Synthesis, Tautomeric States and Crystal Structure of (Z)-Ethyl 2-Cyano-2-(3H-Quinazoline-4-ylidene) Acetate and (Z)-Ethyl 2-Cyano-2-(2-Methyl-3H-Quinazoline-4-ylidene) Acetate
Abstract
:Introduction
Results and Discussion
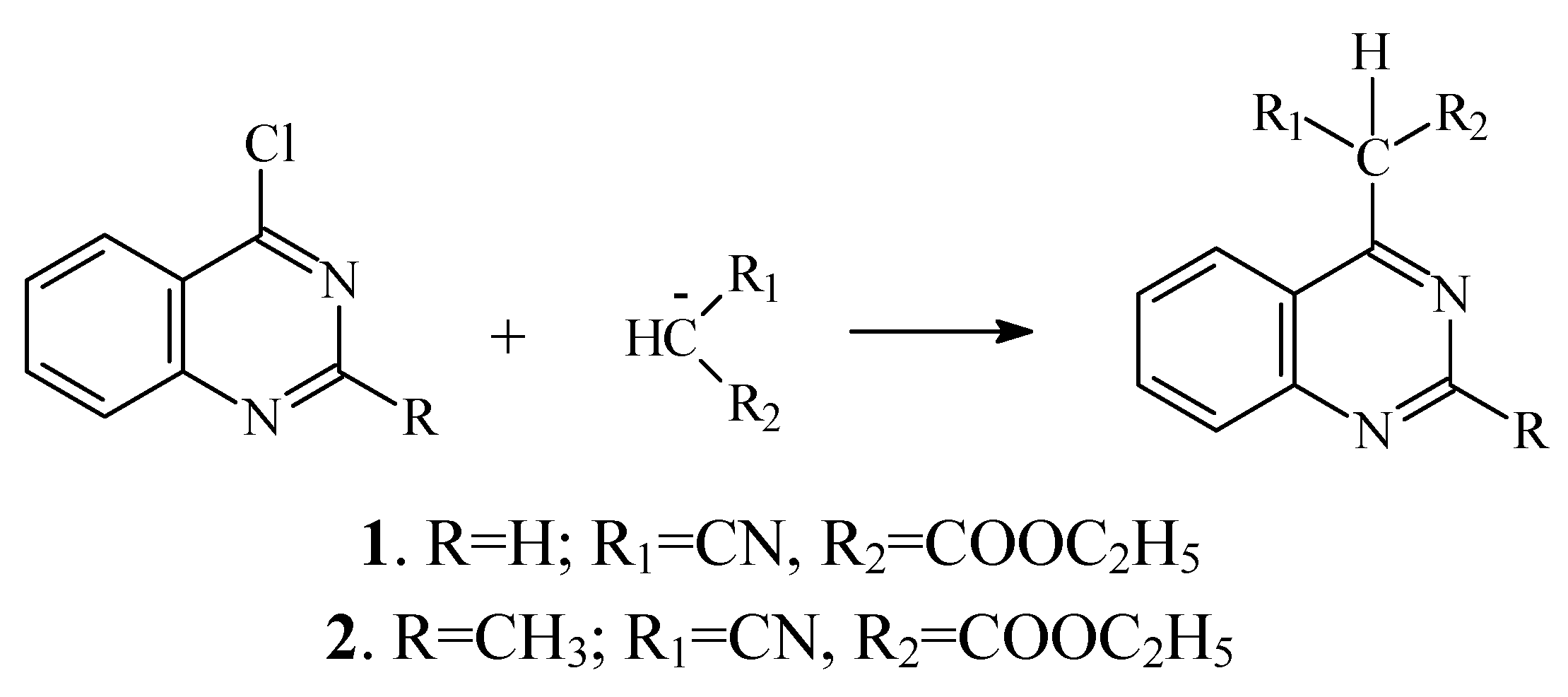

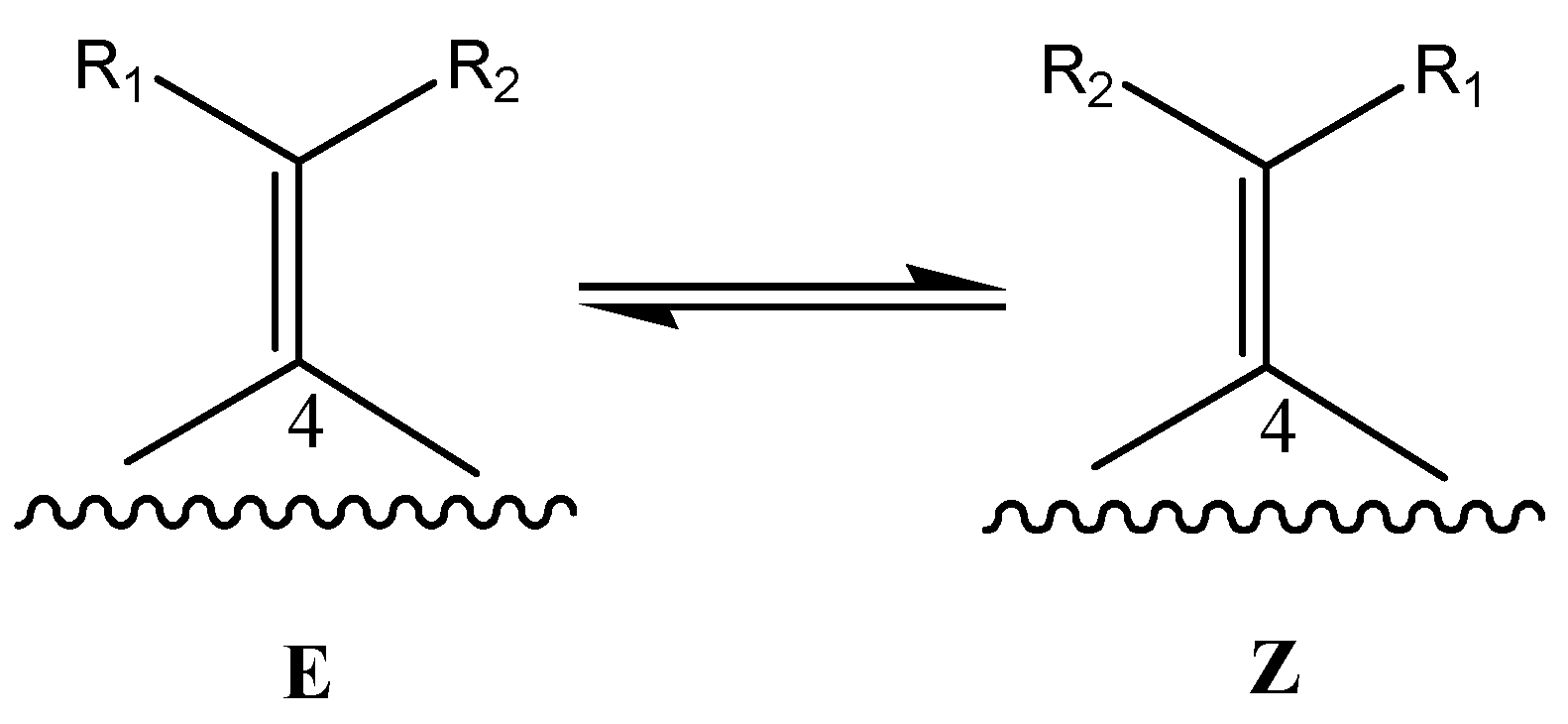
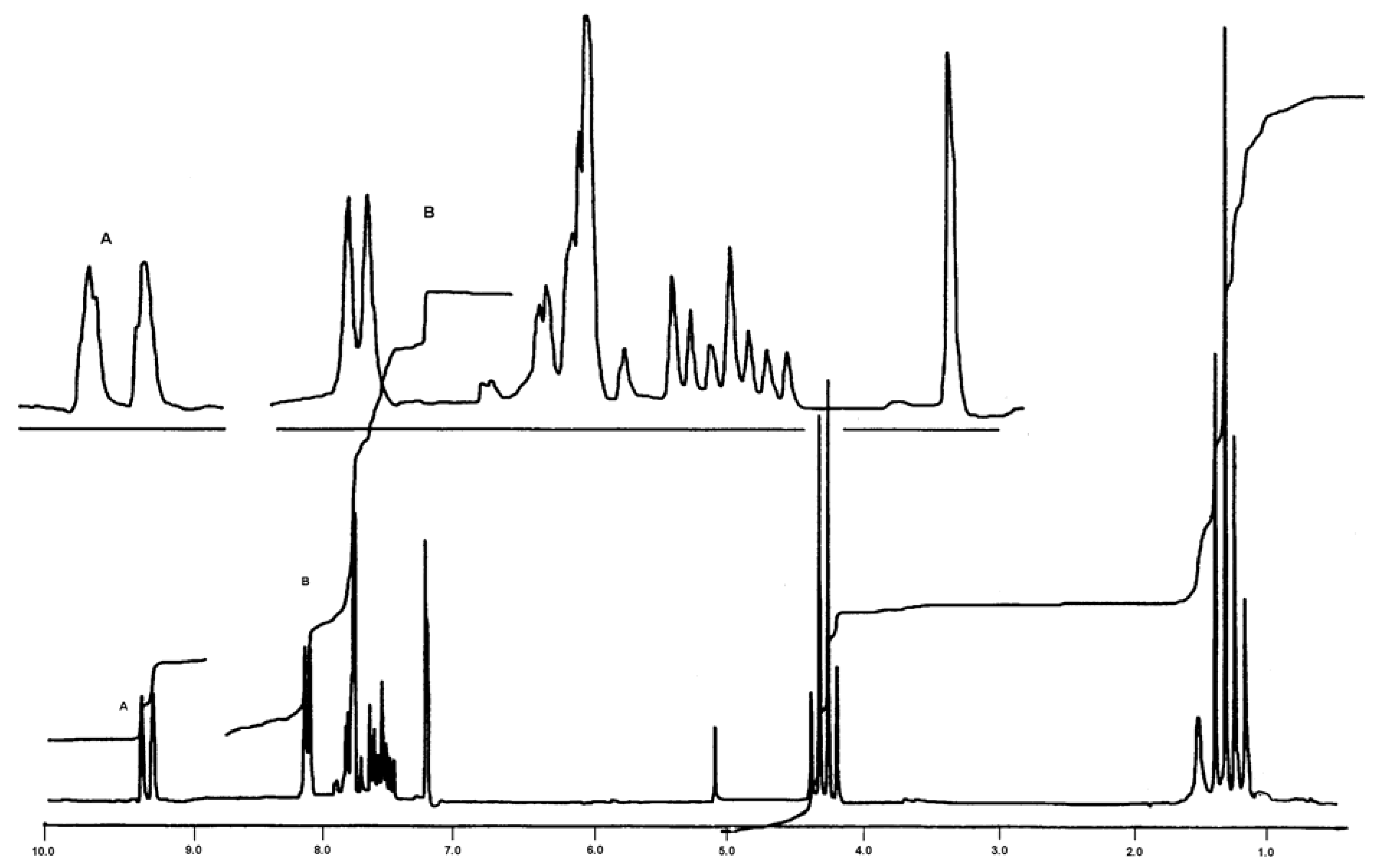

Determination of the most stable tautomeric form of compound 1 by a computational approach.
| Tautomer | Ionization potential | Dipole moment, D | Heat of formation, ∆Hf , (kcal/mol) |
