An Efficient Synthesis and Reactions of Novel Indol-ylpyridazinone Derivatives with Expected Biological Activity
Abstract
:Introduction
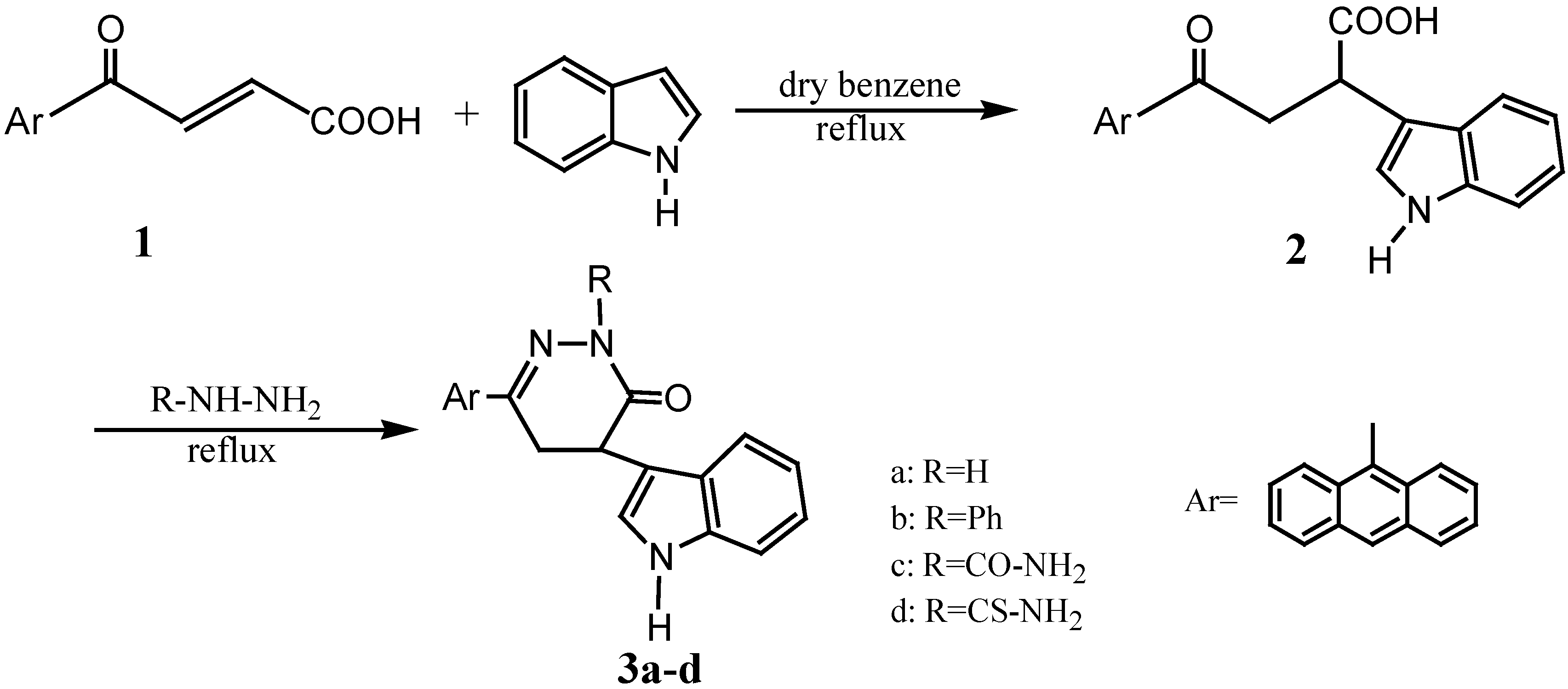
Results and Discussion
| Compound No | M.P. (°C) Cryst. solvent | Mol. Formula Mol. weight | M.W. from MS | Analysis % Calc./ Found | |||
|---|---|---|---|---|---|---|---|
| C | H | N | S | ||||
| 1 | 215-217 Ethanol | C18H12O3 276.29 | 276.08 | 78.25 78.20 | 4.38 4.35 | ----- | ----- |

| Compound No | M.P. (°C) Cryst. solvent | Mol. Formula Mol. Weight | M.W. from MS | Analysis % Calc./ Found | |||
|---|---|---|---|---|---|---|---|
| C | H | N | S | ||||
| 4a | 200 Benzene | C26H16ClN3 405.88 | 405.10 | 76.94 76.99 | 3.97 3.94 | 10.35 10.33 | ----- |
| Compound No | M.P. (°C) Cryst. solvent | Mol. Formula Mol. Weight | M.W. from MS | Analysis % Calc./ Found | |||
|---|---|---|---|---|---|---|---|
| C | H | N | S | ||||
| 8 | 165 Benzene | C18H12N2O 272.30 | 272.30 | 79.39 79.42 | 4.44 4.40 | 10.29 10.32 | ----- |
| Compound No | M.P. (°C) Cryst. solvent | Mol. Formula Mol. Weight | M.W. from MS | Analysis % Calc./ Found | |||
|---|---|---|---|---|---|---|---|
| C | H | N | S | ||||
