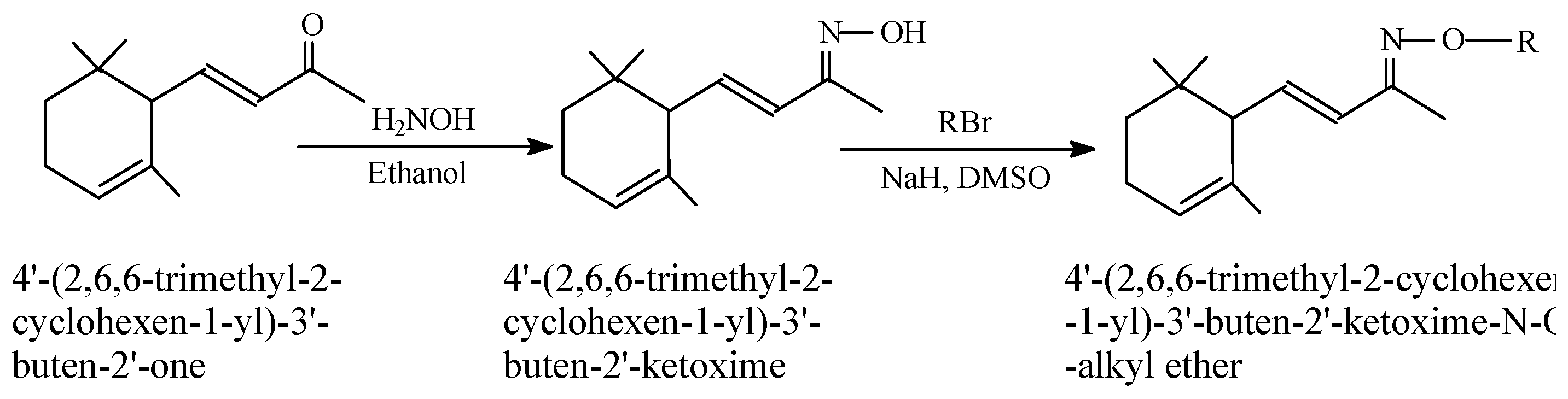
Synthesis of 4'-(2,6,6-Trimethyl -2-Cyclohexen-1-yl) -3'-Buten-2'-Ketoxime-N-O-Alkyl Ethers
Abstract
:Introduction
Results and Discussion

| Isomer | δ (ppm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | h | i | j | k | l | m | |
| 3(I) | 1.57 | 5.43 | 1.57 | 1.57 | 0.94 | 0.92 | 5.83 | 6.10 | 1.94 | 4.12 | 2.02 | 0.90 | 2.23 |
| 3(II) | 1.58 | 5.45 | 1.58 | 1.58 | 1.26 | 1.26 | 5.90 | 6.75 | 1.97 | 4.08 | 2.01 | 0.91 | 2.00 |
Experimental
General
General Procedure for Preparation of 4'-(2,6,6-Trimethyl-2-cyclohexen-1-yl)-3'-buten-2'-ketoxime-N-O-alkyl ethers:

| Comp. No. | R | Rf (TLC)** | 1H-NMR (δ) | MS m/z (%) |
| 1(I) | CH3 | 0.61 | 6.04 (1H, h, d, J=8Hz), 5.81 (1H, g, dd, J=3Hz), 5.43 (1H, b, s), 3.84 (2H, j, s), 2.23 (1H, m, d, J =3Hz), 1.94 (3H, i, s), 1.71 (1H, c2, q, J=3Hz), 1.58 (2H, d, t, J= 3Hz), 1.57 (3H, a, s), 1.42 (1H, c1, q, J=3Hz), 0.94 (3H, e, s), 0.92 (3H, f, s). | 221 (M+, 89), 203 (17), 207 (4), 206 (5), 193 (89), 178 (48), 160 (8), 150 (49), 136 (28), 134 (100), 119 (22), 117 (13), 107 (35), 105 (20), 93 (83), 91 (60), 85 (7), 83 (12), 79 (25), 77 (29). |
| 1(II) | CH3 | 0.33 | 6.57 (1H, h, d, J=8 Hz) 5.79 (1H, g, dd, J=3Hz ), 5.46 (1H, b, s), 3.87 (2H, j, s), 2.00 (1H, m, d, J =3 Hz), 1.97 (3H, i, s), 1.71 (1H, c2, q, J=3 Hz), 1.58(2H, d, t, J=3 Hz), 1.58 (3H, a, s), 1.45 (1H, c1, q, J=3 Hz), 1.26 (3H, e, s), 1.26 (3H, f, s). | 221 (M+, 7), 207 (2), 193 (5), 178 (4), 150 (5), 136 (3), 134 (10), 119 (5), 117 (4), 107 (5), 105 (3), 93 (17), 91 (14), 85 (92), 83 (100), 79 (5), 77 (7). |
| 2(I) | C2H5 | 0.67 | 6.11 (1H, h, d, J=8Hz), 5.85 (1H, g, dd, J=3Hz), 5.42 (1H, b, s), 4.1 (2H, j, q, J=3Hz), 2.23 (1H, m, d, J=3Hz), 2.01 (3H, l, t, J=3Hz), 1.94 (3H, i, s), 1.71 (1H, c2, q, J= 3Hz), 1.58 (2H, d, t, J=3Hz), 1.57 (3H, a, s), 1.42 (1H, c1, q, J= 3Hz), 0.94 (3H, e, s), 0.92 (3H, f, s). | 235 (M+, 89), 217 (17), 207 (4), 206 (5), 193 (89), 178 (48), 160 (8), 150 (49), 136 (28), 134 (100), 119 (22), 117 (13), 107 (35), 105 (20), 93 (83), 91 (60), 85 (7), 83 (12), 79 (25), 77 (29). |