|---|---|---|---|
| A | 8.75 | 6.80 | 15.14 |
| B | 8.91 | 2.79 | 7.09 |
| C | 9.74 | 4.45 | 10.41 |
Conclusions
Experimental
General
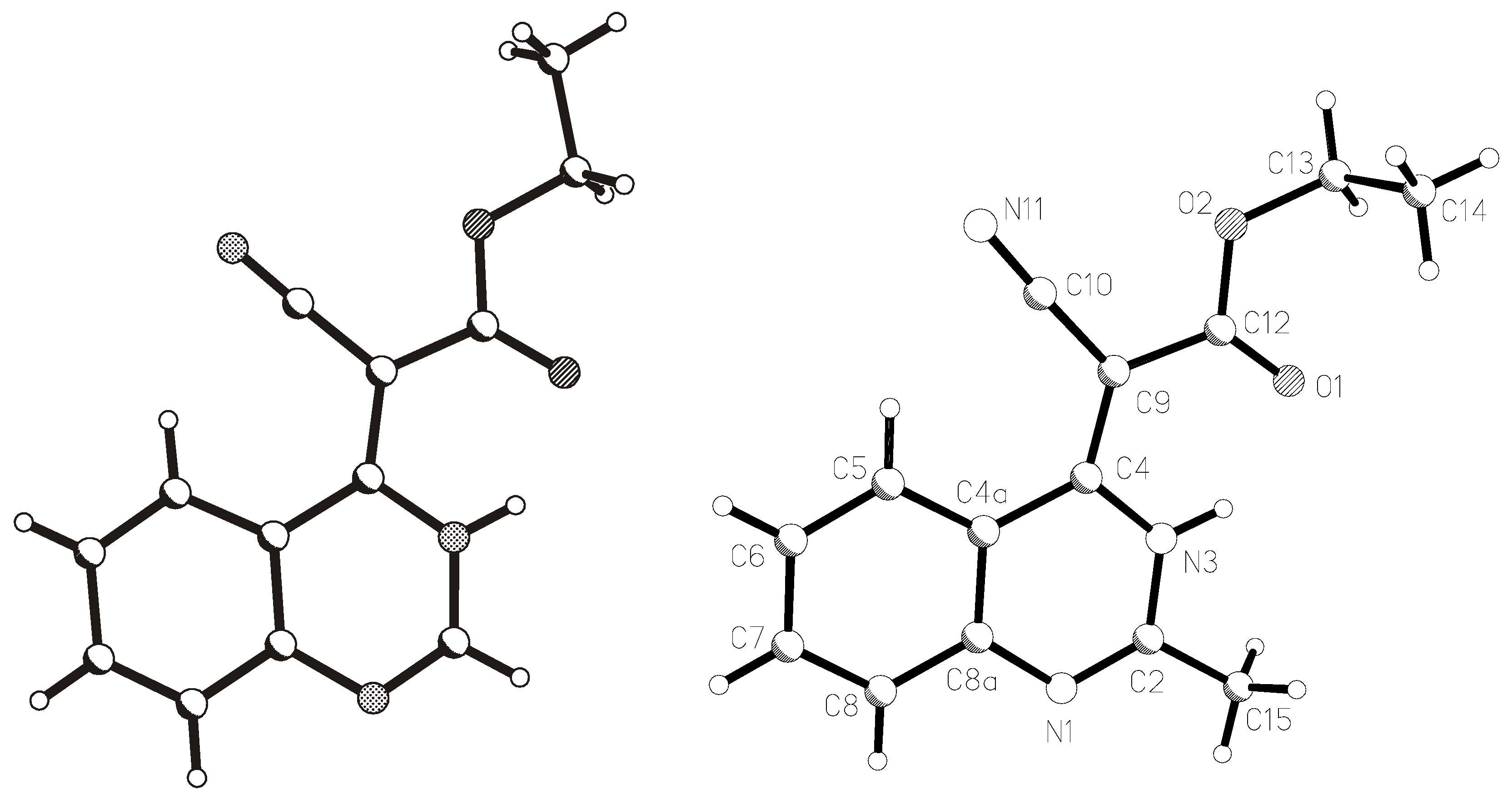
X-ray structural analysis
| Parameter | 1 | 2 |
|---|---|---|
| Empirical formula | C13H11N3O2 | C14H13N3O2 |
| Formula weight | 241.25 | 255.27 |
| Crystal size (mm) | 0.90 х 0.80 х 0.10 | 0.80 х 0.30 х 0.20 |
| Crystal system | Monoclinic | Triclinic |
| Space group | P 21/с | P-1 |
| Z | 4 | 2 |
