Preparation of an Eight-Membered Sesquiterpene Lactone Resulting from Sequential Gif System GoAggIII and MCPBA Oxidation of ( )-10β,14-Dihydroxy-allo-aromadendrane
Abstract
:Introduction
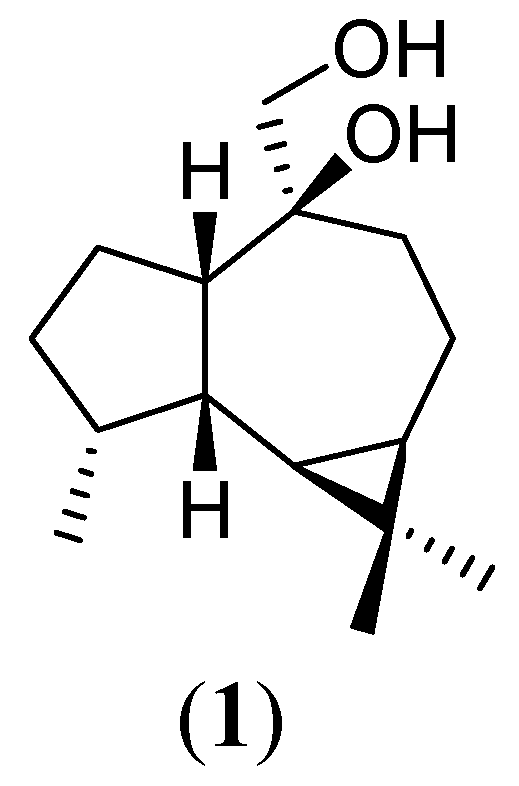
Results and Discussion
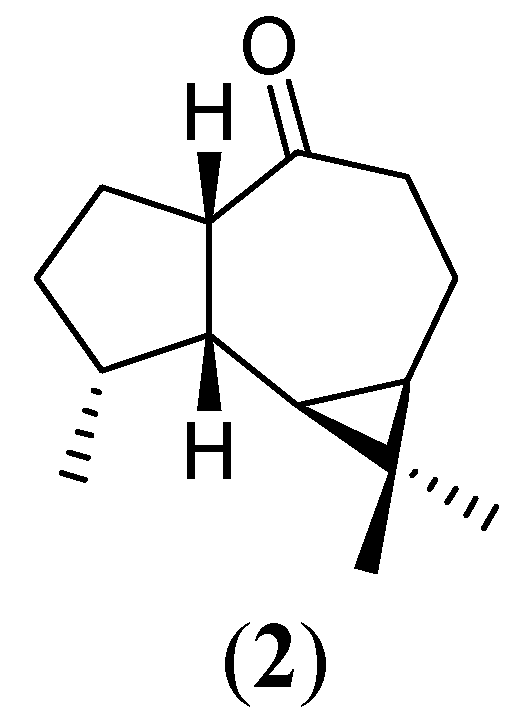
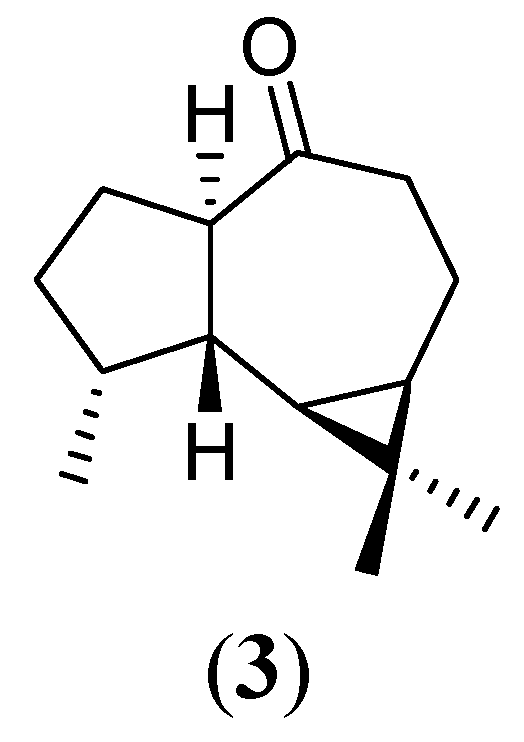
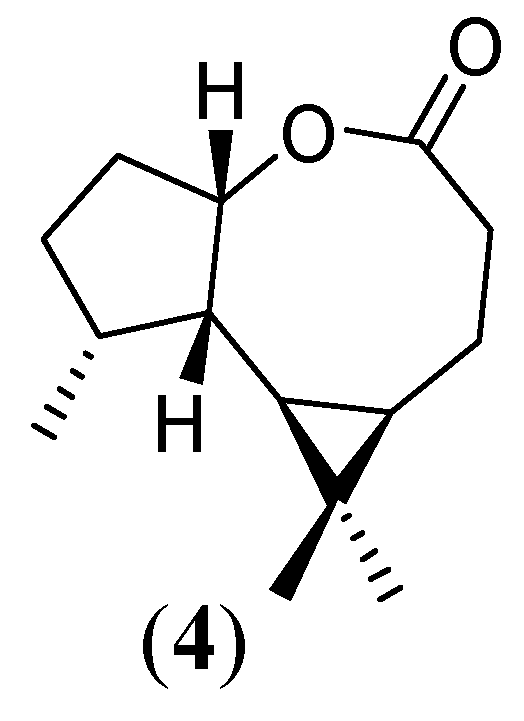
Experimental
General.