| 2(II) | C2H5 | 0.34 | 6.67 (1H, h, d, J=8 Hz) 5.83 (1H, g, dd, J=3Hz ), 5.4 (1H, b, s), 4.08 (2H, j, q, J=3 Hz), 2.06 (3H, l, t, J=3 Hz), 2.00 (1H, m, d, J=3 Hz), 1.97 (3H, i, s), 1.71 (1H, c2, q, J=3 Hz), 1.58 (2H, d, t, J=3 Hz), 1.58 (3H, a, s), 1.45 (1H, c1, q, J=3 Hz), 1.26 (3H, e, s), 1.26 (3H, f, s). | 235 (M+, 7), 207 (2), 193 (5), 178 (4), 150 (5), 136 (3), 134 (10), 119 (5), 117 (4), 107 (5), 105 (3), 93 (17), 91 (14), 85 (92), 83 (100), 79 (5), 77 (7). |
| 3(I) | C3H7 | 0.75 | 6.10 (1H, h, d, J=8Hz), 5.83 (1H, g, dd, J=3Hz), 5.43 (1H, b, s), 4.12 (2H, j, t, J= 3Hz), 2.23 (1H, m, d, J=3Hz), 1.94 (3H, i, s), 2.02 (2H, k, m), 1.71(1H, c2, q, J= 3Hz), 1.58 (2H, d, t, J=3Hz), 1.57 (3H, a, s), 1.42 (1H, c1, q, J=3Hz), 0.94 (3H, e, s), 0.92 (3H, f, s), 0.90 (3H, l, t, J= 3Hz). | 249 (M+, 89), 234 (17), 207 (4), 206 (5), 193 (89), 178 (48), 160 (8), 150 (49), 136 (28), 134 (100), 119 (22), 117 (13), 107 (35), 105 (20), 93 (83), 91 (60), 85 (7), 83 (12), 79 (25), 77 (29). |
| 3(II) | C3H7 | 0.39 | 6.75 (1H, h, d, J=8 Hz) 5.90 (1H, g, dd, J=3Hz ), 5.45 (1H, b, s), 4.08 (2H, j, t, J= 3 Hz), 2.01 (2H, k, m), 2.00 (1H, m, d, J=3 Hz), 1.97 (3H, i, s), 1.71 (1H, c2, q, J=3 Hz), 1.58 (2H, d, t, J=3 Hz), 1.58 (3H, a, s), 1.45 (1H, c1, q, J=3 Hz), 1.26 (3H, e, s), 1.26 (3H, f, s), 0.91 (3H, l, t, J=3 Hz). | 249 (M+, 7), 207 (2), 193 (5), 178 (4), 150 (5), 136 (3), 134 (10), 119 (5), 117 (4), 107 (5), 105 (3), 93 (17), 91 (14), 85 (92), 83 (100), 79 (5), 77 (7). |
| 4(I) | CH(CH3)2 | 0.75 | 6.19 (1H, h, d, J=8Hz), 5.83 (1H, g, dd, J=3Hz), 5.43 (1H, b, s), 4.23 (2H, j, h, J= 3Hz), 2.23 (1H, m, d, J=3Hz), 2.02 (6H, l, d, J=3Hz), 1.94 (3H, i, s), 1.71 (1H, c2, q, J=3Hz), 1.58 (2H, d, t, J=3Hz), 1.57 (3H, a, s), 1.42 (1H, c1, q, J=3Hz), 0.94 (3H, e, s), 0.92 (3H, f, s). | 249 (M+, 89), 234 (17), 207 (4), 206 (5), 193 (89), 178 (48), 160 (8), 150 (49), 136 (28), 134 (100), 119 (22), 117 (13%), 107 (35), 105 (20), 93 (83), 91 (60), 85 (7), 83 (12), 79 (25), 77 (29). |
