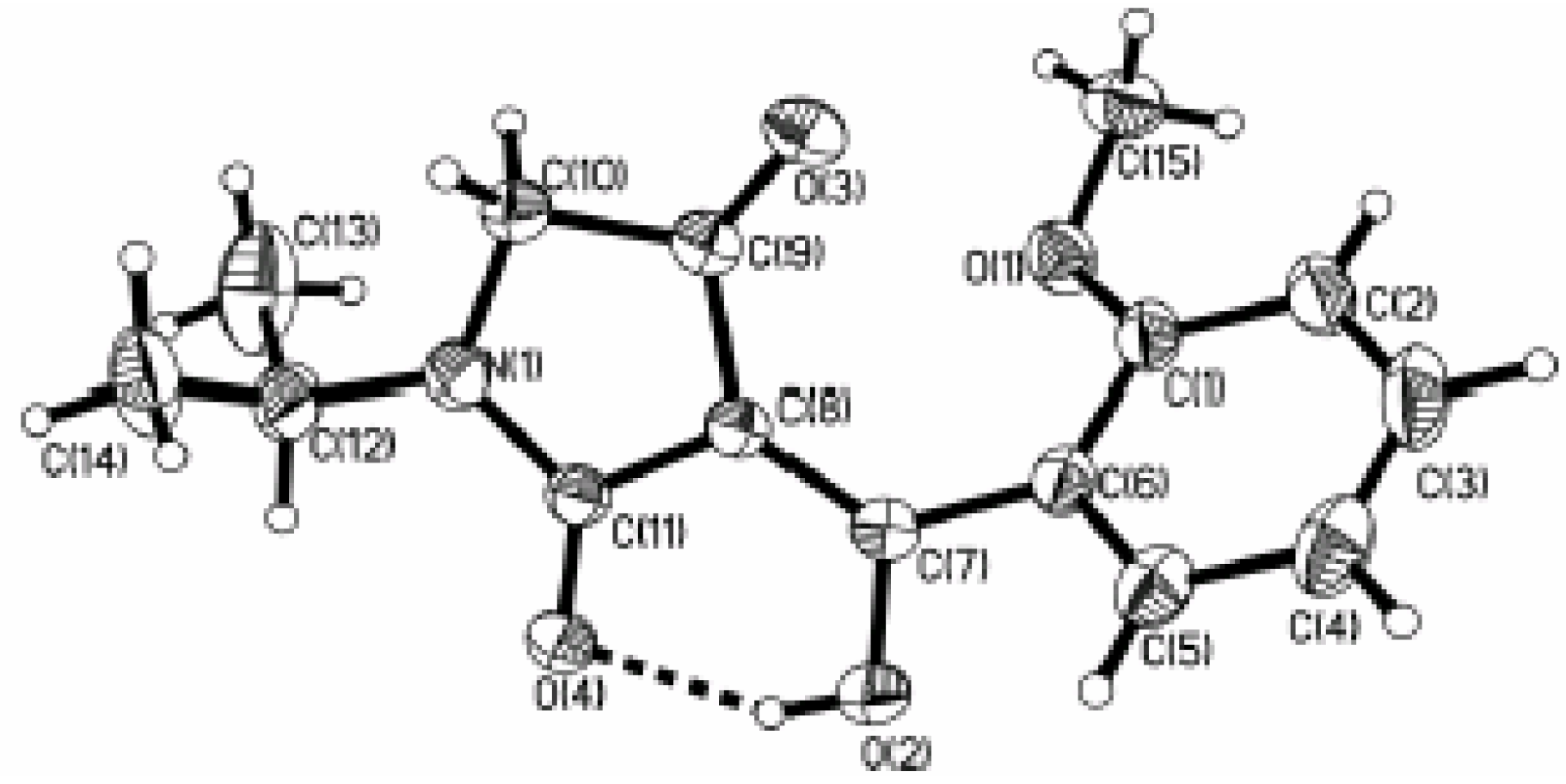
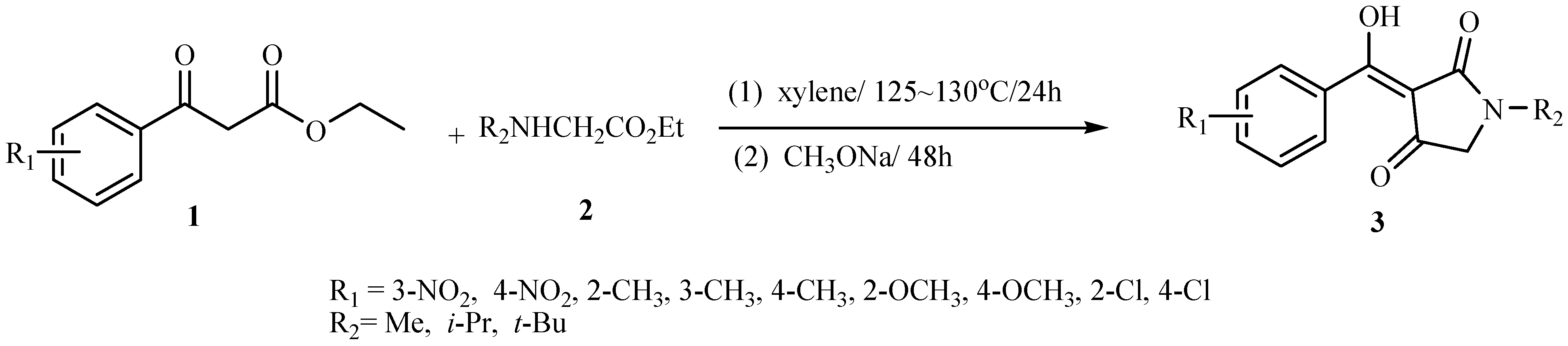General procedure for the synthesis of compounds 3a~3w: Preparation of 1-methyl-3-(α-hydroxy-3’-nitrobenzylidene)pyrrolidine-2,4-dione (3a)
A mixture of 1 (R1=3-NO2, 4.11 mmol) and ethyl benzylamino acetate (2, R2= Me) (4.30 mmol) in dry xylene (15 mL) was heated at 125-130 ºC with stirring for 20 hours. The cooled solution was added to methanolic CH3ONa, prepared from Na metal (0.10 g, 4.35 mmol) and methanol (10mL) at room temperature with stirring. After the above mixture was stirred at room temperature for 48 hours, water (30 mL) was added to the reaction mixture and the organic layer was separated and extracted twice with water. The original water layer and the extracts were combined and acidified to pH 2-3 with 2N HCl under cooling. The acidic solution was extracted three times with chloroform (30 mL) and the extracts were washed with saturated brine and then dried over Na2SO4. The solvent was removed under reduced pressure to give crude product 3a, which was purified by flash column chromatography on silica gel, using 1:1 (v:v) ethyl acetate-petroleum ether as the eluent to afford the target product as white crystals (1.0g, 92.8%) with m.p. 145~146 ºC; IR (KBr) cm-1 3443(O-H), 