Synthesis of Novel N-Sulfonyl Monocyclic β-Lactams as Potential Antibacterial Agents
Abstract
:Introduction
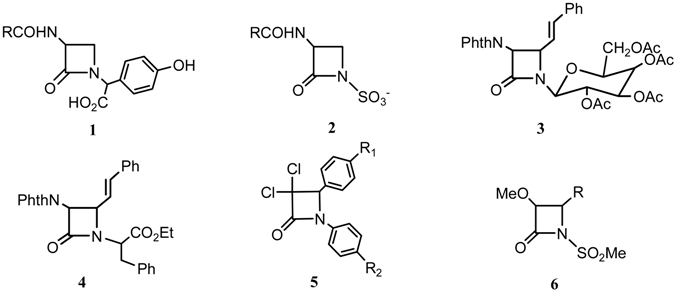
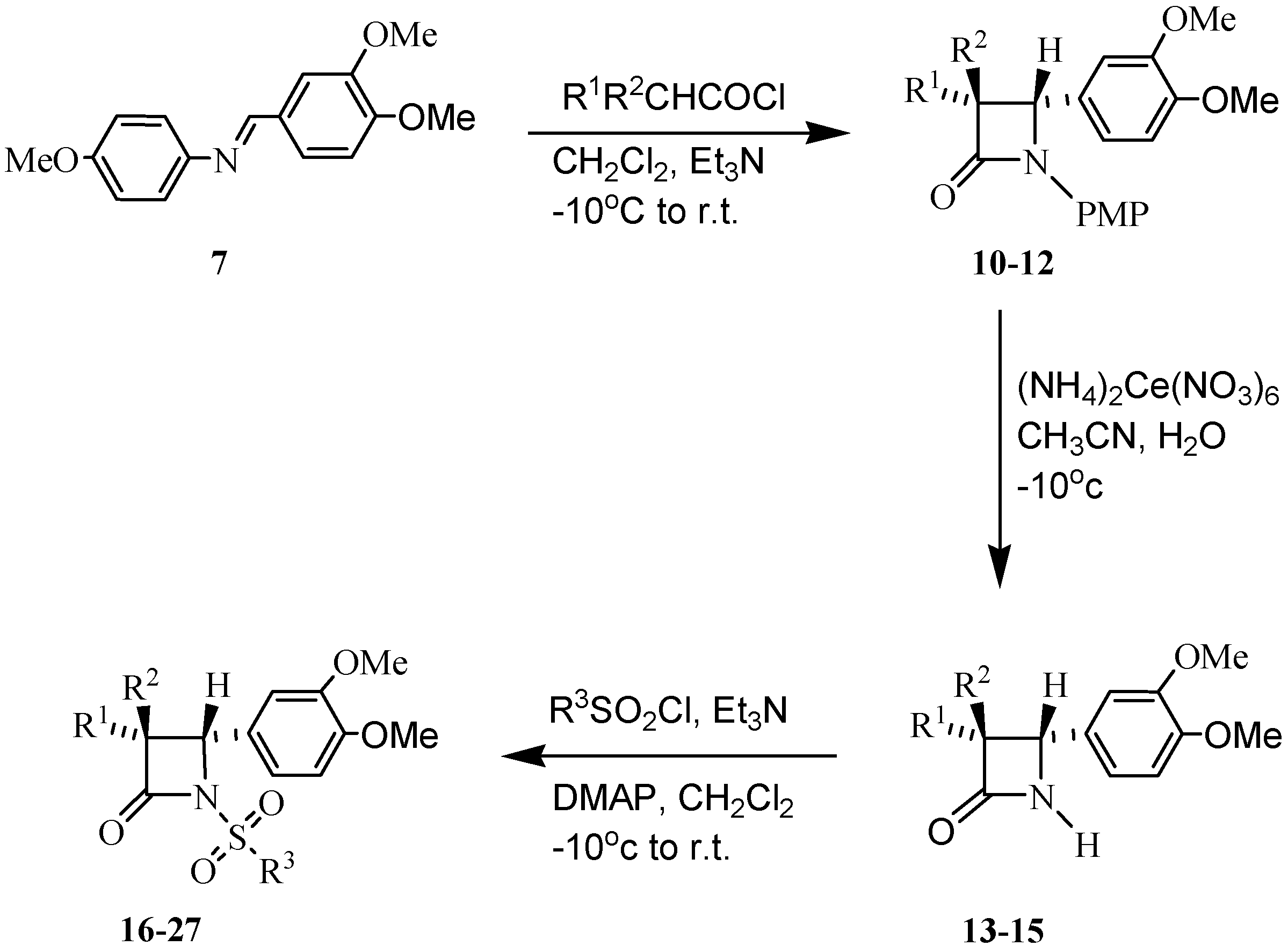
Results and Discussion
Conclusions
| Compound | R1 | R2 | R3 | Yield% |
|---|---|---|---|---|
| 10 | 3-NO2Phth | H | - | 60 |
| 11 | 3-NO2Phth | Me | - | 58 |
| 12 | PhO | H | - | 97 |
| 13 | 3-NO2Phth | H | - | 79 |
| 14 | 3-NO2Phth | Me | - | 88 |
| 15 | PhO | H | - | 83 |
| 16 | 3-NO2Phth | H | Me | 78 |
| 17 | 3-NO2Phth | H | Ph | 85 |
| 18 | 3-NO2Phth | H | 4-MeAr | 88 |
| 19 | 3-NO2Phth | H | 2-naphthalene | 73 |
| 20 | 3-NO2Phth | Me | Me | 79 |
| 21 | 3-NO2Phth | Me | Ph | 76 |
| 22 | 3-NO2Phth | Me | 4-MeAr | 83 |
| 23 | 3-NO2Phth | Me | 2-naphthalene | 82 |
| 24 | PhO | H | Me | 83 |
| 25 | PhO | H | Ph | 86 |
| 26 | PhO | H | 4-MeAr | 74 |
| 27 | PhO | H | 2-naphthalene | 72 |
Experimental
General
Synthesis of (3,4-dimethoxybenzylidene)-(4-methoxyphenyl)amine (7):
Synthesis of 3-nitrophthaloylglycyl chloride (8):
Synthesis of 3-nitrophthaloylalaninyl chloride (9):
General procedure for synthesis of monocyclic β-lactams 10-12:
General procedure for synthesis of N-unsubstituted β-lactams 13-15:
Typical procedure for synthesis of N-sulfonyl-β-lactams 16-27:
Acknowledgments
References
- Southgate, R. Contemp. Org. Synth. 1994, 1, 417.b)Morin, R. B.; Gorman, M. Chemistry and Biology of β-Lactam Antibiotics; Academic Press: New York, 1982. [Google Scholar]
- Mata, E. G.; Fraga, M. A.; Delpiccolo, C. M. L. J. Comb. Chem. 2003, 5, 208.
- Staudinger, H. Liebigs Ann. Chem. 1907, 356, 61.
- Clark, H. T.; Johnson, J. R.; Robinson, R. The Chemistry of Penicillin; Princeton University Press: Princeton, NJ, 1949. [Google Scholar]