| 4(II) | CH(CH3)2 | 0.39 | 6.83 (1H, h, d, J=8 Hz) 5.90 (1H, g, dd, J=3Hz ), 5.45 (1H, b, s), 4.26 (2H, j, h, J=3 Hz), 2.03 (6H, l, d, J=3 Hz), 2.00 (1H, m, d, J=3 Hz), 1.97 (3H, i, s), 1.71 (1H, c2, q, J=3 Hz), 1.58 (2H, d, t, J=3 Hz), 1.58 (3H, a, s), 1.45 (1H, c1, q, J=3 Hz), 1.26 (3H, e, s), 1.26 (3H, f, s), | 249 (M+, 7), 207 (2), 193 (5), 178 (4), 150 (5), 136 (3), 134 (10), 119 (5), 117 (4), 107 (5), 105 (3), 93 (17), 91 (14), 85 (92), 83 (100), 79 (5), 77 (7). |
| 5(I) | C4H9 | 0.76 | 6.16 (1H, h, d, J=8Hz), 5.84 (1H, g, dd, J=3Hz), 5.4 (1H, b, s), 4.0 (2H, j, t, J= 3Hz), 2.23 (1H, m, d, J=3Hz), 1.94 (3H, I, s), 2.02 (4H, k, m), 1.71 (1H, c2, q, J=3Hz), 1.58 (2H, d, t, J=3Hz), 1.57 (3H, a, s), 1.42 (1H, c1, q, J=3Hz), 0.94 (3H, e, s), 0.92 (3H, f, s), 0.90 (3H, l, t, J=3Hz). | 263 (M+, 89), 248 (17), 207 (4), 206 (5), 193 (89), 178 (48), 160 (8), 150 (49), 136 (28), 134 (100), 119 (22), 117 (13), 107 (35), 105 (20), 93 (83), 91 (60), 85 (7), 83 (12), 79 (25), 77 (29). |
| 5(II) | C4H9 | 0.41 | 6.83 (1H, h, d, J=8 Hz) 5.68 (1H, g, dd, J=3Hz ), 5.37 (1H, b, s), 4.12 (2H, j, t, J=3 Hz), 2.01 (4H, k, m), 2.00 (1H, m, d, J=3 Hz), 1.97 (3H, I, s), 1.71 (1H, c2, q, J=3 Hz), 1.58 (2H, d, t, J=3 Hz), 1.58 (3H, a, s), 1.45 (1H, c1, q, J=3 Hz), 1.26 (3H, e, s), 1.26 (3H, f, s), 0.91 (3H, l, t, J=3 Hz). | 263 (M+, 7), 207 (2), 193 (5), 178 (4), 150 (5), 136 (3), 134 (10), 119 (5), 117 (4), 107 (5), 105 (3), 93 (17), 91 (14), 85 (92), 83 (100), 79 (5), 77 (7). |
| 6(I) | CH2CH(CH3)2 | 0.76 | 6.10 (1H, h, d, J=8Hz), 5.87 (1H, g, dd, J=3Hz), 5.4 (1H, b, s), 3.8 (2H, j, d, J=3Hz), 2.23 (1H, m, d, J=3Hz), 1.94 (3H, I, s), 2.02 (1H, k, m), 1.71 (1H, c2, q, J=3Hz), 1.58 (2H, d, t, J=3Hz), 1.57 (3H, a, s), 1.42(1H, c1, q, J=3Hz), 0.94(3H, e, s), 0.92(3H, f, s), 0.90 (6H, l, d, J=3Hz). | 263 (M+, 89), 248 (17), 207 (4), 206 (5), 193 (89), 178 (48), 160 (8), 150 (49), 136 (28), 134 (100), 119 (22), 117 (13), 107 (35), 105 (20), 93 (83), 91 (60), 85 (7), 83 (12), 79 (25), 77 (29). |
