Experimental Section
General
All melting points were determined on a Melt-Temp R apparatus equipped with a digital thermometer and are uncorrected. The combustion analysis was performed on an Elemental Exeter Analytical CE 440 Apparatus. The IR spectra were measured as potassium bromide pellets on a Digilab Scimitar Series spectrophotometer; the wave numbers are given in cm-1. The 1H-NMR spectra were recorded in DMSO-d6 solutions on Brucker ARX-300 spectrometer (1H: 300 MHz) at ambient temperature. Chemical shifts were recorded as δ values in parts per millions (ppm) and were indirectly referenced to tetramethylsilane via the residual solvent signal (2.49 for 1H). All chemical reagents were obtained from the Aldrich Chemical Company.
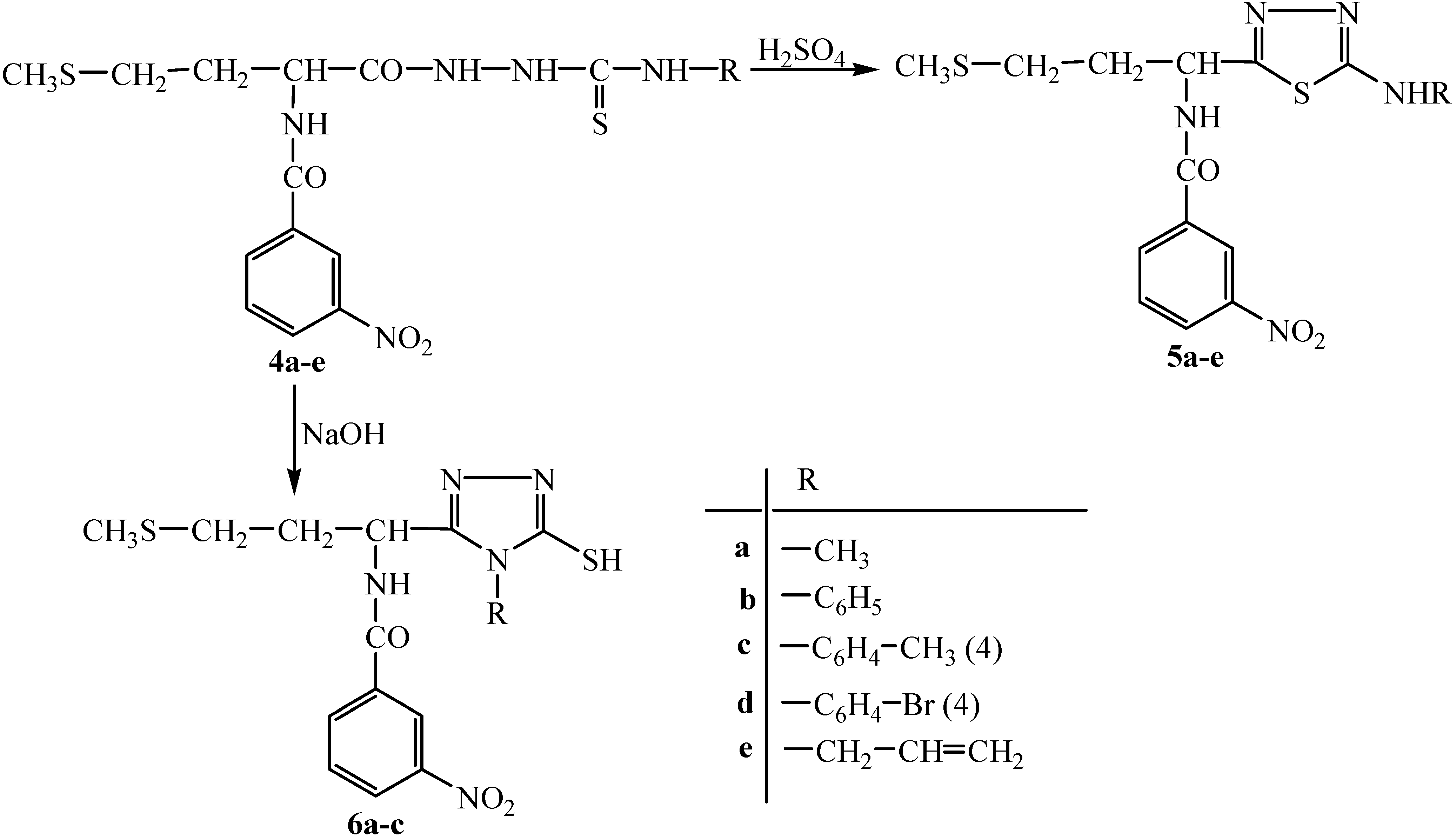
Synthesis of 2-(3-nitrophenyl)-4-(2-methylthioethyl)-Δ2-5-oxazolinone (2).