| 13a | 154 Benzene | C32H23N3O3S 526.61 | 529.15 | 72.57 72.50 | 4.38 4.41 | 7.93 7.90 | 6.05 6.00 |
Biological Screening
| Compound No | Gram positive bacteria | Gram negative bacteria | ||
|---|---|---|---|---|
| Staph. aureus | Staph. epidermis | E. coli | Pr. vulgaris | |
| 1 | +++ | +++ | + | ++ |
Conclusions
Acknowledgements
Experimental
General
4-Anthracen-9-yl-4-oxo-but-2-enoic acid (1)
4-Anthracen-9-yl-2-(1H-indol-3-yl)-4-oxo-butyric acid (2).
General method for the preparation of 3a-d:
6-Anthracen-9-yl-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (3a).
6-Anthracen-9-yl-4-(1H-indol-3-yl)-2-phenyl-4,5-dihydro-2H-pyridazin-3-one (3b).
3-Anthracen-9-yl-5-(1H-indol-3-yl)-6-oxo-5,6-dihydro-4H-pyridazine-1-carboxylic acid amide (3c).
3-Anthracen-9-yl-5-(1H-indol-3-yl)-6-oxo-5,6-dihydro-4H-pyridazine-1-carbothioic acid amide (3d).
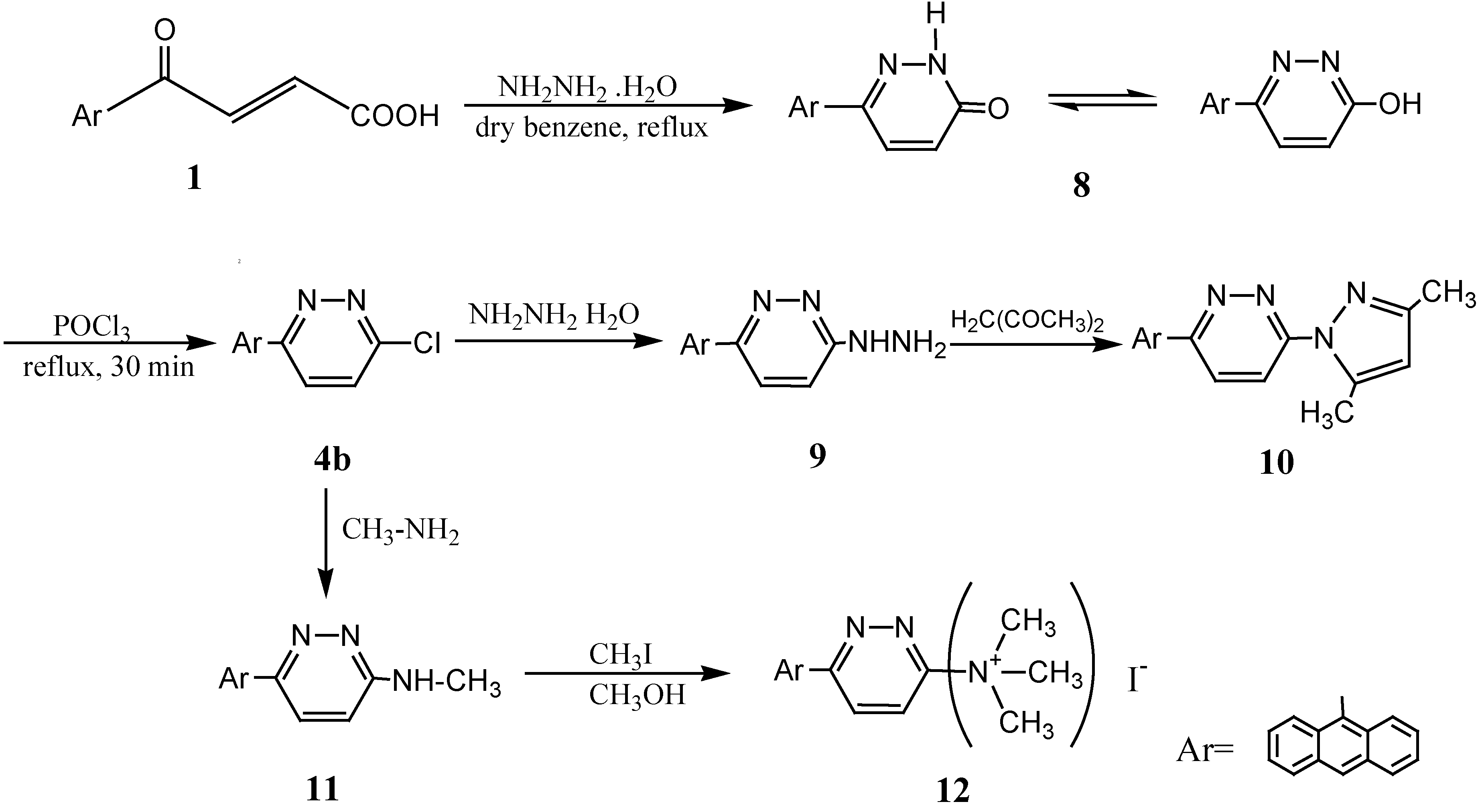
3-(6-Anthracen-9-yl-3-chloropyridazin-4-yl)-1H-indole (4a).
3-Anthracen-9-yl-6-chloropyridazine (4b).
General procedure for the reaction of chloropyridazine 4a with carbohydrate hydrazones.
(2R,3S,4S)-5-{[6-Anthracen-9-yl-4-(1H-indol-3-yl)pyridazin-3-yl]hydrazono}pentane-1,2,3,4,-tetraol (5a).