1678, 1575, 1520(C=O); 1H-NMR δ: 3.06 (s, 3H, N-CH3), 3.78 (s, 2H, C-CH2-N), 8.22-8.33 (m, 4H, C6H4); Anal. Calc. for C12H10N2O5 (262.21) C 54.96, H 3.84, N 10.68; Found: C 55.01, H 3.89, N 10.51. The twenty two other title compounds 3b~3x were synthesized in the same manner.
1-methyl-3-(α-hydroxy-4’-nitrobenzylidene)pyrrolidine-2,4-dione (3b): Yield 67.0%; m.p. 167~169ºC; IR (KBr) cm-1 3450(O-H), 1690, 1585, 1520 (C=O); 1H-NMR δ: 3.06 (s, 3H, N-CH3), 3.78 (s, 2H, C-CH2-N), 8.22-8.33(m, 4H, C6H4); Anal. Calc. for C12H10N2O5 (262.21) C 54.96, H 3.84, N 10.68; Found: C 54.83, H 3.99, N 10.72.
1-methyl-3-(α-hydroxy-4’-chlorobenzylidene)pyrrolidine-2,4-dione (3c). Yield 57.3%; m.p. 104~105 ºC; IR (KBr) cm-1 3463(O-H), 1712, 1670,1598(C=O); 1H-NMR δ: 3.10 (s, 3H, NCH3), 3.81 (s, 2H, CCH2N), 7.46, 7.49, 8.21, 8.24 (d-d, 4H, J=9.04 Hz, C6H4); Anal. Calc. for C12H10ClNO3 (251.66) C 57.27, H 4.00, N 5.57; Found C 57.21, H 4.07, N 5.57.
1-methyl-3-(α-hydroxy-2’-methylbenzylidene)pyrrolidine-2,4-dione (3d). Yield 52.2%; yellow liquid; IR (KBr) cm-1 3422 (O-H), 1719, 1657, 1620 (C=O); 1H-NMR δ: 2.32 (s, 3H, ArCH3), 3.02 (s, 3H, N‑CH3), 3.67 (s, 2H, CCH2N), 7.13-7.42 (m, 4H, C6H4); Anal. Calc. for C13H13NO3 (231.24) C 67.52, H 5.67, N 6.06; Found C 67.42, H 5.51, N 6.01.
