Syntheses, Characterization and Study of the Use of Cobalt (II) Schiff–Base Complexes as Catalysts for the Oxidation of Styrene by Molecular Oxygen
Abstract
:Introduction
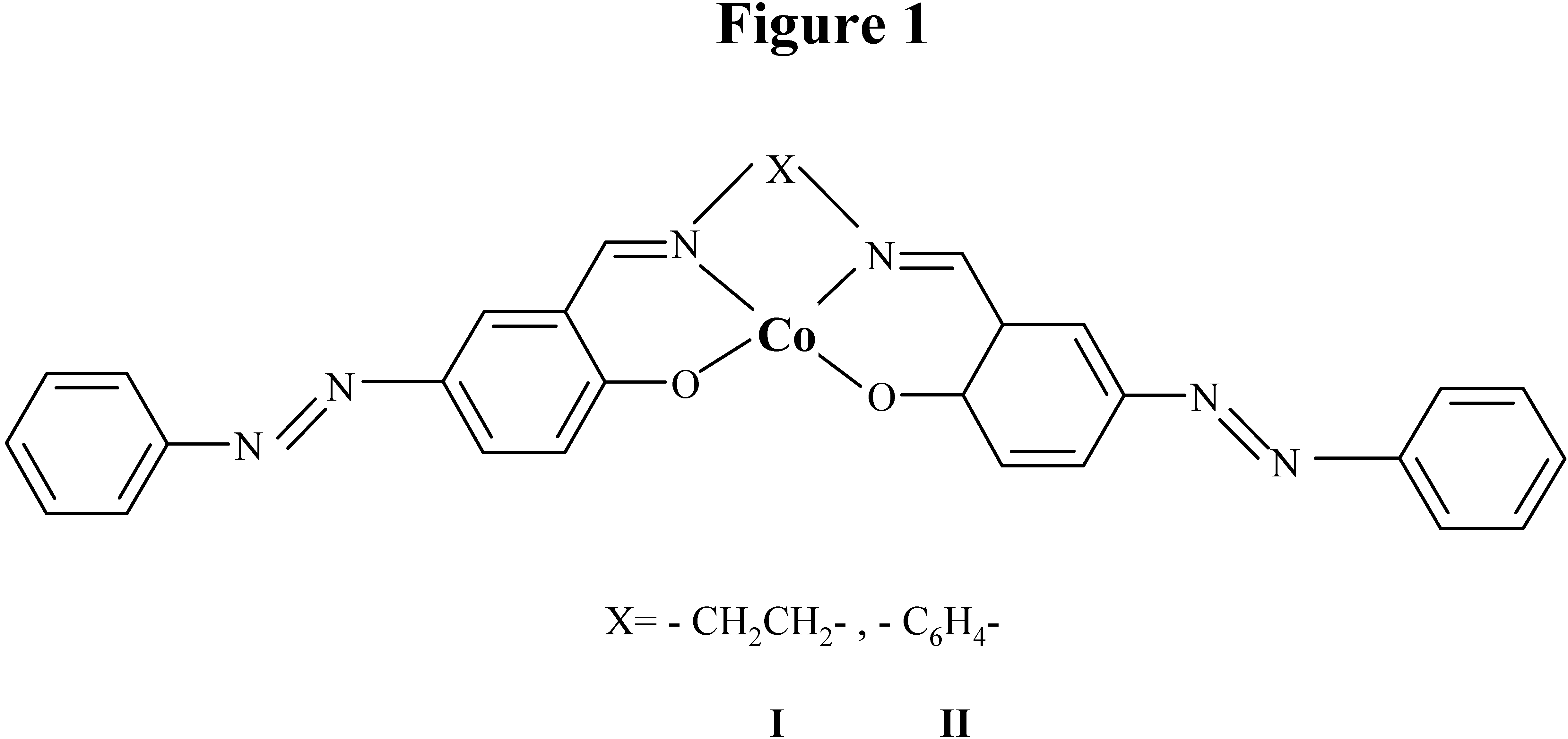
Results and Discussion
Synthesis
| Complex | I | II |
|---|---|---|