(2R,3R,4R,5S)-6-{[6-Anthracen-9-yl-4-(1H-indol-3-yl)pyridazin-3-yl]-hydrazono}-hexane-1,2,3,4,5-pentaol (5b).
(2R,3S,4R,5S)-6-{[6-Anthracen-9-yl-4-(1H-indol-3-yl)pyridazin-3-yl]hydrazono}-hexane-1,2,3,4,5-pentaol (5c).
6-{[6-Anthracen-9-yl-4-(1H-indol-3-yl)pyridazin-3-yl]hydrazono}-3-(3,4,5-trihydroxy-6-hydroxy-methyltetrahydropyran-2-yloxy)-hexane-1,2,4,5-tetraol (5d).
General reaction of chloropyridazine 4a with aliphatic or aromatic amines
[6-Anthracen-9-yl-4-(1H-indol-3-yl)-pyridazin-3-yl]-methylamine (6a).
[6-Anthracen-9-yl-4-(1H-indol-3-yl)-pyridazin-3-yl]-ethylamine (6b).
[6-Anthracen-9-yl-4-(1H-indol-3-yl)-pyridazin-3-yl]-phenylamine (6c).
4-[6-Anthracen-9-yl-4-(1H-indol-3-yl)-pyridazin-3-ylamino] benzenesulfonic acid (6d).
[6-Anthracen-9-yl-4-(1H-indol-3-yl)-pyridazin-3-yl]-naphthalen-1-yl amine (6e).
[6-Anthracen-9-yl-4-(1H-indol-3-yl)-pyridazin-3-yl] diphenylamine (6f).
2-Anthracen-9-yl-4-(1H-indol-3-yl)-1,9-a,10-triaza-anthracen-9-one (7).
6-Anthracen-9-yl-2H-pyridazin-3-one (8).
6-Anthracen-9-yl-pyridazin-3-yl) hydrazine (9).
3-Anthracen-9-yl-6-(3,5-dimethylpyrazol-1-yl)pyridazine (10).
6-Anthracen-9-yl-3-methylamino-pyridazine (11).
(6-Anthracen-9-yl-pyridazin-3-yl)trimethylammonium iodide (12).