1-methyl-3-(α-hydroxy-3’-methylbenzylidene)pyrrolidine-2,4-dione (3e). Yield 86.5%; red liquid; IR (KBr) cm-1 3435 (O-H), 1720, 1654, 1621 (C=O); 1H-NMR δ: 2.36 (s, 3H, ArCH3), 3.02 (s, 3H, N‑CH3), 3.72 (s, 2H, CCH2N), 7.23-7.39, 7.83-8.03 (m, 4H, C6H4); Anal. Calc. for C13H13NO3 (231.24) C 67.52, H 5.67, N 6.06; Found C 67.60, H 5.59, N 6.05.
1-methyl-3-(α-hydroxy-4’-methoxybenzylidene)pyrrolidine-2,4-dione (3f). Yield 62.9%; m.p. 96~97 ºC; IR (KBr) cm-1 3419 (O-H), 1717, 1652, 1616 (C=O); 1H-NMR δ: 3.00 (s, 3H, NCH3), 3.71 (s, 2H, CCH2N), 3.81 (s, 3H, OCH3), 6.88, 6.99, 8.28, 8.31 (d-d, 4H, J=9.04 Hz, C6H4); Anal. Calc. for C13H13NO4 (247.24) C 63.15, H 5.30, N 5.67; Found C 63.14, H 5.43, N 5.78.
1-i-propyl-3-(α-hydroxy-3’-nitrobenzylidene)pyrrolidine-2,4-dione (3g). Yield 74.8%; m.p. 136~137 ºC; IR(KBr) cm-1 3453 (O-H), 1688, 1595, 1532 (C=O); 1H-NMR δ: 1.21(d, 6H, J=6.78Hz, C(CH3)2), 3.72 (s, 2H, C-CH2-N), 4.38-4.68 (m,1H, J=6.78Hz, CH); 7.51-7.70, 8.28-8.60, 9.02-9.13 (m, 4H, J=8.29 Hz, C6H4); Anal. Calc. for C14H14N2O5 (290.26) C 57.93, H 4.86, N 9.65; Found: C 57.96, H 5.08, N 9.75.
1-i-propyl-3-(α-hydroxy-4’-nitrobenzylidene)pyrrolidine-2,4-dione (3h). Yield 69.2%; m.p. 146~147 ºC; IR (KBr) cm-1 3463 (O-H), 1693, 1610, 1553 (C=O); 1H-NMR δ: 1.22 (d, 6H, J=6.78Hz, C(CH3)2), 3.71 (s, 2H, C-CH2-N), 4.47-4.57 (m,1H, J= 6.78Hz,CH), 7.91-7.94 , 8.23-8.31 (m, 4H, J=9.04 Hz, C6H4); Anal. Calc. for C14H14N2O5 (290.26) C 57.93, H 4.86, N 9.65; Found C 57.94, H 4.73, N 9.65.
1-i-propyl-3-(α-hydroxy-2-chlorobenzylidene)pyrrolidine-2,4-dione (3i). Yield 37.2%; m.p. 103~104 ºC. IR (KBr) cm-1 3418(O-H), 1726,1656 1561(C=O). 1H-NMR δ: 1.27-1.29 (d, 6H, J=6.78Hz, C(CH3)2), 3.71 (s, 2H, CCH2N), 4.50-4.74 (m, 1H, J=6.78Hz, CH), 7.31-7.62 (m, 4H, J=9.04 Hz, C6H4). Anal. Calc. for C14H14ClNO3 (279.71) C 60.11, H 5.04, N 5.01; Found C 60.22, H 5.09, N 5.11.
1-i-propyl-3-(α-hydroxy--4’-chlorobenzylidene)pyrrolidine-2,4-dione (3j). Yield 73.1%; m.p. 134~135 ºC; IR (KBr) cm-1 3405 (O-H), 1694 1587, 1544 (C=O); 1H-NMR δ: 1.27 (d, 6H, J=6.78Hz, C(CH3)2), 3.76 (s, 2H, CCH2N), 4.54-4.66 (m, 1H, J=6.78Hz, CH), 7.46-7.48, 8.21-8.28 (m, 4H, C6H4); Anal. Calc. for C14H14ClNO3 (279.71) C 60.11, H 5.04, N 5.01; Found C 60.22, H 5.09, N 5.11.
