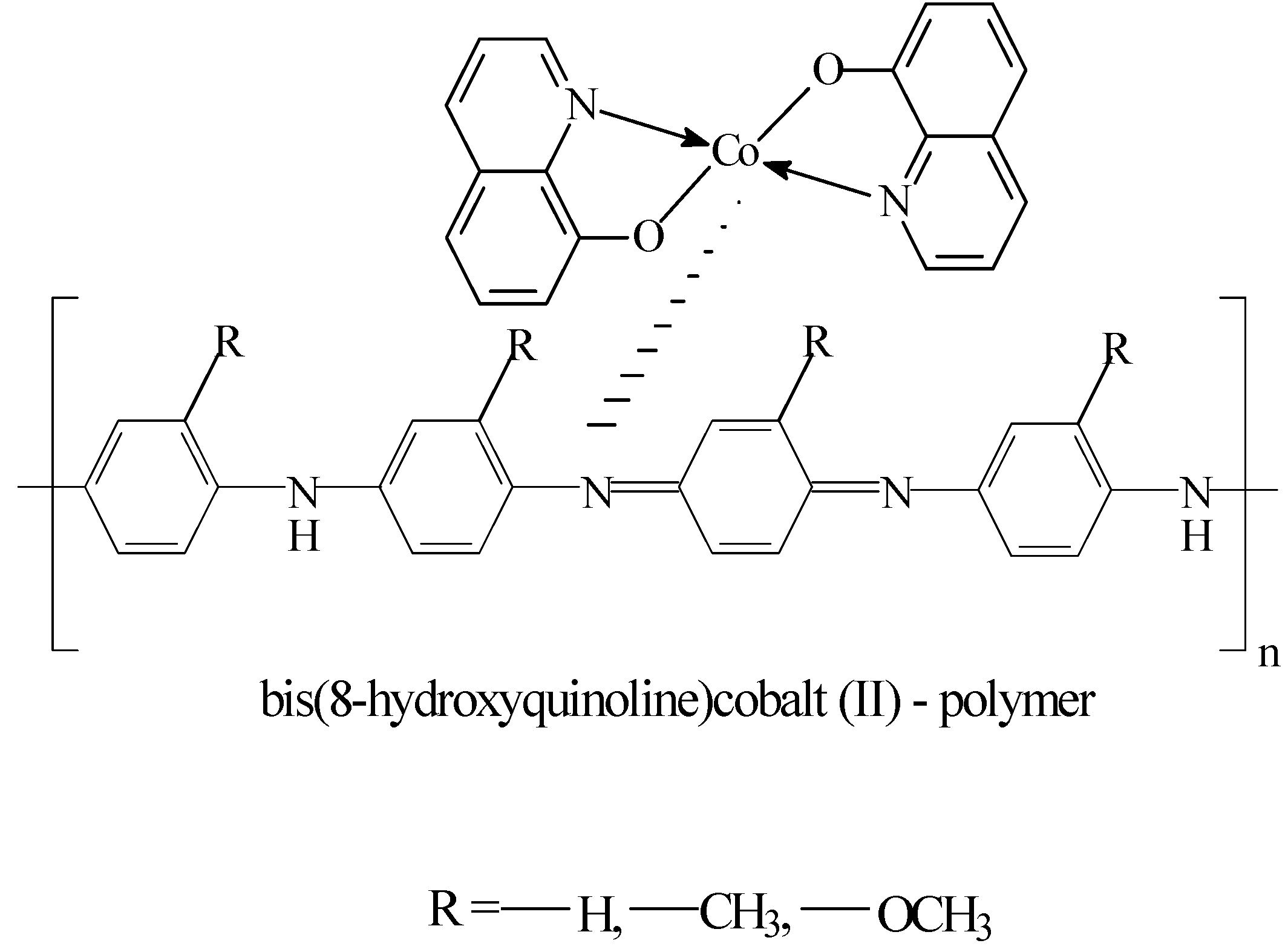
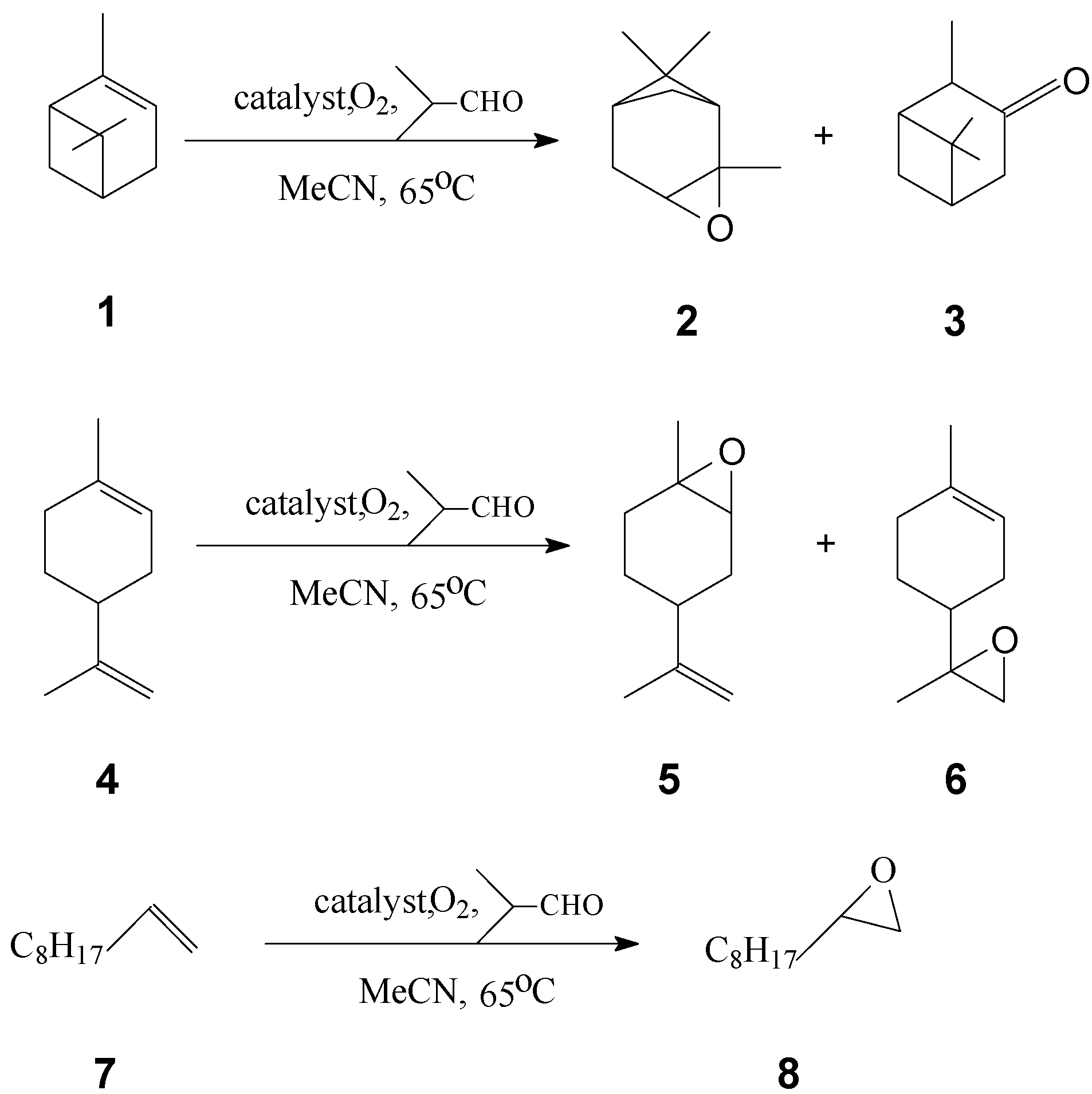
Introduction
Catalytic liquid phase oxidation of compounds is a problem that has been evoking interest for many years. Heterogeneous catalysis allows running of various processes in a selective way and under mild conditions. Oxidation reactions constitute an important group of processes in organic chemistry, but the toxicity of many widely used oxidants and need for a solvent are the main obstacles in further development of the technology. Low selectivity obtained in many processes also makes them more difficult to run. Due to that, new methods of oxidation in organic chemistry and also applications of “friendly” solvents for the natural environment were examined. Molecular oxygen is one of the most interesting oxidizing agents. Immobilized metal complexes with diimines are used as catalysts in oxidation processes of hydrocarbons. A catalyst chemically bound to a support has increased stability and makes it easier to separate after the reaction [
1,
2,
3].