| 6(II) | CH2CH(CH3)2 | 0.44 | 6.8 (1H, h, d, J=8 Hz) 5.90 (1H, g, dd, J=3Hz ), 5.45 (1H, b, s), 3.84 (2H, j, d, J=3 Hz), 2.01 (1H, k, m), 2.00 (1H, m, d, J=3 Hz), 1.97 (3H, i, s), 1.71 (1H, c2, q, J=3 Hz), 1.58(2H, d, t, J=3 Hz), 1.58 (3H, a, s), 1.45 (1H, c1, q, J=3 Hz), 1.26 (3H, e, s), 1.26 (3H, f, s), 0.91 (6H, l, d, J=3 Hz). | 263 (M+, 7%), 207 (2%), 193 (5%), 178 (4%), 150 (5%), 136 (3%), 134 (10%), 119 (5%), 117 (4%), 107 (5%), 105 (3%), 93 (17%), 91 (14%), 85 (92%), 83 (100%), 79 (5%), 77 (7%). |
| 7(I) | C5H11 | 0.83 | 6.23 (1H, h, d, J=8Hz), 5.84 (1H, g, dd, J=3Hz), 5.42 (1H, b, s), 4.0 (2H, j, t, J= 3Hz), 2.23 (1H, m, d, J=3Hz), 1.94 (3H, i, s), 2.02 (6H, k, m), 1.71 (1H, c2, q, J=3Hz), 1.58 (2H, d, t, J =3Hz), 1.57 (3H, a, s), 1.42 (1H, c1, q, J=3Hz), 0.94 (3H, e, s), 0.92 (3H, f, s), 0.90 (3H, l, t, J=3Hz). | 277 (M+, 89), 262 (17), 207 (4), 206 (5), 193 (89), 178 (48), 160 (8), 150 (49), 136 (28), 134 (100), 119 (22), 117 (13), 107 (35), 105 (20), 93 (83), 91 (60), 85 (7), 83 (12), 79 (25), 77 (29). |
| 7(II) | C5H11 | 0.47 | 6.96 (1H, h, d, J=8 Hz) 5.72 (1H, g, dd, J=3Hz ), 5.39 (1H, b, s), 4.08 (2H, j, t, J=3 Hz), 2.01 (6H, k, m), 2.00 (1H, m, d, J=3 Hz), 1.97 (3H, i, s), 1.71 (1H, c2, q, J=3 Hz), 1.58 (2H, d, t, J=3 Hz), 1.58 (3H, a, s), 1.45 (1H, c1, q, J=3 Hz), 1.26 (3H, e, s), 1.26 (3H, f, s), 0.91 (3H, l, t, J=3 Hz). | 277 (M+, 7), 207 (2), 193 (5), 178 (4), 150 (5), 136 (3), 134 (10), 119 (5), 117 (4), 107 (5), 105 (3), 93 (17), 91 (14), 85 (92), 83 (100), 79 (5), 77 (7). |
| 8(I) | C2H4CH(CH3)2 | 0.83 | 6.23 (1H, h, d, J=8Hz), 5.84 (1H, g, dd, J=3Hz), 5.42 (1H, b, s), 4.0 (2H, j, t, J=3Hz), 2.23 (1H, m, d, J=3Hz), 1.94 (3H, i, s), 2.02 (3H, k, m), 1.71 (1H, c2, q, J=3Hz), 1.58(2H, d, t, J= 3Hz), 1.57 (3H, a, s), 1.42 (1H, c1, q, J=3Hz), 0.94 (3H, e, s), 0.92 (3H, f, s), 0.90 (6H, l, d, J=3Hz). | 277 (M+, 89), 262 (17), 207 (4), 206 (5), 193 (89), 178 (48), 160 (8), 150 (49), 136 (28), 134 (100), 119 (22), 117 (13), 107 (35), 105 (20), 93 (83), 91 (60), 85 (7), 83 (12%), 79 (25), 77 (29). |
