Synthesis of Some Phenylpropanoid Monoglycerides via the Mitsunobu Protocol
Abstract
:Introduction
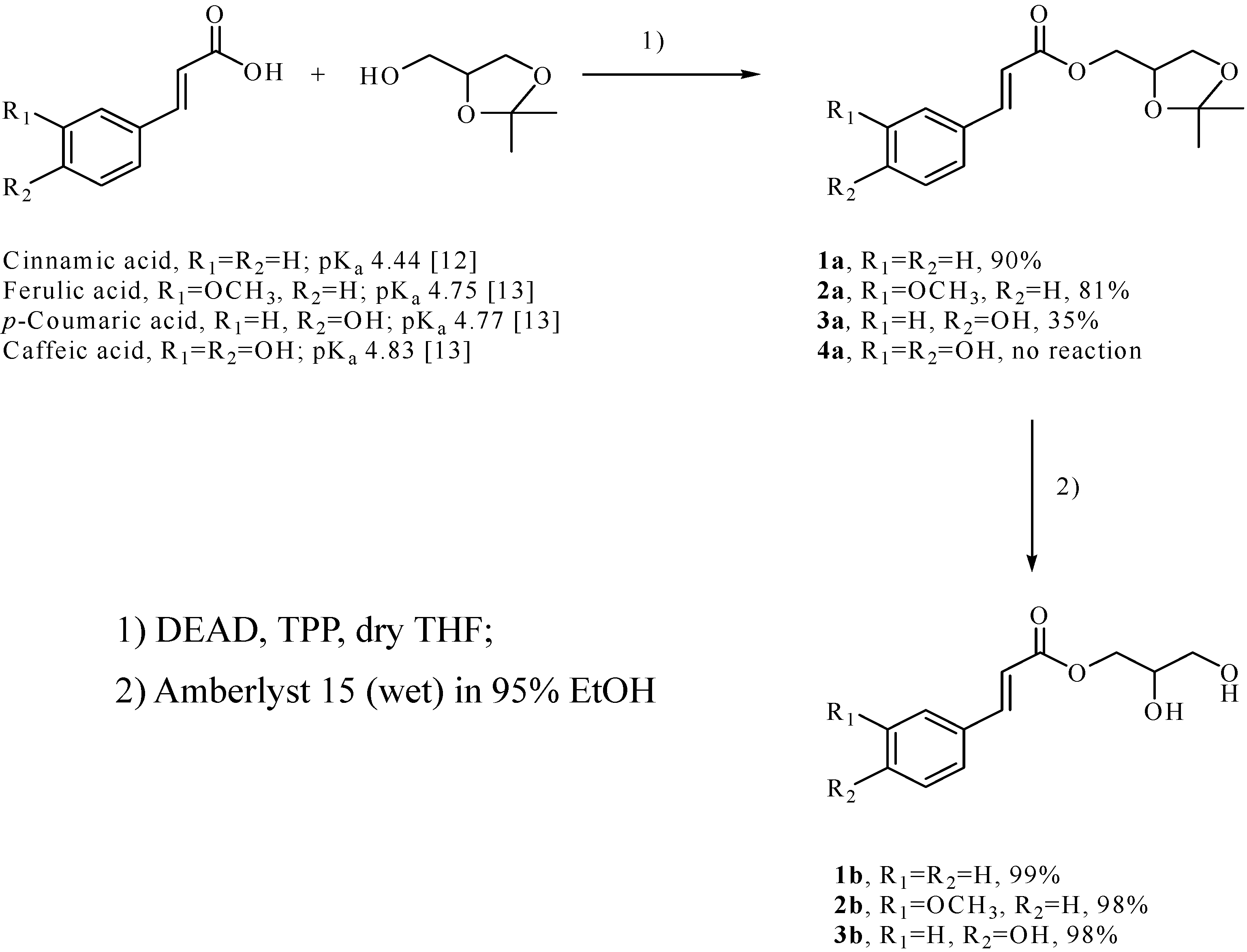
Results and Discussion

Conclusions
Experimental
General
Synthesis of the isopropylidene derivatives of the phenolic acids 1a-3a and octyl caffeate 5 via the Mitsunobu protocol
Deprotection of the isopropylidene derivatives to the monoglycerides 1b-3b [11].