N-(3-Nitrobenzoyl)-D,L-methionine (5.6 g, 0.018 mol) was dissolved in acetic anhydride (15 mL) and heated at 65-70 ºC for half an hour. After cooling the solution was added under stirring to a mixture of petroleum ether (50 mL) and dried ethyl ether (10 mL). The reaction mixture was stirred for 30 minutes, the ethereal layer was removed and the oily product obtained was washed with petroleum ether. The crude product was dissolved in dioxane and crystallized from anhydrous ethyl ether – anhydrous petroleum ether (1:1). The solid compound obtained was dried under vacuum at 40-45 ºC. Yield 76.20 %; m.p. 114-115 ºC; Anal. Calc. for C12H12N2O4S (280.05): 51.42% C, 4.28% H, 10.00% N, 11.42% S; found 51.44% C, 4.01% H, 9.81% N, 11.26% S; IR (ν, cm-1): 725 (CH3S), 819 (aromatic CH), 1350 (symmetric vibrations of NO2), 1433 (-C=N), 1529 (asymmetric vibrations of NO2), 1707 (CO). 1H-NMR δ: 2.09 (s, 3H, CH3,), 2.52 (m, 2H, CH2), 2.65 (m, 2H, CH2) 4.50 (t, 1H, CH), 7.80 (s, 1H, ArH), 8.30 (d, 1H, ArH), 8.40 (d, 1H, ArH), 8.70 (s, 1H, ArH).
N-(3-nitrobenzoyl)-D,L-methionyl-hydrazide (3).
The 2-(3-nitrophenyl)-4-(2-methylthioethyl)-Δ2-5-oxazolinone (2, 1 g, 0.035 mol) was dissolved in dioxane (10 mL) and aqueous hydrazine hydrate solution (98%, 0.172 mL, 0.0035 mol) was added. The reaction mixture was heated at 65-70 ºC for one hour. After cooling the solvent was removed and the obtained oily product was dissolved in absolute ethanol and precipitated with water. The solid compound was dried under vacuum at 40-45 ºC. The crude product was crystallized from ethanol-water. Yield 54.78 %; m.p. 102-104 ºC; Anal. Calc. for C12H16N4O4S (327.11): 46.15% C, 5.12% H, 17.94% N, 11.42% S; found 46.02% C, 4.98% H, 17.89% N, 11.26% S; IR (ν, cm-1): 716 (CH3S); 818 (aromatic CH); 1352 (symmetric vibrations of NO2); 1527 (asymmetric vibrations of NO2); 1638 (CO); 2916, 3103, 3368 (NH); 1H-NMR δ: 2.10 (s, 3H, CH3); 2.50 (m, 4H, CH2); 4.50 (t, 1H, CH); 7.80 (t, 1H, Ar); 8.34 (d, 1H, Ar); 8.42 (d, 1H, Ar); 8.74 (s, 1H, Ar); 9.05 (d, 1H, NH); 10.80 (s, 1H, NH); 11.20 (s, 2H, NH2).
General procedure for the synthesis of 1,4-disubstituted thiosemicarbazides 4a-e.
N-(3-nitrobenzoyl)-D,L-methionylhydrazide (3, 0.78 g, 0.0025 mol) was dissolved in methanol (10 mL) and a solution of the appropriate isothiocyanate (0.0025 mol) in methanol (10 mL) was added. The reaction mixture was heated at 70-80 ºC for two hours. After cooling the solvent was evaporated under reduced pressure and the solid was dried under vacuum at room temperature. The crude product was purified by crystallization from ethanol.