| a, Å | 7.970(6) | 8.196(5) |
| b, Å | 7.061(2) | 8.997(6) |
| c, Å | 20.537(7) | 9.435(4) |
| α | 90 | 74.22(4) |
| β | 97.69(5) | 89.75(4) |
| γ | 90 | 74.07(5) |
| V, Å3 | 1145.3(10) | 641.9(6) |
| dcall, g/cm3 | 1.399 | 1.321 |
| Theta range | 2.4<θ<26.0° | 2.3<θ<26.0° |
| μexp (cm-1) | 0.098 | 0.091 |
| Reflections collected | 2247 | 2526 |
| Number of reflections with | 1644 | 1674 |
| I>2σ (I) | ||
| R1 (I>2σ(I) and for all) | 0.0523 (0.0777) | 0.0714 (0.1092) |
| WR2 (I>2σ(I) and for all) | 0.1197 (0.1387) | 0.1539 (0.1821) |
| Max. diff. peak and hole | 0.166 and –0.181 eÅ-3 | 0.209 и –0.261 eÅ-3 |
Acknowledgments
References
- Shakhidoyatov, Kh. M.; Irisbaev, A.; Abdullaev, N. P. Synthesis of potential growth regulators of plants in series of the N-substituted lactams and quinazolines. In Growth regulators of plants and herbicides; Fan: Tashkent, 1978; pp. 166–195. [Google Scholar]
- Yaxontev, L. N.; Liberman, S. S.; Jiharyova, G. P.; Kuzmina, K. K. Chem.-Pharm. J. 1977, 5, 14–26.