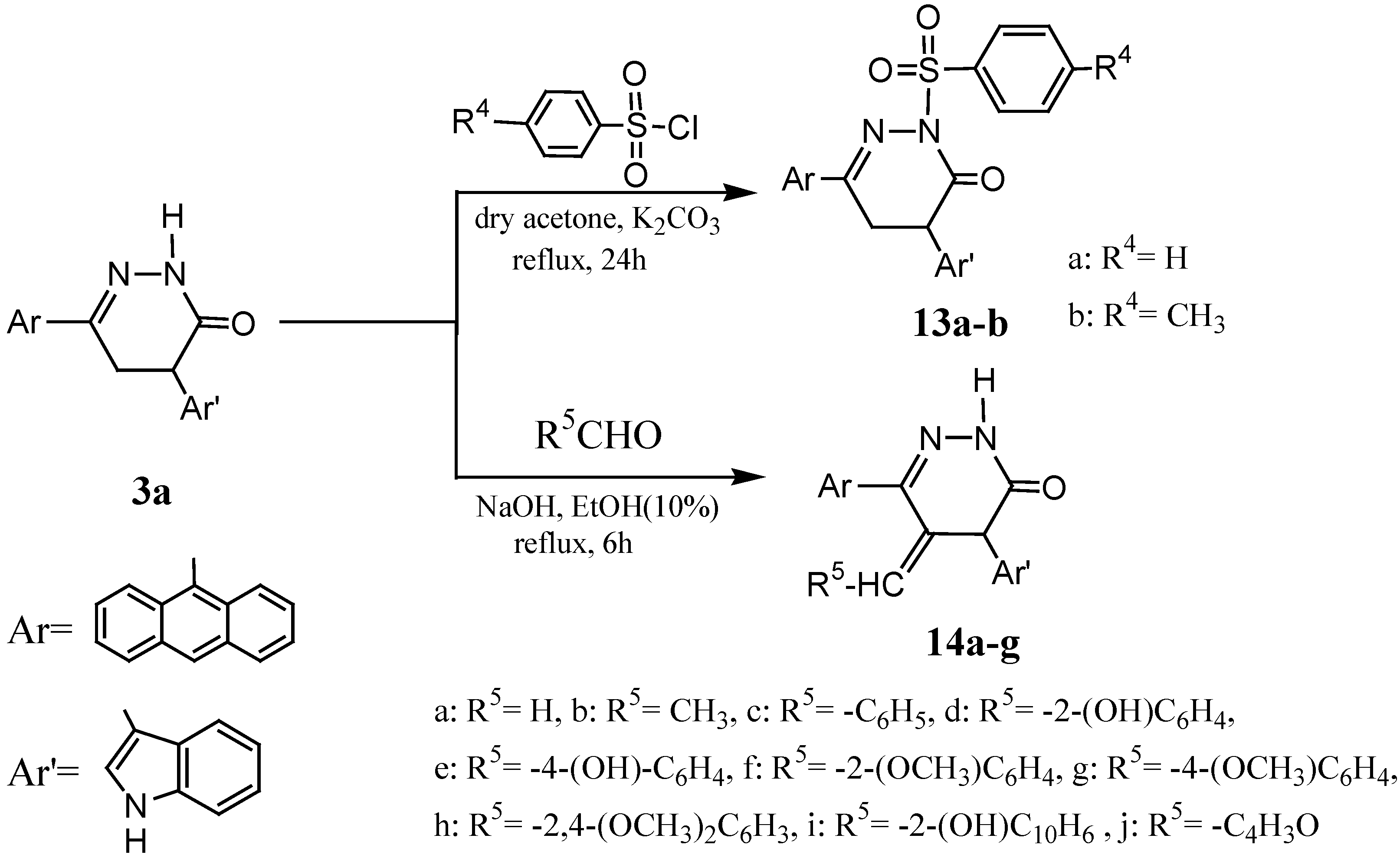
6-Anthracen-9-yl-2-benzenesulfonyl-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (13a).
6-Anthracen-9-yl-4-(1H-indol-3-yl)-2-(toluene-4-sulfonyl)-4,5-dihydro-2H-pyridazin- 3-one (13b).
General reaction of pyridazinone 3a with some aliphatic or aromatic aldehydes
6-Anthracen-9-yl-4-(1H-indol-3-yl)5-methylene-4,5-dihydro-2H-pyridazin-3-one (14a).