1-[N-(3-nitrobenzoyl)-D,L-methionyl]-4-methyl-thiosemicarbazide (4a). Yield 58.33 %; m.p. 121-122 ºC; Anal. Calc. for C14H19N5O4S2 (385.09): 43.63% C, 4.93% H, 18.18% N, 16.62% S; found 43.59% C, 4.75% H, 18.03% N, 16.41% S; IR (ν, cm-1): 720 (CH3S); 819 (aromatic CH); 1173 (C=S); 1351 (symmetric vibrations of NO2); 1528 (asymmetric vibrations of NO2); 1643 (CO); 3302-3367 (NH-CO); 1H-NMR δ: 2.11 (s, 3H, CH3); 2.51 (d, 3H, CH3 ); 2.54 (m, 4H, CH2); 4.50 (t, 1H, CH); 7.80 (t, 1H, Ar); 8.30 (d, 1H, Ar); 8.40 (d, 1H, Ar); 8.70 (s, 1H, Ar); 9.07 (s, 1H, NH); 9.80 (m, 1H, NH); 10.10 (s, 1H, NH).
1-[N-(3-nitrobenzoyl)-D,L-methionyl]-4-phenyl-thiosemicarbazide (4b). Yield 68 %; m.p. 110-111 ºC; Anal. Calc. for C19H21N5O4S2 (447.10): 51.00% C, 4.69% H, 15.65% N, 14.31% S; found 49.72% C, 4.48% H, 15.37% N, 14.25% S; IR (ν, cm-1): 721 (CH3S); 823 (aromatic CH); 1173 (C=S); 1349 (symmetric vibrations of NO2); 1532 (asymmetric vibrations of NO2); 1643 (CO); 3181- 3301 (NH-CO); 1H-NMR δ: 2.09 (s, 3H, CH3); 2.50 (m, 4H, CH2); 4.50 (t, 1H, CH); 7.19 (t, 1H, Ar); 7.29 (t, 2H, Ar); 7.38 (d, 2H, Ar); 7.81 (t, 1H, Ar); 8.41 (d, 1H, Ar); 8.47 (d, 1H, Ar); 8.70 (s, 1H, Ar); 9.07 (s, 1H, NH); 9.81 (s, 1H, NH); 10.10 (s, 1H, NH).
1-[N-(3-nitrobenzoyl)-D,L-methionyl]-4-(4-methylphenyl)-thiosemicarbazide (4c). Yield 78.35 %; m.p. 112-113 ºC; Anal. Calc. for C20H23N5O4S2 (461.12): 52.06% C, 4.98% H, 15.18% N, 13.88% S; found 51.92% C, 4.75% H, 15.01% N, 13.58% S; IR (ν, cm-1): 721 (CH3S); 819 (aromatic CH); 1173 (C=S); 1350 (symmetric vibrations of NO2); 1528 (asymmetric vibrations of NO2); 1644 (CO); 3301-3366 (NH-CO); 1H-NMR δ: 2.11 (s, 3H, CH3); 2.43 (s, 3H, CH3); 2.50 (m, 4H, CH2); 4.50 (t, 1H, CH); 7.80 (t, 1H, Ar); 8.12 (d, 2H, Ar); 8.32 (d, 2H, Ar); 8.39 (d, 1H, Ar); 8.41 (d, 1H, Ar); 8.74 (s, 1H, Ar); 9.07 (d, 1H, NH); 9.80 (s, 1H, NH); 10.10 (s, 1H, NH).
1-[N-(3-nitrobenzoyl)-D,L-methionyl]-4-(4-bromophenyl)-thiosemicarbazide (4d). Yield 73.05 %; m.p. 95-96 ºC; Anal. Calc. for C19H20N5O4S2Br (525.01): 43.34% C, 3.80% H, 13.30% N, 12.16% S; found 43.18% C, 3.72% H, 13.05% N, 12.11% S; IR (ν, cm-1): 718 (CH3S); 819 (aromatic CH); 1171 (C=S); 1350 (symmetric vibrations of NO2); 1528 (asymmetric vibrations of NO2); 1641 (CO); 3101- 3300 (NH-CO); 1H-NMR δ: 2.11 (s, 3H, CH3); 2.50 (m, 4H, CH2); 4.50 (t, 1H, CH); 7.80 (t, 1H, Ar); 8.17 (d, 2H, Ar); 8.30 (d, 2H, Ar); 8.39 (d, 1H, Ar); 8.45 (d, 1H, Ar); 8.70 (s, 1H, Ar); 9.07 (d, 1H, NH); 9.81 (s, 1H, NH); 10.10 (s, 1H, NH).