- Michael, J. P. Nat. Prod. Rep. 2002, 19, 742–760. [PubMed]
- Bhattacharjee, A.K.; Skanchy, D.J.; Jennings, B.; Hudson, T.H.; Brendle, J.J.; Werbovetz, K.A. Bioorg. Med. Chem. 2003, 10, 1979–89.
- Gupta, C. M.; Bhaduri, A.P.; Khanna, N. M. J. Med. Chem. 1968, 11, 392–395. [PubMed]
- Abdullaev, N. P.; Kayumov, K.; Shakhidoyatov, K. M. Uzb. Khim. J. 1997, 2, 29–34.
- Fathalla, W.; Cajan, M.; Marek, J.; Pazdera, P. Proceedings of ECSOC-5, The Fifth International Electronic Conference on Synthetic Organic Chemistry, September 1-30; 2001. A0049. http://www.mdpi.org/ecsoc-5.htm.
- Tashkhodjaev, B.; Turgunov, K. K.; Dyakonov, A. L.; Belova, G. A.; Shakhidoyatov, Kh. M. Khim. Prirod. Soedin. 1995, 410.
- Turgunov, K. K.; Tashkhodjaev, B.; Molchanov, L. V.; Aripov, Kh. N. Khim. Prirod. Soedin. 1995, 849.
- Pople, J. A.; Santry, J. P.; Segal, G. A. J. Chem. Phys. 1965, 43, S129.
- Dewar, M. J. S.; Zoebisch, E. G.; Healy, E. F.; Stewart, J. J. P. J. Am. Chem. Soc. 1985, 107, 3902–3909.
- Stewart, J. P. P. J. Comput. Chem. 1989, 10, 209.
- Zahedi, M. J. Mol. Struct. (THEOCHEM) 1998, 452, 125–131.
- Sygula, A.; Buda, A. J. Mol. Struct. (THEOCHEM) 1985, 121, 133.
- Sygula, A. J. Chem. Res., Synop. 1989, 56.
- Koryakova, O. V.; Sattarova, V. V.; Sevenard, D. V.; Khomutov, O. G.; Pashkevich, K. I. Russ. J. Gen. Chem. 2003, 73, 141–144.
- Csányi, D.; Hajós, G.; Timári, G.; Riedl, Z.; Kotschy, A.; Kappe, T.; Párkányi, L.; Egyed, O.; Kajtár-Peredy, M.; Holly, S. Eur. J. Org. Chem. 2002, 1, 133–138.
- ХRED 1.09. Data Reduction program for Stadi-4 and IPDS. STOE & Cie GmbH (1997).
- Sheldrick, G. M. Acta Crystallogr. 1993, A49, 53.
- CCDC 240414 and CCDC 240415 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223 336033; e-mail: [email protected] or via the URL http://www.ccdc.cam.ac.uk/conts/retrieving.html).
- Sample availability: Available from the authors.
© 2005 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Tulyasheva, M.; Rasulev, B.; Tojiboev, A.; Turgunov, K.; Tashkhodjaev, B.; Abdullaev, N.; Shakhidoyatov, K. Synthesis, Tautomeric States and Crystal Structure of (Z)-Ethyl 2-Cyano-2-(3H-Quinazoline-4-ylidene) Acetate and (Z)-Ethyl 2-Cyano-2-(2-Methyl-3H-Quinazoline-4-ylidene) Acetate. Molecules 2005, 10, 1209-1217. https://doi.org/10.3390/10091209
Tulyasheva M, Rasulev B, Tojiboev A, Turgunov K, Tashkhodjaev B, Abdullaev N, Shakhidoyatov K. Synthesis, Tautomeric States and Crystal Structure of (Z)-Ethyl 2-Cyano-2-(3H-Quinazoline-4-ylidene) Acetate and (Z)-Ethyl 2-Cyano-2-(2-Methyl-3H-Quinazoline-4-ylidene) Acetate. Molecules. 2005; 10(9):1209-1217. https://doi.org/10.3390/10091209
Chicago/Turabian StyleTulyasheva, M., B. Rasulev, A. Tojiboev, K. Turgunov, B. Tashkhodjaev, N. Abdullaev, and K Shakhidoyatov. 2005. "Synthesis, Tautomeric States and Crystal Structure of (Z)-Ethyl 2-Cyano-2-(3H-Quinazoline-4-ylidene) Acetate and (Z)-Ethyl 2-Cyano-2-(2-Methyl-3H-Quinazoline-4-ylidene) Acetate" Molecules 10, no. 9: 1209-1217. https://doi.org/10.3390/10091209