- Page, E. I. The Chemistry of β-Lactams; Blackie Academic and Professional: New York, 1992. [Google Scholar]
- Niccolai, D.; Trasi, L.; Thomas, R. J. Chem. Commun. 1997, 2233.Chu, D. T. W.; Ptattner, J. I.; Katz, L. J. Med. Chem. 1996, 39, 3853.
- Van der Steen, F. H.; Van Koten, G. Tetrahedron 1991, 47, 7503.
- Rode, J. E.; Dobrowolski, J. C. J. Molecular Struc. 2003, 651, 705.
- Turos, E.; Ren, R. X.; Lim, D. V. Tetrahedron 2000, 56, 5571.
- Beauve, C.; Bouchet, M.; Touillaux, R.; Fastrez, J.; Marchand-Brynaert, J. Tetrahedron 1999, 55, 13301.Page, M. I. Acc. Chem. Res. 1984, 17, 144.
- Jarrahpour, A. A.; Shekarriz, M.; Taslimi, A. Molecules 2004, 9, 29. [PubMed]
- Jarrahpour, A. A.; Shekarriz, M.; Taslimi, A. Molecules 2004, 9, 939. [PubMed]
- Guner, V.; Yildirir, S.; Ozcelik, B.; Abbasoglu, U. IL Farmaco 2000, 55, 147. [PubMed]
- Freitag, D.; Schwab, P.; Metz, P. Tetrahedron Lett. 2004, 45, 3589.Hanessian, S.; Saiks, H.; Therrien, E. Tetrahedron 2003, 59, 7047.
- Hassan, H. H. A. M.; Soliman, R. Synth. Commun. 2000, 30, 2465.
- Turos, E.; Long, T. E. Curr. Med. Chem.-Anti-Infective Agents 2002, 1, 251.
- Ren, R. X.; Turos, E. J. Org. Chem. 1995, 60, 4980.
- Ren, R. X. F.; Konaklieva, M. I.; Shi, H.; Dickey, S.; Lim, D.V.; Gonzalez, J.; Turos, E. J. Org. Chem. 1998, 63, 8898.Konaklieva, M. J.; Shi, H.; Turos, E. Tetrahedron Lett. 1997, 38, 8647.
- Gonzalez-Muniz, R.; De Clercq, E.; Balzarini, J.; Navarro, G. G.; De Vega, J. P.; Anderi, C. Bioorg. Med. Chem. Lett. 2004, 14, 2253.Powers, J. C.; Asgian, J. L.; Ekici, O. D.; James, K. B. E. Chem. Rev. 2002, 102, 4693.Bonneau, P.; Hasani, F.; Deziel, R. J. Am. Chem. Soc. 1999, 121, 2965.