1-[N-(3-nitrobenzoyl)-D,L-methionyl]-4-allyl-thiosemicarbazide (4e). Yield 76.27 %; m.p. 117-118 ºC; Anal. Calc. for C16H21N5O4S2 (411.10): 46.71% C, 5.10% H, 17.03% N, 15.57% S; found 46.48% C, 5.02% H, 16.98% N, 15.23% S; IR (ν, cm-1): 768 (CH3S); 823 (aromatic CH); 1172 (C=S); 1348 (symmetric vibrations of NO2); 1529 (asymmetric vibrations of NO2); 1644 (CO); 3082-3302 (NH-CO); 1H-NMR δ: 1.80 (d, 2H, CH2); 2.11 (s, 3H, CH3); 2.50 (m, 4H, CH2); 4.50 (t, 1H, CH); 5.90 (t, 2H, CH2); 6.32 (m, 1H, CH); 7.80 (t, 1H, Ar); 8.34 (d, 1H, Ar); 8.47 (d, 1H, Ar); 8.74 (s, 1H, Ar); 9.07 (d, 1H, NH); 9.82 (t, 1H, NH); 10.10, 10.30 (s, 1H, NH).
General procedure for the synthesis of 1,3,4-thiadiazole compounds 5a-e.
To corresponding thiosemicarbazide 4a-e (0.006 mol) concentrated H2SO4 (1 mL) was added under stirring. The reaction mixture was stirred at room temperature for one hour and then added dropwise to cold water. The obtained solid was dried under vacuum at 45 ºC. The crude product was purified by crystallization from ethanol.
2-[1-(3-nitrobenzoylamino)-3-(methylthio)]-propyl-5-(methylamino)-1,3,4-thiadiazole (5a). Yield 69.76 %; m.p. 116-117 ºC; Anal. Calc. for C14H17N5O3S2 (367.08): 45.77% C, 4.63% H, 19.07% N, 17.43% S; found 45.55% C, 4.58% H, 18.92% N, 17.21% S; IR (ν, cm-1): 725 (CH3S); 821 (aromatic CH); 1350 (symmetric vibrations of NO2); 1433 (C=N); 1527 (asymmetric vibrations of NO2); 1645 (CO); 3302 (NH-CO); 1H-NMR δ: 2.06 (s, 3H, CH3); 2.50 (m, 4H, CH2); 2.80 (d, 3H, CH3); 4.50 (t, 1H, CH); 7.06 (m, 1H, NH); 7.80 (t, 1H, Ar); 8.30 (d, 1H, Ar); 8.40 (d, 1H, Ar); 8.70 (s, 1H, Ar); 9.07 (d, 1H, NH).
2-[1-(3-nitrobenzoylamino)-3-(methylthio)]-propyl-5-(phenylamino)-1,3,4-thiadiazole (5b). Yield 68.72 %; m.p. 105-106 ºC; Anal. Calc. for C19H19N5O3S2 (429.09): 53.11% C, 4.42% H, 16.31% N, 14.91% S; found 52.98% C, 4.38% H, 16.22% N, 14.81% S; IR (ν, cm-1): 725 (CH3S); 821 (aromatic CH); 1350 (symmetric vibrations of NO2); 1433 (C=N); 1527 (asymmetric vibrations of NO2); 1645 (CO); 2916-3302 (NH-CO); 1H-NMR δ: 2.09 (s, 3H, CH3); 2.50 (m, 4H, CH2); 4.50 (t, 1H, CH); 7.10 (t, 1H, Ar); 7.20 (t, 2H, Ar); 7.30 (d, 2H, Ar); 7.80 (t, 1H, Ar); 8.41 (d, 1H, Ar); 8.47 (d, 1H, Ar); 8.74 (s, 1H, Ar); 9.06 (d, 1H, NH); 10.43 (s, 1H, NH).