6-Anthracen-9-yl-5-ethylidene-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (14b).
6-Anthracen-9-yl-5-benzylidene-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (14c).
6-Anthracen-9-yl-5-(2-hydroxybenzylidene)-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (14d).
6-Anthracen-9-yl-5-(4-hydroxybenzylidene)-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (14e).
6-Anthracen-9-yl-4-(1H-indol-3-yl)-5-(2-methoxybenzylidene)-4,5-dihydro-2H-pyridazin-3-one (14f).
6-Anthracen-9-yl-4-(1H-indol-3-yl)-5-(4-methoxybenzylidene)-4,5-dihydro-2H-pyridazin-3-one (14g).
6-Anthracen-9-yl-5-(2,4-dimethoxybenzylidene)-4-(1H-indol-3-yl-4,5-dihydro-2H-pyridazin-3-one (14h).
6-Anthracen-9-yl-5-(2-hydroxynaphthalen-1-yl-methylene)-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (14i).
6-Anthracen-9-yl-5-furan-2-yl-methylene-4-(1H-indol-3-yl)-4,5-dihydro-2H-pyridazin-3-one (14j).
References
- Sayed, G.H.; Sayed, M.A.; Mahmoud, M.R.; Shaaban, S.S. Synthesis and Reactions of New Pyidazinone Derivatives of Expected Antimicrobial Activities. Egypt. J. Chem. 2002, 45, 767–776. [Google Scholar]
- Katrusiak, A.; Katrusiak, A.; Baloniak, S. Reactivity of 6-Chloro-4- and 5-Hydrazino-2- Phenyl-3(2H)-Pyridazinones with Vilsmeier Reagent. Tetrahedron 1994, 50, 12933–12940. [Google Scholar] [CrossRef]
- Okcelik, B.; Unlu, S.; Banoglu, E.; Kupeli, E.; Yesilada, E.; Sahin, M.F. Investigation of New Pyridazinone Derivatives for the Synthesis of Potent Analgesic and Anti-Inflammatory Compounds with Cyclooxygenase Inhibitory Activity. Arch. Pharm. Pharm. Med. Chem. 2003, 336, 406–412. [Google Scholar]
- Dogruer, D.S.; Sahin, M.F.; Kupeli, E.; Yesilada, E. Synthesis and Analgesic and Anti-Inflammatory Activity of New Pyridazinones. Turk. J. Chem. 2003, 27, 727–738. [Google Scholar]
- Frolov, E.B.; Lakner, F.J.; Khvat, A.V.; Ivachtchenko, A.V. An Efficient Synthesis of Novel 1,3-oxazolo [4,5-d] Pyridazinones. Tetrahedron Lett. 2004, 45, 4693–4696. [Google Scholar]
- Banoglu, E.; Akoglu, C.; Unlu, S.; Kupeli, E.; Yesilada, E.; Sahin, M.F. Amide Derivatives of [6-(5-Methyl-3-phenylpyrazole-1-yl)-3(2H)-Pyridazinone-2-yl]acetic Acids as Potential Analgesic anti-Inflammatory Compounds. Arch. Pharm. Pharm. Med. Chem. 2004, 337, 7–14. [Google Scholar] [CrossRef]
- Gokce, M.; Dogruer, D.; Sahin, F. Synthesis and Antinociceptive Activity of 6-Substituted-3-pyridazinone Derivatives. Il Farmaco 2001, 56, 233–237. [Google Scholar] [CrossRef]
- Cao, S.; Qian, X.; Song, G.; Chai, B.; Jiang, Z. Synthesis and Antifeedant Activity of New Oxadiazolyl 3(2H)-pyridazinones. J. Agric. Food Chem. 2003, 51, 152–155. [Google Scholar] [CrossRef]
- Piaz, V.D.; Ciciani, G.; Giovannoni, M.P. 5-Acetyl-2-Methyl-4-Nitro-6-Phenyl-3(2H)-Pyriazinone: Versatile Precursor to Hetero-Condensed Pyridazinones. Synthesis 1994, 669–671. [Google Scholar]