1-i-propyl-3-(α-hydroxy-2’-methoxybenzylidene)pyrrolidine-2,4-dione (3k). Yield 79.9%; m.p. 99~100 ºC; IR (KBr) cm-1 3441 (O-H), 1741, 1600, 1559 (C=O); 1H-NMR δ: 1.25 (d, 6H, J=6.78Hz, C(CH3)2), 3.66 (s, 2H, CCH2N), 3.86 (s, 3H, Ar-OCH3), 4.57-4.62 (m, 1H, J=6.78Hz, CH), 7.00-7.06, 7.45-7.53 (m, 4H, C6H4); Anal. Calc. for C15H17NO4 (275.29) C 65.44, H 6.22, N 5.09; Found C 65.45, H 6.27, N 4.98.
1-i-propyl-3-(α-hydroxy-4’-methoxybenzylidene)pyrrolidine-2,4-dione (3l). Yield 85.4%; m.p. 145~146 ºC ; IR (KBr) cm-1 3463 (O-H), 1695, 1590, 1546 (C=O); 1H-NMR δ: 1.17 (d, 6H, J=6.78Hz, C(CH3)2), 3.65 (s, 2H, C-CH2-N), 3.82 (s, 3H, Ar-OCH3), 4.45-4.55 (m,1H, J=6.78Hz, CH), 6.89, 6.92 , 8.27, 8.30 (d-d, 4H, J=9.04Hz, C6H4); Anal. Calc. for C15H17NO4 (275.29) C 65.44, H 6.22, N 5.09; Found C 65.39, H 6.25, N 5.07.
1-i-propyl-3-(α-hydroxy-2’-methylbenzylidene)pyrrolidine-2,4-dione (3m). Yield 90.8%; m.p. 79~80 ºC ; IR (KBr) cm-1 3427 (O-H), 1719, 1646, 1601 (C=O); 1H-NMR δ: 1.37 (d, 6H, J=6.78Hz, C(CH3)2); 2.42 (s, 3H, Ar-CH3), 3.70 (s, 2H, CCH2N), 4.56-4.66 (m, 1H, J=6.78Hz, CH), 7.28-7.49 (m, 4H, C6H4); Anal. Calc. for C15H17NO3 (259.29) C 69.48, H 6.61, N 5.40; Found C 69.56, H 6.58, N 5.35.
1-i-propyl-3-(α-hydroxy-3’-methylbenzylidene)pyrrolidine-2,4-dione (3n). Yield 75.7%; m.p. 64~65 ºC; IR (KBr) cm-1 3477 (O-H), 1745, 1685, 1591 (C=O); 1H-NMR δ: 1.18 (d, 6H, J=6.78Hz, C(CH3)2), 2.24 (s, 3H, Ar-CH3), 3.68 (s, 2H, CCH2N), 4.48-4.55 (m, 1H, J=6.78Hz, CH), 7.19-7.95 (m, 4H, C6H4); Anal. Calc. for C15H17NO3 (259.29) C 69.48, H 6.61, N 5.40; Found C 69.35, H 6.45, N 5.48.
1-i-propyl-3-(α-hydroxy-4’-methylbenzylidene)pyrrolidine-2,4-dione (3o). Yield 85.3%; m.p. 101~102 ºC; IR (KBr) cm-1 3378 (O-H), 1692, 1652, 1590 (C=O); 1H-NMR δ: 1.37 (d, 6H, J=6.78Hz, C(CH3)2), 2.45 (s, 3H, Ar-CH3), 3.74 (s, 2H, CCH2N), 4.57-4.62 (m, 1H, J=6.78Hz, CH), 7.28-7.49 (m, 4H, C6H4); Anal. Calc. for C15H17NO3 (259.29) C 69.48, H 6.61, N 5.40; Found C 69.69, H 6.65, N 5.56.
1-t-butyl-3-(α-hydroxy-3’-nitrobenzylidene)pyrrolidine-2,4-dione (3p). Yield 35.1%; m.p. 168~169ºC. IR (KBr) cm-1 3463(O-H), 1698, 1651,1557(C=O). 1H-NMR δ: 1.55 (s, 9H, C(CH3)3), 3.91 (s, 2H, C-CH2-N), 7.61-7.85, 8.35-8.67, 9.07-9.20 (m, 4H, J=8.29 Hz, C6H4); Anal. Calc. for C15H16N2O5 (304.29) C 59.20, H 5.30, N 9.31; Found C 59.31, H 5.28, N 9.20.