Typical procedure for the oxidation by the GoAggIII system.
Reaction with CHMO.
Reaction with MCPBA.
Acknowledgments
References
- San Feliciano, A.; Medarde, M.; Gordaliza, M.; Del Olmo, E.; Del Corral, J. M. M. Tetrahedron 1989, 28, 2717–2721.
- De Siqueira, J. M.; De Oliveira, C. D.; Boaventrua, M. A. D. Fitoterapia 1997, LXVIII, 89–90.
- De Lima, D. P.; Beatriz, A.; Ramos, A. A.; De Siqueira, J. M.; De Oliveira, C. C.; Marques, M. R. Quím. Nova 1997, 20, 616–620.
- De Lima, D. P.; Carnell, A. J.; Roberts, S. M. J. Chem. Soc. Res. (S) 1999, 396–397.
- Blanco, M.; De Lima, D. P.; Maia, G. J. Electroanal. Chem. 2001, 512, 49–55.
- Vizzotto, L.; Muzzi, R. M.; De Lima, D. P. Mag. Res. Chem. 2003, 41, 1034–1037.
- Barton, D. H. R.; Hay-Motherwell, R. S.; Motherwell, W. B. Tetrahedron Lett. 1983, 24, 1979–1982.
- Barton, D. H. R.; Doller, D. Acc. Chem. Res. 1992, 25, 504–512.
- Barton, D. H. R.; Hu, B. Tetrahedron 1996, 52, 10313–10326.
- Barton, D. H. R.; Csuhai, E.; Doller, D.; Geletti, Y. V. Tetrahedron 1991, 47, 6561–6570.
- Doller, D.; Chackalamannil, S.; Stamford, A.; McKittrick, B.; Czarniecki, M. Bioorg. Med. Chem. Lett. 1997, 7, 1381–1386.
- Roberts, A. U.; Wan, P. W. H. J. Mol. Cat. B: Enzymatic 1998, 4, 111–136.
- Faber, K. Biotransformations in Organic Chemistry; Springer: Berlin, 1997; pp. 224–225. [Google Scholar]
- Sheng, D.; Ballou, D. P.; Massey, V. Biochemistry 2001, 40, 11156–11167. [PubMed]
- Massey, V. J. Biol. Chem. 1994, 22, 459–462.
- Chi-Huey, W.; Whitesides, G. M. Enzymes in Synthetic Organic Chemistry; Pergamon: Oxford, 1995; p. 169. [Google Scholar]
- Barton, D. H. R.; Bévière, S. D.; Chabot, B. M.; Chavasiri, W.; Taylor, D. K. Tetrahedron Lett. 1994, 35, 4681–4684.
- Lamers, Y. M. A. W. PhD. Thesis, Wageningen University, Wageningen, The Netherlands, 2003.
- Gijsen, H. J. M. PhD. Thesis, Wageningen University, Wageningen, The Netherlands, 1993.
- Sample Availability: Available from the authors.
© 2005 by MDPI (http:www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
De Bodas, M.; Marques, M.; Beatriz, A.; De Lima, D. Preparation of an Eight-Membered Sesquiterpene Lactone Resulting from Sequential Gif System GoAggIII and MCPBA Oxidation of ( )-10β,14-Dihydroxy-allo-aromadendrane. Molecules 2005, 10, 1010-1014. https://doi.org/10.3390/10081010
De Bodas M, Marques M, Beatriz A, De Lima D. Preparation of an Eight-Membered Sesquiterpene Lactone Resulting from Sequential Gif System GoAggIII and MCPBA Oxidation of ( )-10β,14-Dihydroxy-allo-aromadendrane. Molecules. 2005; 10(8):1010-1014. https://doi.org/10.3390/10081010
Chicago/Turabian StyleDe Bodas, M., M. Marques, A. Beatriz, and D. De Lima. 2005. "Preparation of an Eight-Membered Sesquiterpene Lactone Resulting from Sequential Gif System GoAggIII and MCPBA Oxidation of ( )-10β,14-Dihydroxy-allo-aromadendrane" Molecules 10, no. 8: 1010-1014. https://doi.org/10.3390/10081010