2-[1-(3-nitrobenzoylamino)-3-(methylthio)]-propyl-5-(4-methylphenylamino)-1,3,4-thiadiazole (5c). Yield 62.43 %; m.p. 98-99 ºC; Anal. Calc. for C20H21N5O3S2 (443.11): 54.17% C, 4.74% H, 15.80% N, 14.44% S; found 54.12% C, 4.38% H, 15.72% N, 14.28% S; IR (ν, cm-1): 725 (CH3S); 819 (aromatic CH); 1350 (symmetric vibrations of NO2); 1433 (C=N); 1527 (asymmetric vibrations of NO2); 1645 (CO); 3084-3302 (NH-CO); 1H-NMR δ: 2.09 (s, 3H, CH3); 2.50 (m, 4H, CH2); 4.50 (t, 1H, CH); 7.10 (s, 3H, CH3); 7.78 (t, 1H, Ar); 7.82 (d, 2H, Ar); 7.94 (d, 2H, Ar); 8.30 (d, 1H, Ar); 8.40 (d, 1H, Ar); 8.00 (s, 1H, Ar); 9.07 (d, 1H, NH); 10.43 (s, 1H, NH).
2-[1-(3-nitrobenzoylamino)-3-(methylthio)]-propyl-5-(4-bromophenylamino)-1,3,4-thiadiazole (5d). Yield 61.18 %; m.p. 106-107 ºC; Anal. Calc. for C19H18N5O3S2Br (507.00): 44.88% C, 3.54% H, 13.77% N, 12.59% S; found 44.81% C, 3.25% H, 13.51% N, 12.42% S; IR (ν, cm-1): 769 (CH3S); 825 (aromatic CH); 1349 (symmetric vibrations of NO2); 1432 (C=N); 1529 (asymmetric vibrations of NO2); 1644 (CO); 3082-3302 (NH-CO); 1H-NMR δ: 2.11 (s, 3H, CH3); 2.50 (m, 4H, CH2); 4.50 (t, 1H, CH); 7.90 (t, 1H, Ar); 8.05 (d, 2H, Ar); 8.08 (d, 2H, Ar); 8.41 (d, 1H, Ar); 8.47 (d, 1H, Ar); 8.74 (s, 1H, Ar); 9.07 (d, 1H, NH); 10.43 (s, 1H, NH).
2-[1-(3-nitrobenzoylamino)-3-(methylthio)]-propyl-5-(allylamino)-1,3,4-thiadiazole (5e). Yield 71.03 %; m.p. 112-113 ºC; Anal. Calc. for C16H19N5O3S2 (393.09): 48.85% C, 4.83% H, 17.81% N, 16.28% S; found 48.69% C, 4.78% H, 17.62% N, 16.01% S; IR (ν, cm-1): 723 (CH3S); 819 (aromatic CH); 1350 symmetric vibrations of (NO2); 1431 (C=N); 1527 (asymmetric vibrations of NO2); 1645 (CO); 3082-3300 (NH-CO); 1H-NMR δ: 2.06 (s, 3H, CH3); 2.50 (m, 4H, CH2); 3.70 (d, 2H, CH2); 4.50 (t, 1H, CH); 5.90 (t, 2H, CH2); 6.30 (m, 1H, CH); 7.80 (t, 1H, Ar); 8.30 (d, 1H, Ar); 8.40 (d, 1H, Ar); 8.70 (s, 1H, Ar); 9.07 (d, 1H, NH); 10.42 (t, 1H, NH).
General procedure for the synthesis of 1,2,4-triazole compounds 6a-c.
To corresponding thiosemicarbazide 4a-e (0.0014 mol) a solution of NaOH 2N (10 mL) was added. The reaction mixture was heated at 80 °C for one hour, diluted with water (1:1) and then a solution of HCl 1N was added till pH 4.5. The obtained solids were dried at 45 ºC and then recristalized from ethanol.