1-t-butyl-3-(α-hydroxy-4’-nitrobenzylidene)pyrrolidine-2,4-dione (3q). Yield 39.6%; m.p. 142~144 ºC; IR (KBr) cm-1 3463 (O-H), 1698, 1651, 1557 (C=O); 1H-NMR δ: 1.46 (s, 9H, C(CH3)3), 3.81 (s, 2H, C-CH2-N), 8.15-8.38 (m, 4H, C6H4); Anal. Calc. for C15H16N2O5 (304.29) C 59.20, H 5.30, N 9.31; Found C 61.50, H 4.85, N 11.28.
1-t-butyl-3-(α-hydroxy--4’-chlorobenzylidene)pyrrolidine-2,4-dione (3r). Yield 73.1%; m.p. 134~135 ºC; IR (KBr) cm-1 3405(O-H), 1694 1587,1544(C=O). 1H-NMR δ : 1.27 (d, 6H, J=6.78Hz, C(CH3)2), 3.76 (s, 2H, CCH2N), 4.54-4.66 (m, 1H, J=6.78Hz, CH), 7.46-7.48, 8.21-8.28 (m, 4H, C6H4); Anal. Calc. for C14H14ClNO3 (279.71) C 60.11, H 5.04, N 5.01; Found C 60.22, H 5.09, N 5.11.
1-t-butyl-3-(α-hydroxy-2’-methoxybenzylidene)pyrrolidine-2,4-dione (3s). Yield 30%; m.p. 66~67 ºC; IR (KBr) cm-1 3381 (O-H), 1748, 1711, 1624 (C=O); 1H-NMR δ: 1.52 (s, 9H, C(CH3)3), 3.88 (s, 3H, Ar-OCH3), 3.78 (s, 2H, C-CH2-N), 6.99-7.06, 7.44-7.48 (m, 4H, J=9.04Hz, C6H4); Anal. Calc. for C16H19NO4 (289.32) C 66.42, H 6.62, N 4.84; Found C 66.35, H 6.57, N 5.01.
1-t-butyl-3-(α-hydroxy-4’-methoxybenzylidene)pyrrolidine-2,4-dione (3t). Yield 40.7%; m.p. 117~118 ºC; IR (KBr) cm-1 3431 (O-H), 1743, 1683, 1594 (C=O); 1H-NMR δ: 1.43 (s, 9H, C(CH3)3), 3.80 (s, 3H, Ar-OCH3), 3.75 (s, 2H, C-CH2-N), 6.88, 6.91, 8.25, 8.28 (d-d, 4H, J=9.04Hz, C6H4); Anal. Calc. for C16H19NO4 (289.32) C 66.42, H 6.62, N 4.84; Found C 66.32, H 6.67, N 5.04.
1-t-butyl-3-(α-hydroxy-2’-methylbenzylidene)pyrrolidine-2,4-dione (3u). Yield 60.4%; m.p. 69~70 ºC; IR (KBr) cm-1 3430 (O-H), 1718, 1651, 1611 (C=O); 1H-NMR δ: 1.45 (s, 3H, NCH3), 2.33 (s, 2H, CCH2N), 3.72 (s, 3H, OCH3), 7.16-7.38 (m, 4H, J=6.028 Hz, C6H4); Anal. Calc. for C16H19NO3 (273.32) C 70.31, H 7.01, N 5.12; Found C 70.16, H 6.98, N 5.08.
1-t-butyl-3-(α-hydroxy-3’-methyl benzylidene)pyrrolidine-2,4-dione (3v). Yield 53.8%; m.p. 137~138 ºC; IR (KBr) cm-1 3412 (O-H), 1708, 1648, 1564 (C=O); 1H-NMR δ: 1.53 (s, 3H, NCH3), 2.44 (s, 2H, CCH2N), 3.84 (s, 3H, OCH3), 7.32-8.04 (m, 4H, C6H4); Anal. Calc. for C16H19NO3 (273.32) C 70.31, H 7.01, N 5.12; Found C 70.32, H 7.08, N 5.15.
1-t-butyl-3-(α-hydroxy-4’-methylbenzylidene)pyrrolidine-2,4-dione (3w). Yield 32.4%; m.p. 124~125 ºC; IR (KBr) cm-1 3378 (O-H), 1709, 1665, 1588 (C=O); 1H-NMR δ: 1.53 (s, 3H, NCH3), 2.44 (s, 2H, CCH2N), 3.84 (s, 3H, OCH3), 7.26-8.14 (m, 4H, C6H4); Anal. Calc. for C16H19NO3 (273.32) C 70.31, H 7.01, N 5.12; Found C 70.30, H 6.99, N 5.15.