3) in the presence of β-cyclodextrin: an efficient route to 1,2-azido alcohols. Tetrahedron: Asymmetry 1999, 10, 4261–4264. [Google Scholar] [CrossRef]
- Reddy, L. R.; Reddy, M. A.; Bhanumathi, N.; Rao, K. R. Cerium Chloride-Catalysed Cleavage of Epoxides with Aromatic Amines. Synthesis 2001, 831–832. [Google Scholar]
- Chakraborti, A. K.; Rudrawar, S.; Kondaskar, Atul. An efficient synthesis of 2-amino alcohols by silica gel catalysed opening of epoxide rings by amines. Org. Biomol. Chem. 2004, 1277–1280. [Google Scholar] [CrossRef]
- Xue, W. M.; Kung, M. C.; Kozlov, A. I.; Popp, K. E.; Kung, H. H. Catalytic aminolysis of epoxide by alumina prepared from amine-protected Al precursor. Catal. Today 2003, 85, 219–224. [Google Scholar] [CrossRef]
- Horváth, A.; Skoda-Földes, R.; Mahó, S.; Berente, Z.; Kollár, L. Facile ring opening of 2,3-epoxy-steroids with aromatic amines in ionic liquids. Steroids 2006, 71, 706–711. [Google Scholar] [CrossRef]
- Azizi, N.; Saidi, M. R. Highly Chemoselective Addition of Amines to Epoxides in Water. Org. Lett. 2005, 7, 3649–3651. [Google Scholar] [CrossRef]
- Azizi, N.; Saidi, M. R. LiClO4.3H2O promoted highly regioselective ring-opening of epoxides with thiols under neutral conditions. Cat. Commun. 2006, 7, 224–227. [Google Scholar] [CrossRef]
- Babu, K. S.; Raju, B. C.; Kumar, S. P.; Mallur, Shanta G.; Reddy, S. V.; Rao, J. M. Tungstophosphoric Acid (H3PW12O40)-Catalyzed Regioselective Ring Opening of Epoxides with Amines. Synth. Commun. 2005, 35, 879–885. [Google Scholar] [CrossRef]
- Azizi, N.; Saidi, M. R. Highly efficient ring opening reactions of epoxides with deactivated aromatic amines catalyzed by heteropoly acids in water. Tetrahedron 2007, 63, 888–891. [Google Scholar] [CrossRef]
- Rafiee, E.; Tangestaninejad, S.; Habibi, M. H.; Mirkhani, V. Potassium Dodecatungstocobaltate Trihydrate (K5CoW12O40.3H2O) as an Efficient Catalyst for Aminolysis of Epoxides. Synth. Commun. 2004, 34, 3673–3681. [Google Scholar] [CrossRef]
- Angeles-Beltrán, D.; Lomas-Romero, L.; Lara-Corona, V. H.; González-Zamora, E.; Negrón-Silva, G. Sulfated Zirconia-Catalyzed Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones (DHPMs) under Solventless Conditions: Competitive Multicomponent Biginelli vs. Hantzsch Reactions. Molecules 2006, 11, 731–738. [Google Scholar] [CrossRef]
- Negrón, G.; Ángeles, D.; Lomas, L.; Martínez, Á.; Ramírez, M.; Martínez, Roberto. An Efficient Synthesis Of 6,6-Dimethyl-2-(4-Nitrophenyl)-1-(R-Phenyl)-4,5,6,7-Tetrahydro-1h-4-Indolones using a solid sulfated zirconia as catalyst. Heterocycles 2004, 63, 367–371. [Google Scholar] [CrossRef]
- Negrón, G. E.; Palacios, L. N.; Angeles, D.; Lomas, L.; Gaviño, R. A Mild and Efficient Method for the Chemoselec tive Synthesis of Acylals from Aromatic Aldehydes and their Deprotections Catalyzed by Sulfated Zirconia. J. Braz. Chem. Soc. 2005, 16, 490–494. [Google Scholar] [CrossRef]
- Klose, B. S.; Jentoft, R. E.; Hahn, A.; Ressler, T.; Kröhnert, J.; Wrabetz, S.; Yang, X.; Jentoft, F. C. Mechanical stress induced activity and phase composition changes in sulfated zirconia catalysts. J. Catal. 2003, 217, 487–490. [Google Scholar]
- Webb, P. A.; Orr, C. Analytical methods in fine particle technology; Micromeritics Instrument Corporation: Norcross, GA, USA, 1997. [Google Scholar]
- Du, Y.; Sun, Y.; Di, Y.; Zhao, L.; Liu, S.; Xiao, F. S. Ordered mesoporous sulfated silica-zirconia materials with high zirconium contents in the structure. J. Porous Mater. 2006, 13, 163–171. [Google Scholar] [CrossRef] Li, M.; Feng, Z.; Xiong, G.; Ying, P.; Xin, Q.; Li, Can. J. Phys. Chem. B. 2001, 105, 8107–8111. [CrossRef]
- Cepanec, I.; Litvić, M.; Mikuldaš, H.; Bartolinčića, A.; Vinkovićb, V. Calcium trifluoromethanesulfonate-catalysed aminolysis of epoxides. Tetrahedron 2003, 59, 2435–2439. [Google Scholar] [CrossRef]