3-mercapto-4-methyl-5-[1-(3-nitrobenzoylamino)-3-methylthio]-propyl-1,2,4-triazole (6a). Yield 62.43 %; m.p. 280-281 ºC; Anal. Calc. for C14H17N5O3S2 (367.08): 45.77% C, 4.63% H, 19.07% N, 17.43% S; found 45.62% C, 4.48% H, 18.93% N, 17.21% S; IR (ν, cm-1): 686 (CH3S); 866 (aromatic CH); 1350 (symmetric vibrations of NO2); 1436 (C=N); 1580 (asymmetric vibrations of NO2); 1648 (CO); 2592 (SH); 2974-3271 (NH-CO); 1H-NMR δ: 2.11 (s, 3H, CH3); 2.50 (m, 4H, CH2); 2.80 (s, 3H, CH3); 4.50 (t, 1H, CH); 7.80 (t, 1H, Ar); 8.41 (d, 1H, Ar); 8.47 (d, 1H, Ar); 8.74 (s, 1H, Ar); 9.05 (d, 1H, NH); 12.60 (s, 1H, SH).
3-mercapto-4-phenyl-5-[1-(3-nitrobenzoylamino)-3-methylthio]-propyl-1,2,4-triazole (6b). Yield 60.31 %; m.p. 233-234 ºC; Anal. Calc. for C19H19N5O3S2 (429.09): 53.14% C, 4.42% H, 16.31% N, 14.91% S; found 53.02% C, 4.16% H, 16.28% N, 14.85% S; IR (ν, cm-1): 684 (CH3S); 866 (aromatic CH); 1348 (symmetric vibrations of NO2); 1440 (C=N); 1550 (asymmetric vibrations of NO2); 1691 (CO); 2589 (SH); 2981-3462 (NH-CO); 1H-NMR δ: 2.08 (s, 3H, CH3); 2.50 (m, 4H, CH2); 4.50 (t, 1H, CH); 7.18 (t, 1H, Ar); 7.27 (t, 2H, Ar); 7.38 (d, 2H, Ar); 7.80 (t, 1H, Ar); 8.30 (d, 1H, Ar); 8.40 (d, 1H, Ar); 8.70 (s, 1H, Ar); 9.07 (d, 1H, NH); 12.80 (s, 1H, SH).
3-mercapto-4-(4-methylphenyl)-5-[1-(3-nitrobenzoylamino)-3-methylthio]-propyl-1,2,4-triazole (6c). Yield 68.38 %; m.p. 285-286 ºC; Anal. Calc. for C20H19N5O3S2 (443.11): 54.17% C, 4.74% H, 15.80% N, 14.44% S; found 54.03% C, 4.58% H, 15.52% N, 14.39% S; IR (ν, cm-1): 686 (CH3S); 866 (aromatic CH); 1350 (symmetric vibrations of NO2); 1450 (C=N); 1580 (asymmetric vibrations of NO2); 1698 (CO); 259 (SH); 2976-3486 (NH-CO); 1H-NMR δ: 2.06 (s, 3H, CH3); 2.50 (m, 4H, CH2); 4.50 (t, 1H, CH); 7.10 (s, 3H, CH3); 7.80 (t, 1H, Ar); 7.90 (d, 2H, Ar); 8.08 (d, 2H, Ar); 8.41 (d, 1H, Ar); 8.47 (d, 1H, Ar); 8.74 (s, 1H, Ar); 9.07 (d, 1H, NH); 12.70 (s, 1H, SH).
Antimicrobial activity assessment
The test microorganisms used to evaluate the potential antimicrobial activity of the new synthesized compounds were:
Staphylococcus aureus ATCC 25923,
Bacillus antracis ATCC 8705,
Bacillus cereus ATCC 10987,
Sarcina lutea ATCC 9341 and
Escherichia coli ATCC 25922. All the new compounds were weighed and dissolved in dimethylsulphoxide (DMSO) to prepare an extract stock solution of 100 mg/mL. The antimicrobial effects of the substances were quantitatively tested in the respective broth media by using double dilution and the Minimal Inhibitory Concentration (MIC) values (μg/mL) were determined [
17]. The antibacterial assays were performed in Mueller-Hinton broth (MH) at pH 7.3. The MIC was defined as the lowest concentration that showed no growth. Dimethylsulfoxide (DMSO) with dilution of 1:10 was used as solvent control.
Toxicity study
The acute toxicity was estimated by intraperitoneal administration of the compounds as a suspension in Tween 80 to groups of fourteen mice, each weighting 20-25 g, according to the classical laboratory methodology [
18]. The animals were observed and the death rate ascertained after 7 days.