| 8(II) | C2H4CH(CH3)2 | 0.47 | 6.96 (1H, h, d, J=8 Hz) 5.72 (1H, g, dd, J=3Hz ), 5.39 (1H, b, s), 4.08 (2H, j, t, J=3 Hz), 2.01 (3H, k, m), 2.00 (1H, m, d, J=3 Hz), 1.97 (3H, i, s), 1.71 (1H, c2, q, J=3 Hz), 1.58 (2H, d, t, J=3 Hz), 1.58 (3H, a, s), 1.45 (1H, c1, q, J=3 Hz), 1.26 (3H, e, s), 1.26 (3H, f, s), 0.91 (6H, l, d, J=3 Hz). | 277 (M+, 7), 207 (2), 193 (5), 178 (4), 150 (5), 136 (3), 134 (10), 119 (5), 117 (4), 107 (5), 105 (3), 93 (17), 91 (14), 85 (92), 83 (100%), 79 (5), 77 (7). |
| 9(I) | C6H13 | 0.86 | 6.13 (1H, h, d, J=8Hz), 5.78 (1H, g, dd, J=3Hz), 5.43 (1H, b, s), 4.0 (2H, j, t, J=3Hz), 2.23 (1H, m, d, J=3Hz), 1.94 (3H, i, s), 2.02 (8H, k, m), 1.71 (1H, c2, q, J=3Hz), 1.58 (2H, d, t, J=3Hz), 1.57 (3H, a, s), 1.42 (1H, c1, q, J=3Hz), 0.94 (3H, e, s), 0.92 (3H, f, s), 0.90 (3H, l, t, J=3Hz). | 291 (M+, 89), 276 (17), 207 (4), 206 (5), 193 (89), 178 (48), 160 (8), 150 (49), 136 (28), 134 (100), 119 (22), 117 (13), 107 (35), 105 (20), 93 (83), 91 (60), 85 (7), 83 (12), 79 (25), 77 (29). |
| 9(II) | C6H13 | 0.54 | 6.65 (1H, h, d, J=8 Hz) 5.90 (1H, g, dd, J=3Hz ), 5.45 (1H, b, s), 4.08 (2H, j, t, J=3 Hz), 2.01 (8H, k, m), 2.00 (1H, m, d, J=3 Hz), 1.97 (3H, i, s), 1.71 (1H, c2, q, J=3 Hz), 1.58 (2H, d, t, J=3 Hz), 1.58 (3H, a, s), 1.45 (1H, c1, q, J=3 Hz), 1.26 (3H, e, s), 1.26 (3H, f, s), 0.91 (3H, l, t, J=3 Hz). | 291 (M+, 7), 207 (2), 193 (5), 178 (4), 150 (5), 136 (3), 134 (10), 119 (5), 117 (4), 107 (5), 105 (3), 93 (17), 91 (14), 85 (92), 83 (100), 79 (5), 77 (7). |
| 10(I) | C7H15 | 0.86 | 6.10 (1H, h, d, J=8Hz), 5.87 (1H, g, dd, J=3Hz), 5.43 (1H, b, s), 4.0 (2H, j, t, J=3Hz), 2.23 (1H, m, d, J=3Hz), 1.94 (3H, i, s), 2.02 (10H, k, m), 1.71 (1H, c2, q, J=3Hz), 1.58 (2H, d, t, J=3Hz), 1.57 (3H, a, s), 1.42 (1H, c1, q, J=3Hz), 0.94 (3H, e, s), 0.92 (3H, f, s), 0.90 (3H, l, t, J=3 Hz). | 305 (M+, 89), 290 (17), 207 (4), 206 (5), 193 (89), 178 (48), 160 (8), 150 (49), 136 (28), 134 (100), 119 (22), 117 (13), 107 (35), 105 (20), 93 (83), 91 (60), 85 (7), 83 (12), 79 (25), 77 (29). |