It has been reported in the literature that the epoxidation of alkenes by various oxidants can proceed very efficiently when a polymer-supported catalyst is used. The use of polymer-supported catalysts offers several advantages in preparation procedures. The polymer is typically very stable (with regards to thermal properties) even in an oxidative atmosphere [
4]. Polyaniline (PANI) is one of the polymers which can be used as a matrix for catalysts. It is cheap, easy to synthesize and insoluble in a large majority of commonly used solvents, which is the main advantage of supported catalysts [
5]. Moreover, PANI is thermally stable to 300°C and chemical resistant to oxidation [
6]. A polyaniline-supported cobalt (II) catalyst was prepared first by Pielichowski and Iqbal [
7] and used for the aerobic epoxidation of alkenes. For example, a polyaniline-supported catalyst has been used for epoxidation of
trans-stilbene with very high yield and selectivity [
8]. In this paper, we report a specific series of reactions leading to novel catalysts based on a cobalt (II) 8-hydroxyquinoline complex supported by polyaniline or polyaniline derived polymers. These catalysts have been designed and tested in oxidation reactions of α-pinene, limonene and 1-decene.
Results and Discussion
In our experiments the catalysts were prepared via a three-step sequence and were then used in a fourth one for the oxidation reactions:
Synthesis of 8-hydroxyquinoline complex with cobalt (II) acetate.
PANI, POT and POA were obtained via oxidative polymerization.
Cobalt-based catalysts were supported on the polymers (see
Scheme 1).
The oxidation of hydrocarbons in the presence of the obtained catalysts.
Alkene oxidations reactions (
Scheme 2) were carried out by refluxing and stirring the reagents in acetonitrile in a glass reactor thermostated at 65°C. Molecular oxygen which was bubbled through the reaction medium was used as the oxidizing agent and isobutyraldehyde was used as reductant and co-catalyst. During the reactions samples were periodically drawn for analysis and the conversion ratios of hydrocarbon into the main product(s) was examined. The results are collected in
Table 2.
Table 1.
Results of the Polymer-Supported Cobalt Catalysts Oxidation of α-Pinene, Limonene and 1-Decene with Molecular Oxygen a
Table 1.
Results of the Polymer-Supported Cobalt Catalysts Oxidation of α-Pinene, Limonene and 1-Decene with Molecular Oxygen a
| Catalysts | Substrates |
|---|
| α-pinene | limonene | 1-decene |
|---|
Time
[h] | Yield
[%] | Time
[h] | Yield
[%] | Time
[h] | Yield
[%] |
|---|
| PANI + Co (II) 8-hydroxyquinoline | 1 | 2 - 50
3 - 36 | 1 | 5 - 69
6 - 17 | 16 | 63 |
| POT + Co (II) 8-hydroxyquinoline | 1 | 2 - 55
3 - 41 | 2 | 5 - 58
6 - 28 | 16 | 70 |
| POA + Co (II) 8-hydroxyquinoline | 1 | 2 - 59
3 - 32 | 2 | 5 - 58
6 - 28 | 16 | 65 |
All the catalysts appeared to be active in the tested reactions, although their relative activity and selectivity depended on the nature of the hydrocarbon. Catalysts were selective in the oxidation of 1-decene, which gave only one product, the epoxide derivative 8, in high yield, but aliphatic hydrocarbons are less reactive. In these reactions α-pinene was transformed into two derivatives – 2 and 3. The conversions and molar ratios of the α-pinene derivatives were similar for all the catalysts. The oxidation of limonene involved longer reaction times.