- Sutton, J. C.; Bolton, S. A.; Harti, K. S.; Huang, M. H.; Jacobs, G.; Meng, W.; Zhao, G.; Bisacchi, G. S. Bioorg. Med. Chem. Lett. 2004, 14, 2233. [PubMed]Bengalia, M.; Annunziata, R.; Clinquini, M.; Cozzi, F. J. J. Org. Chem. Lett. 2003, 68, 2952.Sutton, J. C.; Bolton, S. A.; Harti, K. S.; Huang, M. H.; Jacobs, G.; Meng, W.; Zhao, G.; Bisacchi, G. S. Bioorg. Med. Chem. 2002, 12, 3229.
- Delpiccolo, C. M. L.; Mata, E. G. Tetrahedron Lett. 2004, 45, 4085.Karmer, W.; Girbig, F.; Muller, G. Biochim. Biophys. Acta 2003, 1633, 13.Altmann, S. W.; Davis, R. D.; Butnett, D. A. Biochim. Biophys. Acta 2002, 77, 1580.
- Marchand-Brynaert, J.; Dive, G.; Galleni, M.; Gerard, S. Bioorg. Med. Chem. 2004, 12, 129.Gerard, S.; Dive, G.; Clamet, B.; Touillaux, R.; Marchand-Brynaert, J. Tetrahedron 2002, 58, 242.Storace, L.; Anzalone, L.; Li, H. Y.; Wood, C. C. Org. Process Res. Dev. 2002, 6, 54.
- Wurtle, M.; Hahn, M.; Hilpert, K.; Hohne, W. Acta Crystallogr. 2000, D56, 520.Bode, W.; Meyer, E. F.; Powers, J. C. Biochemistry 1989, 28, 1951. [PubMed]
- Banik, B. K.; Becker, F. F.; Banik, I. Bioorg. Med. Chem. 2004, 12, 2523. [PubMed]Banik, B. K. Curr. Med. Chem. 2004, in press.Banik, B. K.; Samajdar, S.; Banik, I.; Hann, J. Heterocycles 2003, 61, 97.Banik, I.; Becker, F. F.; Banik, B. K. J. Med. Chem. 2003, 46, 12. [CrossRef] [PubMed]
- Alcaide, B.; Almendros, P. Synlett 2002, 381.Palomo, C.; Aizupura, J. M.; Ganoba, I.; Oiarbide, M. Synlett 2001, 1813.Alcaide, B.; Almendros, P. Org. Prep. Proced. Int. 2001, 33, 315. [CrossRef]Palomo, C.; Aizpurua, J. M.; Ganboa, I.; Oiarbide, M. Amino-acids 1999, 16, 321.
- Botta, M.; Corell, F.; Armaroli, S.; Castagnolo, D. Tetrahedron: Asymmetry 2004, 15, 941.Kingston, D. I. Chem. Commun. 2001, 867, and references cited therein.
- Singh, G. S. Tetrahedron 2003, 59, 7631.Ward, M. F. Annu. Rep. Prog. Chem., Sect. B, 2000, 96, 157. [CrossRef]Palomo, C.; Aizpurua, J. M.; Ganboa, L.; Oiarbide, M. Eur. J. Org. Chem. 1999, 3223.d)Dekimpe, N. Comprehensive Heterocyclic Chemistry; Katritzky, A. R., Rees, C. W., Scriven, E. F. V., Padwa, A., Eds.; Pergamon: Oxford, 1996; Vol. 1B, pp. 507–589. [Google Scholar]
- Bianchi, L.; Tavani, C.; Dell Erba, C.; Maccagno, M.; Mugnoli, A.; Petrillo, G.; Sancassan, F.; Novi, M. Tetrahedron 2003, 59, 10195.
- Fu, G. C.; Hodous, B. L. J. Am. Chem. Soc. 2002, 124, 1578.b)Georg, G. I. The Organic Chemistry of β-Lactams; VCH: New York, 1993; and references cited therein. [Google Scholar]
- Lopez, J. R.; Martinez, J. C. G.; Barra, E.D. Synlett 2003, 1587.Buttero, P.D.; Molteni, G.; Papagani, A. Tetrahedron: Asymmetry 2003, 14, 3949.Kumar, Y.; Tewari, N.; Nizar, H.; Singh, S. K. Org. Process Res. Develop. 2003, 7, 933. [CrossRef]
- Ha, D.C.; Hart, D.J. Tetrahedron Lett. 1987, 28, 4489.
- Lyman, R. C.; Yang, K. C. C. J. Chem. Eng. Data 1968, 291.
- Sample availability: Contact the authors or MDPI
© 2006 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes
Share and Cite
Jarrahpour, A.; Zarei, M. Synthesis of Novel N-Sulfonyl Monocyclic β-Lactams as Potential Antibacterial Agents. Molecules 2006, 11, 49-58. https://doi.org/10.3390/11010049
Jarrahpour A, Zarei M. Synthesis of Novel N-Sulfonyl Monocyclic β-Lactams as Potential Antibacterial Agents. Molecules. 2006; 11(1):49-58. https://doi.org/10.3390/11010049
Chicago/Turabian StyleJarrahpour, Aliasghar, and Maaroof Zarei. 2006. "Synthesis of Novel N-Sulfonyl Monocyclic β-Lactams as Potential Antibacterial Agents" Molecules 11, no. 1: 49-58. https://doi.org/10.3390/11010049