| 10(II) | C7H15 | 0.56 | 6.81 (1H, h, d, J=8 Hz) 5.90 (1H, g, dd, J=3Hz ), 5.4 (1H, b, s), 4.0 (2H, j, t, J=3 Hz), 2.01 (10H, k, m), 2.00 (1H, m, d, J=3 Hz), 1.97 (3H, i, s), 1.71 (1H, c2, q, J=3 Hz), 1.58 (2H, d, t, J=3 Hz), 1.58 (3H, a, s), 1.45 (1H, c1, q, J=3 Hz), 1.26 (3H, e, s), 1.26 (3H, f, s), 0.91 (3H, l, t, J=3 Hz). | 305 (M+, 7), 207 (2), 193 (5), 178 (4), 150 (5), 136 (3), 134 (10), 119 (5), 117 (4), 107 (5), 105 (3), 93 (17), 91 (14), 85 (92), 83 (100), 79 (5), 77 (7). |
| 11(I) | C8H17 | 0.87 | 6.16 (1H, h, d, J=8 Hz), 5.80 (1H, g, dd, J=3 Hz), 5.43 (1H, b, s), 4.0 (2H, j, t, J=3 Hz), 2.23 (1H, m, d, J=3Hz), 1.94 (3H, i, s), 2.02 (12H, k, m), 1.71 (1H, c2, q, J=3 Hz), 1.58 (2H, d, t, J=3 Hz), 1.57 (3H, a, s), 1.42 (1H, c1, q, J=3 Hz), 0.94 (3H, e, s), 0.92 (3H, f, s), 0.90 (3H, l, t, J=3 Hz). | 319 (M+, 89), 304 (17), 207 (4), 206 (5), 193 (89), 178 (48), 160 (8), 150 (49), 136 (28), 134 (100), 119 (22), 117 (13), 107 (35), 105 (20), 93 (83), 91 (60), 85 (7), 83 (12), 79 (25), 77 (29). |
| 11(II) | C8H17 | 0.57 | 6.75 (1H, h, d, J=8 Hz) 5.73 (1H, g, dd, J=3Hz ), 5.4 (1H, b, s), 4.0 (2H, j, t, J=3Hz), 2.01 (12H, k, m), 2.00 (1H, m, d, J=3 Hz), 1.97 (3H, i, s), 1.71 (1H, c2, q, J=3 Hz), 1.58 (2H, d, t, J=3 Hz), 1.58 (3H, a, s), 1.45 (1H, c1, q, J=3 Hz), 1.26 (3H, e, s), 1.26 (3H, f, s), 0.91 (3H, l, t, J=3 Hz). | 319 (M+, 7), 207 (2), 193 (5), 178 (4), 150 (5), 136 (3), 134 (10), 119 (5), 117 (4), 107 (5), 105 (3), 93 (17), 91 (14), 85 (92), 83 (100), 79 (5), 77 (7). |
| 12(I) | C10H21 | 0.88 | 6.13 (1H, h, d, J=8 Hz), 5.87 (1H, g, dd, J=3 Hz), 5.5 (1H, b, s), 4.0 (2H, j, t, J= 3 Hz), 2.23 (1H, m, d, J=3 Hz), 1.94 (3H, i, s), 2.02 (16H, k, m), 1.71 (1H, c2, q, J=3 Hz), 1.58 (2H, d, t, J=3 Hz), 1.57 (3H, a, s), 1.42 (1H, c1, q, J=3 Hz), 0.94 (3H, e, s), 0.92 (3H, f, s), 0.90 (3H, l, t, J=3 Hz). | 347 (M+, 89), 332 (17), 207 (4), 206 (5), 193 (89), 178 (48), 160 (8), 150 (49), 136 (28), 134 (100), 119 (22), 117 (13), 107 (35), 105 (20), 93 (83), 91 (60), 85 (7), 83 (12), 79 (25), 77 (29). |