- De, S. K.; Gibbs, R. A. Ruthenium(III) Chloride–Catalyzed Ring Opening of Epoxides with Aromatic Amines. Synth. Commun. 2005, 35, 2675–2680. [Google Scholar] [CrossRef]
- Westermann, J.; Schneider, M.; Platzek, J.; Petrov, O. Practical Synthesis of a Heterocyclic Immunosuppressive Vitamin D Analogue. Org. Process Res. Dev. 2007, 11, 200–205. [Google Scholar] [CrossRef]
- Kondaskar, C.; Kondaskar, A. ZrCl4 as a new and efficient catalyst for the opening of epoxide rings by amines. Tetrahedron Lett. 2003, 44, 8315–8319. [Google Scholar] [CrossRef]
- Fujiwara, M.; Imada, M.; Bab, A.; Matsuda, H. Tetraphenylstibonium triflate as a regio- and chemoselective catalyst in the reaction of oxiranes with amines. Tetrahedron Lett. 1989, 30, 739–742. [Google Scholar] [CrossRef] Alam, M. M.; Varala, R.; Enugala, R.; Adapa, S. R. Synthesis of -Amino Alcohols by Regioselective Ring Opening of Epoxides with Aromatic Amines Catalyzed by Tin (II) Chloride. Lett. Org. Chem. 2006, 3, 187–190. [Google Scholar] Swamy, N. R.; Goud, T. V.; Reddy, S. M.; Krishnaiah, P.; Venkateswarlu, Y. Zirconium (IV) Chloride Catalyzed Ring Opening of Epoxides with Aromatic Amines. Synth. Commun. 2004, 34, 727–734. [Google Scholar]
- Gupta, R.; Paul, S.; Gupta, A. K.; Kachroo, P. L.; Dandia, A. Opening of oxirane ring with N-nucleophiles under microwave irradiation. Ind. J. Chem. Sect. B. 1997, 36B, 281–283. [Google Scholar] Yadav, J. S.; Reddy, B. V. S.; Basak, A. K.; Narsaiah, A. V. [Bmim]BF4 ionic liquid. A novel reaction medium for the synthesis of β-amino alcohols. Tetrahedron Lett. 2003, 44, 1047–1050. [Google Scholar] Kumar, S. R.; Leelavathi, P. Cadmium chloride-catalyzed regioselective opening of oxiranes with aromatic amines–Animproved protocol for the synthesis of 2-amino alcohols. Can. J. Chem. 2007, 85, 37–41. [Google Scholar] Chakraborti, A. K.; Rudrawar, S.; Kondaskar, A. Lithium bromide, an inexpensive and efficient catalyst for opening of epoxide rings by amines at room temperature under solvent-free condition. Eur. J. Org. Chem. 2004, 17, 3597–3600. [Google Scholar]
- Chakraborti, A. K.; Kondaskar, A.; Rudrawar, S. Scope and limitations of montmorillonite K 10 catalysed opening of epoxide rings by amines. Tetrahedron 2004, 60, 9085–9091. [Google Scholar] [CrossRef]
- Greenwood, D. T.; Mallion, K. B.; Todd, A. H.; Turner, R. W. 2-Aryloxymethyl-2,3,5,6-tetrahydro-1,4-oxazines, a new class of antidepressants. J. Med. Chem. 1975, 18(6), 573–577. [Google Scholar]
- Matsuhashi, H.; Tanaka, M.; Nakamura, H.; Arata, K. Formation of acid sites in ordered pores of FSM-16 by modification with sulfated zirconia. Appl. Catal. A. 2001, 208, 1–5. [Google Scholar] [CrossRef]
- Luo, Y.; Lu, G. Z.; Guo, Y. L.; Wang, Y. S. Study on Ti-MCM-41 zeolites prepared with inorganic Ti sources: synthesis, characterization and catalysis. Catal. Commun. 2002, 3, 129–134. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Negrón-Silva, G.; Hernández-Reyes, C.X.; Angeles-Beltrán, D.; Lomas-Romero, L.; González-Zamora, E.; Méndez-Vivar, J. Comparative Study of Regioselective Synthesis of β-Aminoalcohols under Solventless Conditions Catalyzed by Sulfated Zirconia and SZ/MCM-41. Molecules 2007, 12, 2515-2532. https://doi.org/10.3390/12112515
Negrón-Silva G, Hernández-Reyes CX, Angeles-Beltrán D, Lomas-Romero L, González-Zamora E, Méndez-Vivar J. Comparative Study of Regioselective Synthesis of β-Aminoalcohols under Solventless Conditions Catalyzed by Sulfated Zirconia and SZ/MCM-41. Molecules. 2007; 12(11):2515-2532. https://doi.org/10.3390/12112515
Chicago/Turabian StyleNegrón-Silva, Guillermo, C. Xochitl Hernández-Reyes, Deyanira Angeles-Beltrán, Leticia Lomas-Romero, Eduardo González-Zamora, and Juan Méndez-Vivar. 2007. "Comparative Study of Regioselective Synthesis of β-Aminoalcohols under Solventless Conditions Catalyzed by Sulfated Zirconia and SZ/MCM-41" Molecules 12, no. 11: 2515-2532. https://doi.org/10.3390/12112515