- Ogretir, C.; Yarligan, S.; Demirayak, S. Spectroscopic Determination of Acid Dissociation Constants of Some Biologically Active 6-Phenyl-4,5-Dihydro-3(2H)-Pyridazinone Derivatives. J. Chem. Eng. Data 2002, 47, 1396–1400. [Google Scholar] [CrossRef]
- Barbaro, R.; Betti, L.; Botta, M.; Corelli, F.; Giannaccini, G.; Maccari, L.; Manetti, F.; Strappaghetti, G.; Corsano, S. Synthesis Biological Evaluation and Pharmacophore Generation of New Pyridazinone Derivatives with Affinity Toward α1- and α2- Adrenoceptors. J. Med. Chem. 2001, 44, 2118–2132. [Google Scholar]
- Sircar, I. Synthesis of New 1,2,4-Triazolo[4,3-b]pyridazines and Related Compound. J. Hetero-cyclic Chem. 1985, 22, 1045–1048. [Google Scholar]
- Coelho, A.; Sotelo, E.; Fraiz, N.; Yanez, M.; Laguna, R.; Cano, E.; Ravina, E. Pyridazines. Part 36: Synthesis and Antiplatelet Activity of 5-Substituted-6-Phenyl-3(2H)- Pyridazinones. Bioorg. Med. Chem. Lett. 2004, 14, 321–324. [Google Scholar] [CrossRef]
- Sotelo, E.; Centeno, N.B.; Rodrigo, J.; Ravina, E. Pyridazine Derivatives. Part 27: A joint Theoretical and Experimental approach to the Synthesisi of 6-Phenyl-4,5-disubstituted-3(2H)Pyridazinones. Tetrahedron Lett. 2002, 58, 2389–2395. [Google Scholar] [CrossRef]
- Sotelo, E.; Fraiz, N.; Yanez, M.; Terrades, V.; Laguna, R.; Cano, E.; Ravina, E. Pyridazines. Part XXIX: Synthesis and Platelet Aggregation Inhibition Activity of 5-Substituted-6-Phenyl-3(2H)-Pyridazinones Novel Aspects of their Biological Action. Bioorg. Med. Chem. 2002, 10, 2873–2882. [Google Scholar] [CrossRef]
- Malinka, W.; Redzicka, A.; Lozach, O. New Derivatives of Pyrrolo[3,4-d]Pyridazinone and their Anticancer Effects. Il Farmaco 2004, 59, 457–462. [Google Scholar] [CrossRef]
- Youssef, A.S.; Marzouk, M.I.; Madkour, H.M.F.; El-Soll, A.M.A.; El-Hashash, M.A. Synthesis of some heterocyclic systems of anticipated biological activities via 6-aryl-4-pyrazol-1-yl-pyridazin-3-one. Can. J. Chem. 2005, 83, 251–259. [Google Scholar]
- Sotelo, E.; Pita, B.; Ravina, E. Pyridazines. Part 22:1 Highly Efficient Synthesis of Pharmacologically Useful 4-Cyano-6-Phenyl-5-Substituted-3(2H)-Pyridazinones. Tetrahedron Lett. 2000, 41, 2863–2866. [Google Scholar] [CrossRef]
- Sotelo, E.; Coelho, A.; Ravina, E. Pyridazine Derivatives 321): Stille-Based Approaches in the Synthesis of 5-Substituted-6-Phenyl-3(2H)-Pyridazinones. Chem. Pharm. Bull. 2003, 51, 427–430. [Google Scholar] [CrossRef]
- Kassab, R.R. Simple Synthesis and Reactions of Some New Pyridazinono Derivatives and their Antimicrobial Activity. Egypt. J. Chem. 2002, 45, 1055–1073. [Google Scholar]
- Toshiki, S.; Hiroshi, Y. Jpn. Kokai Tokkyo Koho JP 01,100,157, 1989. [Chem Abstr. 1989, 111, 153626 u].
- Masanori, S.; Toshiharu, O.; Hiroko, T. Jpn. Kokai Tokkyo Koho JP 01,31,763, 1989. [Chem Abstr. 1989, 111, 15623 r].
- Tsutomu, F.; Yoichiro, N.; Tomokayu, G.; Amid Kazuimasa, Y. Jpn. Kokai Tokkyo Koho JP 61,148,160, 1987. [Chem Abstr. 1987, 106, 50037 v].