| 12(II) | C10H21 | 0.60 | 7.0 (1H, h, d, J=8 Hz) 5.8 (1H, g, dd, J= 3 Hz), 5.45 (1H, b, s), 4.0 (2H, j, t, J=3 Hz), 2.01 (16H, k, m), 2.00 (1H, m, d, J=3 Hz), 1.97 (3H, i, s), 1.71 (1H, c2, q, J=3 Hz), 1.58(2H, d, t, J=3 Hz), 1.58 (3H, a, s), 1.45 (1H, c1, q, J=3 Hz), 1.26 (3H, e, s), 1.26 (3H, f, s), 0.91 (3H, l, t, J=3 Hz). | 347 (M+, 7), 207 (2), 193 (5), 178 (4), 150 (5), 136 (3), 134 (10), 119 (5), 117 (4), 107 (5), 105 (3), 93 (17), 91 (14), 85 (92), 83 (100), 79 (5), 77 (7). |
References
- Henrick, C.A.; Staal, G.B.; Siddall, J.B. Alkyl 3,7,11-trimethyl-2, 4-dodecadieoates, A new class of potent insect growth regulators with juvenile hormone activity. J. Agric. Food. Chem. 1973, 21, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Wigglesworth, V. B. Insect Hormones; W.H. Freeman: San Francisco, 1970; pp. 1–159. [Google Scholar]
- Bowers, W.S. Naturally Occurring Insecticides; Jacobson, M., Crosby, D. G., Eds.; Marcel Dekker: New York, 1971; p. 307. [Google Scholar]
- Pawar, P.V.; Tungikar, B. V.; Sharma, R.N.; Suresh, S.; Padalkar, S. N.; Patwardhan, S. A. Pesticide Res. Jour. 1995, 7, 128–131.
- Benson, W.R.; Pohland, A.E. J. Org. Chem 1965, 30, 1129–1132.
- Sample availability: Available on request from the corresponding author.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Banerjee, T.; Dureja, P. Synthesis of 4'-(2,6,6-Trimethyl -2-Cyclohexen-1-yl) -3'-Buten-2'-Ketoxime-N-O-Alkyl Ethers. Molecules 2005, 10, 990-999. https://doi.org/10.3390/10080990
Banerjee T, Dureja P. Synthesis of 4'-(2,6,6-Trimethyl -2-Cyclohexen-1-yl) -3'-Buten-2'-Ketoxime-N-O-Alkyl Ethers. Molecules. 2005; 10(8):990-999. https://doi.org/10.3390/10080990
Chicago/Turabian StyleBanerjee, T., and P. Dureja. 2005. "Synthesis of 4'-(2,6,6-Trimethyl -2-Cyclohexen-1-yl) -3'-Buten-2'-Ketoxime-N-O-Alkyl Ethers" Molecules 10, no. 8: 990-999. https://doi.org/10.3390/10080990