Acknowledgements
References
- Bankova, V.; Popova, M.; Bogdanov, St.; Sabatini, A.-G. Chemical composition of European propolis: expected and unexpected results. Z. Naturforsch. 2002, 57c, 530–533. [Google Scholar]
- Banskota, A.H.; Nagaoka, T.; Sumioka, L.Y.; Tezuka, Y.; Awale, S.; Midorikawa, K.; Matsushige, K.; Kadota, S. Antiproliferative activity of the Netherlands propolis and its active principles in ccancer cell lines. J. Etnopharmacol. 2000, 80, 67–73. [Google Scholar]
- Shimomura, H.; Sashida, Y.; Mimaki, Y. Phenolic glycerides from Lilium auratum. Phytochemistry 1987, 26, 844–845. [Google Scholar]
- Dellagreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Sorrentino, M. Antialgal phenylpropane glycerides from Juncus effusus. Nat. Prod. Lett. 1998, 12, 263–270. [Google Scholar]
- Fenz, R.; Ludger, E.; Galensa, R. Phenolic acids and their glycerides in maize grits. Z. Lebensmitt.-Untersuch. Forsch. 1992, 194, 252–8. [Google Scholar]
- Gunasekera, S.P.; Kinghorn, A.D.; Cordell, G.A.; Farnsworth, N. R. Plant anticancer agents. XIX Constituents of Aquilaria malaccensis. J. Nat. Prod. 1981, 44, 569–72. [Google Scholar]
- Laszlo, J.A.; Compton, D.L.; Eller, F.J.; Taylor, S.L.; Isbell, T.A. Packed-bed bioreactor synthesis of feruloylated monoacyl- and diacylglycerols: clean production of a ‘green’ sunscreen. Green Chem. 2003, 5, 382–386. [Google Scholar]
- Armesto, N.; Ferrero, M.; Fernandez, S.; Gotor, V. Novel enzymatic synthesis of 4-O-cinnamoyl quinic and shikimic acid derivatives. J. Org. Chem. 2003, 68, 5784–5787. [Google Scholar]
- Fureby, A. M.; Adlercreutz, P.; Mattiasson, B. Ann. N. Y. Acad. Sci. 1996, 799, 231–237.
- Appendino, G.; Minassi, A.; Daddario, N.; Bianchi, F.; Tron, G. C. Chemoselective esterification of phenolic acids and alcohols. Org. Lett. 2002, 4, 3839–3841. [Google Scholar]
- Yu, C.C.; Lee, Y.-S.; Cheon, B.S.; Lee, S.H. Synthesis of glycerol monostearate with high purity. Bull. Kor. Chem. Soc. 2003, 24, 1229–1231. [Google Scholar]
- http://www.csudh.edu/oliver/chemdata/data-ka.htm.
- Beltran, J.L.; Sanli, N.; Fonrodona, G.; Barron, D.; Özkan, G.; Barbosa, J. Spectrophotometric, potentiometric and chromatographic pKa values of polyphenolic acids in water and acetonitrile-water media. Anal. Chim. Acta 2003, 484, 253–264. [Google Scholar]
- Dodge, J.A.; Trujillo, J.I.; Presnell, M. Effect of the acidic component on the Mitsunobu inversion of a sterically hindered alcohol. J. Org. Chem. 1994, 59, 234–236. [Google Scholar]
- Hughes, D.L.; Reamer, R A. The effect of acid strength on the Mitsunobu esterification reaction: carboxyl vs hydroxyl reactivity. J. Org. Chem. 1996, 61, 2967–2971. [Google Scholar]
- Camp, D.; Jenkins, I.D. Mechanism of the Mitsunobu esterification reaction. 1. The involvement of phosphoranes and oxyphosphonium salts. J. Org. Chem. 1989, 54, 3045–3049. [Google Scholar]
- Hughes, D.L.; Reamer, R.A.; Bergan, J.J.; Grabowski, E.J.J. A mechanistic study of the Misunobu esterification reaction. J. Am. Chem. Soc. 1988, 110, 6487–6491. [Google Scholar]
- Thewlis, B.H. New synthetic antioxidants based on caffeic acid. J. Food Technol. 1967, 2, 83–7. [Google Scholar]
- Sample availability: Contact the authors.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Batovska, D.I.; Kishimoto, T.; Bankova, V.S.; Kamenarska, Z.G.; Ubukata, M. Synthesis of Some Phenylpropanoid Monoglycerides via the Mitsunobu Protocol. Molecules 2005, 10, 552-558. https://doi.org/10.3390/10030552
Batovska DI, Kishimoto T, Bankova VS, Kamenarska ZG, Ubukata M. Synthesis of Some Phenylpropanoid Monoglycerides via the Mitsunobu Protocol. Molecules. 2005; 10(3):552-558. https://doi.org/10.3390/10030552
Chicago/Turabian StyleBatovska, Daniela I., Takao Kishimoto, Vassya S. Bankova, Zornitsa G. Kamenarska, and Makoto Ubukata. 2005. "Synthesis of Some Phenylpropanoid Monoglycerides via the Mitsunobu Protocol" Molecules 10, no. 3: 552-558. https://doi.org/10.3390/10030552