- John, B.E.; James, C.N.; Bayer, A.C. Eur. Pat. Appl. EP 409,027, 1991. [Chem. Abstr. 1991, 114, 228729 c].
- Kamat, G.A.; Joshi, G.R.; Gadaginamath, S.G. Proc. Indian Acad. Sci. Chem. Sci. 1993, 105, 189.
- Halasz, B.D.; Monsieurs, K.; Elias, O.; Karolyhazy, L.; Tapolcsanyi, P.; Maes, B.U.; Riedl, Z.; Hajos, G.; Dommisse, R.A.; Lemiere, G.L.; Kosmrlj, J.; Matyus, P. Synthesis of 5H-Pyridazino[4,5-b]Indoles and their benzofurane analogues utilizing an intramolecular Heck-Type Reaction. Tetrahedron. 2004, 60, 2283–2291. [Google Scholar] [CrossRef]
- Sayed, G.H.; El-Kady, M.Y.; Abd Elhalim, M.S. Synthesis and Reactions of Some α–aryl-(4-bromobenzoyl)propionic acids. Indian J. Chem. 1981, 20, 845–848. [Google Scholar]
- Sayed, G.H.; Hamed, A.A.; Meligi, G.A.; Boraie, W.E.; Shafik, M. The Use of 4-(3,4- Dichlorophenyl)-4-Oxo-2-(4-Antipyrinyl)-Butonoic Acid in the Preparation of Some New Heterocyclic Compounds With Expected Biological Activity. Molecules 2003, 8, 322–332. [Google Scholar] [CrossRef]
- Toth, G.; Molnar, S.; Tamas, T.; Borbely, I. An Efficient Synthesis of 4,5-Dihydro-3(2H)- Pyridazinone Derivative. Synth. Commun. 1997, 27, 3513–3524. [Google Scholar]
- Sayed, G.H.; Sayed, M.A.; Shaaban, S.S.; Mahmoud, M.R. Synthesis and Reactions of 4-(p-Bromophenyl)-4-Oxo-2-(4-Antipyrinyl)Butanoic Acid and Some Unexpected Products. Egypt. J. Chem. 2000, 43, 17–29. [Google Scholar]
- Coates, W.J.; McKillop, A. One Pot Preparation of 6-Substituted 3(2H)-Pyridazinones from Ketones. Synthesis 1993, 334–342. [Google Scholar] [CrossRef]
- Kassab, R.R.; Sayed, G.H.; Radwan, A.M.; Abd El-Azzez, N. Some reactions with (biphenyl)-4-(5-oxo-1,3-diphenyl-2-pyrazolin-4-yl)-4,5-dihydropyridazin-3-(2H)ones. Rev. Roum. Chim. 2001, 46, 649–655. [Google Scholar]
- Jaihne, H.; Sayed, A.; Zaher, H.A.; Sherif, O. Reaction of 3-Chloro- and 3-Hydrazino-6-(p- tolyl)pyridazines. Indian J. Chem. 1977, 250–251. [Google Scholar]
- El-Hashash, M.A.; Amine, M.S.; Soliman, F.M.; Morsi, M.A. Behavior of aroylacyclic acids toward hydrazine hydrate and some on the cyclized products. J.Serb. Chem. Soc. 1992, 57, 563–569. [Google Scholar]
- Chung, K.T.; Chen, S.C.; Wong, T.Y.; Wei, C.I. Effects of Benzidine and Benzidine Analogues on Growth of Bacteria Including Azotobacter Vinelandii. Environ. Toxicol. Chem. 1998, 17, 271–275. [Google Scholar] [CrossRef]
- Sample Availability: Available from the author.
© 2007 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Abubshait, S.A. An Efficient Synthesis and Reactions of Novel Indol-ylpyridazinone Derivatives with Expected Biological Activity. Molecules 2007, 12, 25-42. https://doi.org/10.3390/12010025
Abubshait SA. An Efficient Synthesis and Reactions of Novel Indol-ylpyridazinone Derivatives with Expected Biological Activity. Molecules. 2007; 12(1):25-42. https://doi.org/10.3390/12010025
Chicago/Turabian StyleAbubshait, Samar A. 2007. "An Efficient Synthesis and Reactions of Novel Indol-ylpyridazinone Derivatives with Expected Biological Activity" Molecules 12, no. 1: 25-42. https://doi.org/10.3390/12010025