The main advantage of the prepared catalysts is the simplicity of separating off the heterogeneous catalysts from the reaction medium by simple filtration. Oxidation reactions of alkenes were carried out using synthesized the PANI, POT and POA catalysts at atmospheric pressure in the presence of molecular oxygen. These polymers can be used as a new group of effective oxidations catalysts, as shown by the research conducted. We also studied the total Co(II) content in all tested polymers before and after the oxidation reaction. The results summarized in
Table 2 show that the Co(II) content varies on the different supports and decreased during the oxidation reactions.
Table 2.
Total Co(II) content in catalysts before and after oxidation reaction.
Table 2.
Total Co(II) content in catalysts before and after oxidation reaction.
| Catalyst | Co(II) content [wt.%] |
| before | after |
| PANI+Co(II)8-hydroxyquinoline | 10.04 | 9.96 |
| POT+ Co(II) 8-hydroxyquinoline | 9.30 | 8.89 |
| POA+ Co(II) 8-hydroxyquinoline | 9.28 | 9.19 |
Taking into consideration the Co(II) content in the polymers, which ranged between 9.28 and 10.04 wt.%, and the fact that only insignificant changes in product yields were observed, it could be supposed that only some of the Co species are active in the oxidation reaction. This is probably due to the heterogeneous character of the catalyst that makes contact between the active catalytic sites and substrate taking part in oxidation reaction difficult. It was also observed that only a small part of the initial amount of catalyst is deposited into the polymer matrix.
Conclusions
We have described the synthesis of conjugated polymer-supported cobalt based catalysts, which are very effective in oxidizing alkenes under mild conditions. Molecular oxygen used as oxidant is a cheap, effective and environmentally friendly (“Green Chemistry”) material. The main advantage of our catalysts is the simplicity of separating heterogeneous catalysts from the reaction medium by filtration.
Experimental
General
The synthesised substances were identified by means FT-IR, GC-MS and atomic absorption spectroscopy (AAS). All chemicals were of reagent grade (Aldrich, Fluka), and were used without purification.
Synthesis of the 8-hydroxyquinoline complex with cobalt (II)acetate:
N,N-Dimethylformamide (50 mL) and 8-hydroxyquinoline (7.3 g, 50 mmol) were mixed and heated to achieve dissolution of the 8-hydroxyquinoline. Cobalt (II) acetate (6.2 g, 25 mmol) was then added to the flask and the mixture was heated to boiling for 10 hours. After cooling the crystals of Co(II)8-hydroxyquinoline complex were filtered and dried at 60°C under vacuum overnight.
Preparation of polymer supported Co (II) 8-hydroxyquinoline:
A mixture of polymer (500 mg) and Co(II) 8-hydroxyquinoline (500 mg) was stirred in acetonitrile (50 mL) for 48 h at room temperature. The reaction mixture was filtered and the solid filtrate was washed with acetonitrile (5 x 5 mL). The catalyst was dried at 110°C for 24 h.
The oxidation of α-pinene, limonene and 1-decene:
The products were obtained by refluxing and stirring the reagents in a glass thermostatic reactor at 65°C in the presence of oxygen to give the products. Substrates, i.e. α-pinene, limonene and 1-decene (5 mmoles) were dissolved in acetonitrile (20 mL) and then isobutyraldehyde (10 mmoles) and catalyst (20 mg) were added to the mixture. After completion of the oxidation of alkenes the solvent was evaporated to yield a residue which was dissolved in ethyl acetate. Then the mixture was extracted successively with aqueous NaHCO
3 and water. Finally, the organic layer was dried and the solvent was evaporated to give the desired product. All the products were characterized by analytical and spectroscopic data [
9].