| Yield (%) | 90 | 89 |
| IR data(cm-1) | ||
| υC-H(aromatic) | 3040 | 3050 |
| Analyses, found (calc.) | ||
| C | 62.81(63.04) | 65.73(66.09) |
Catalytic Properties
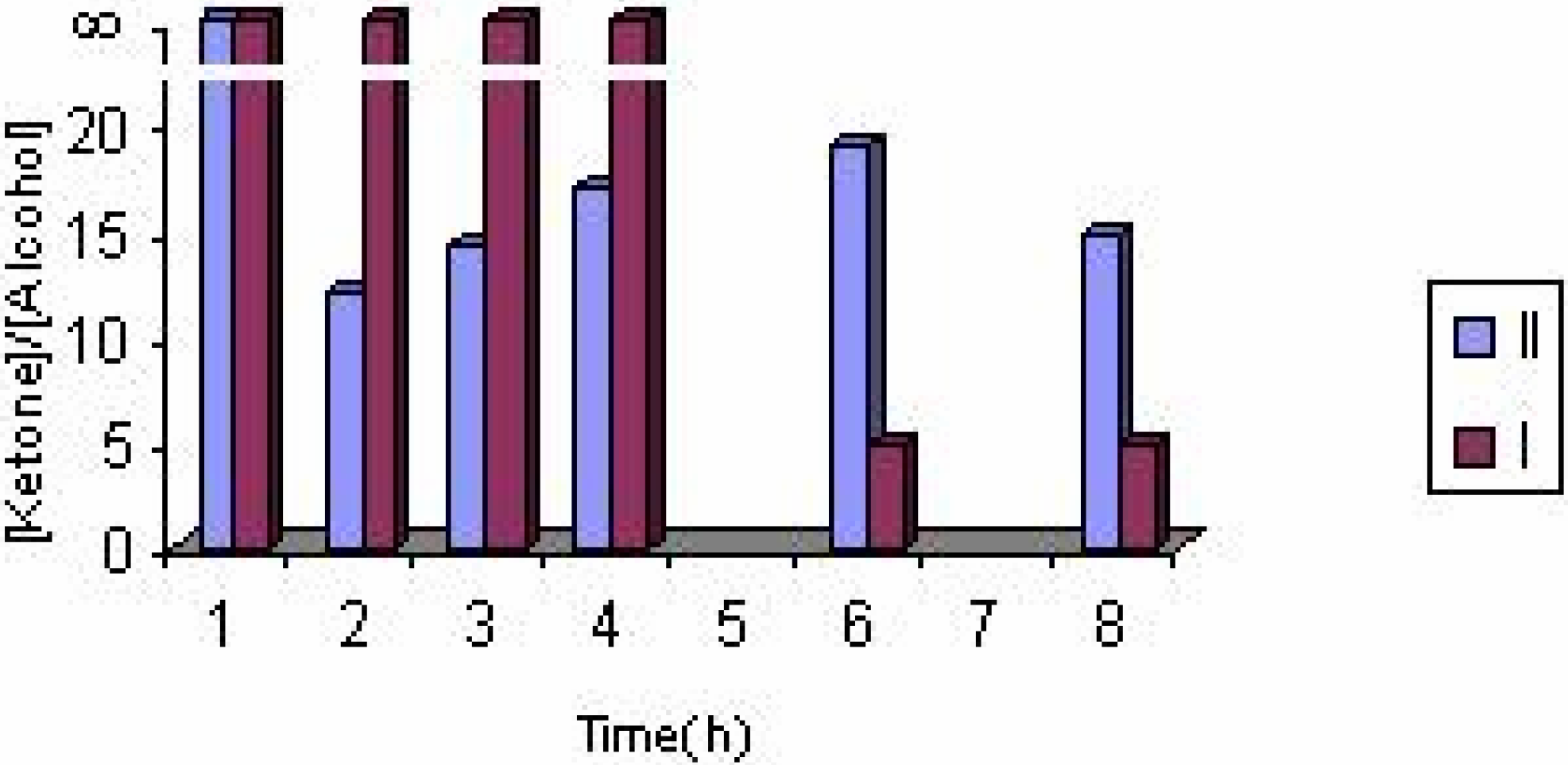
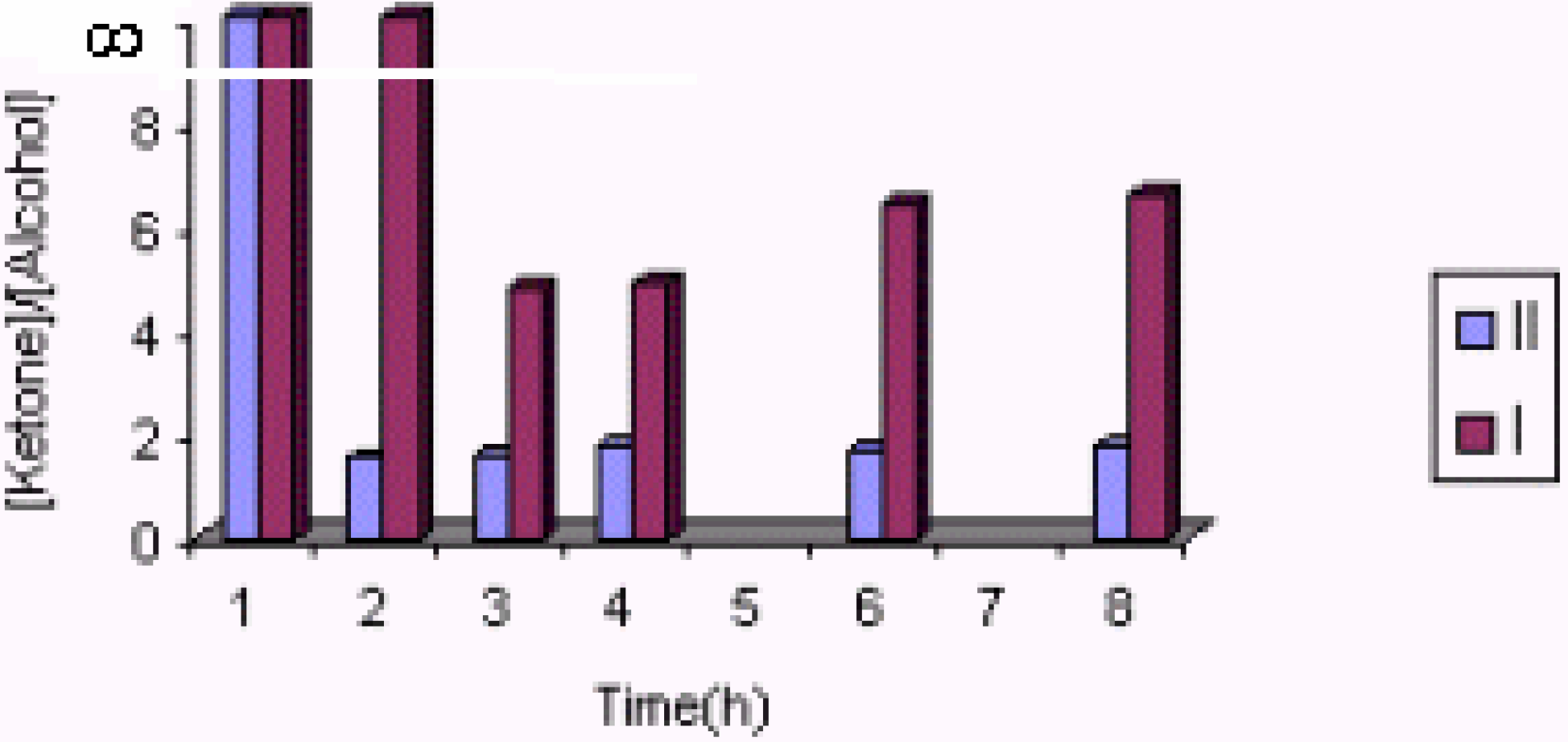
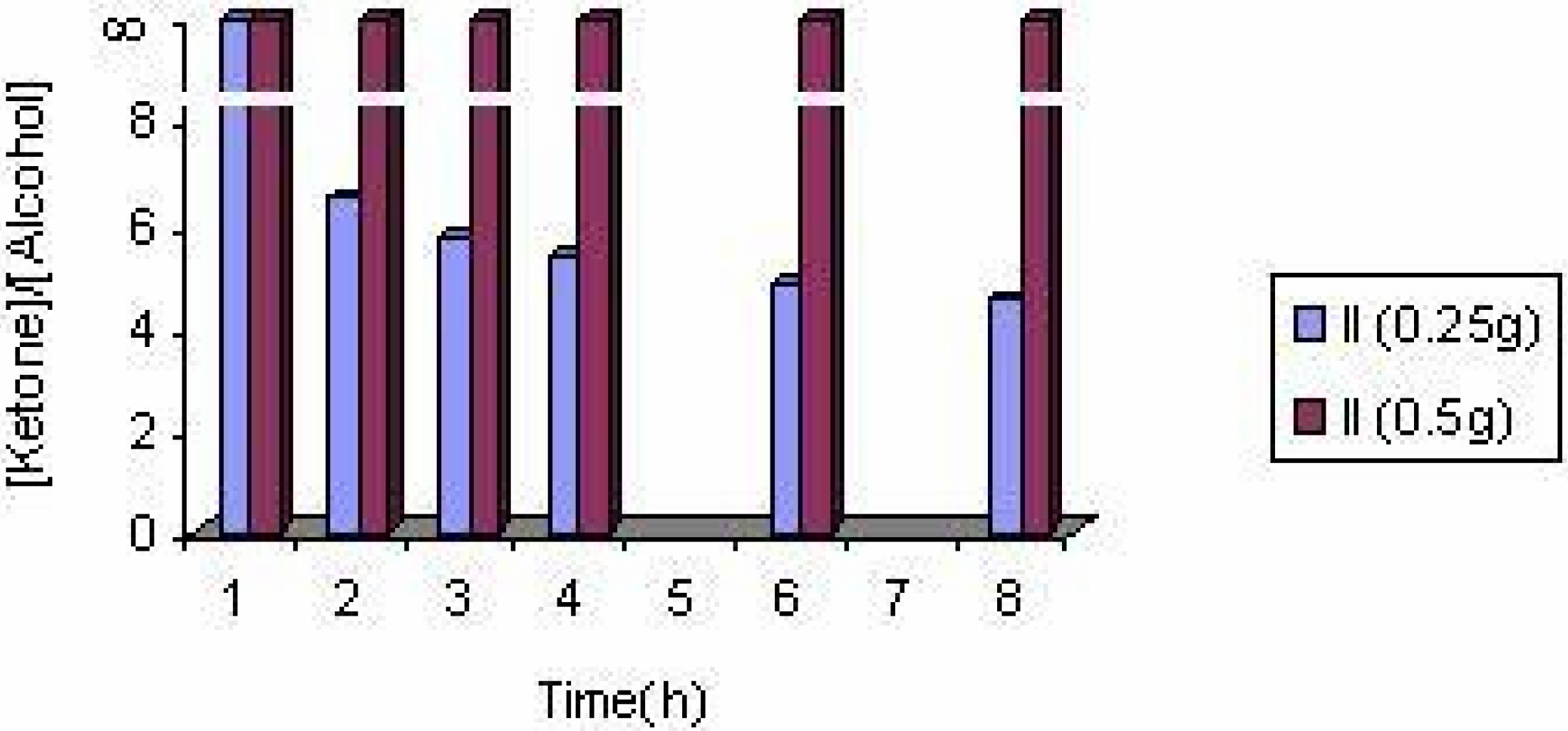
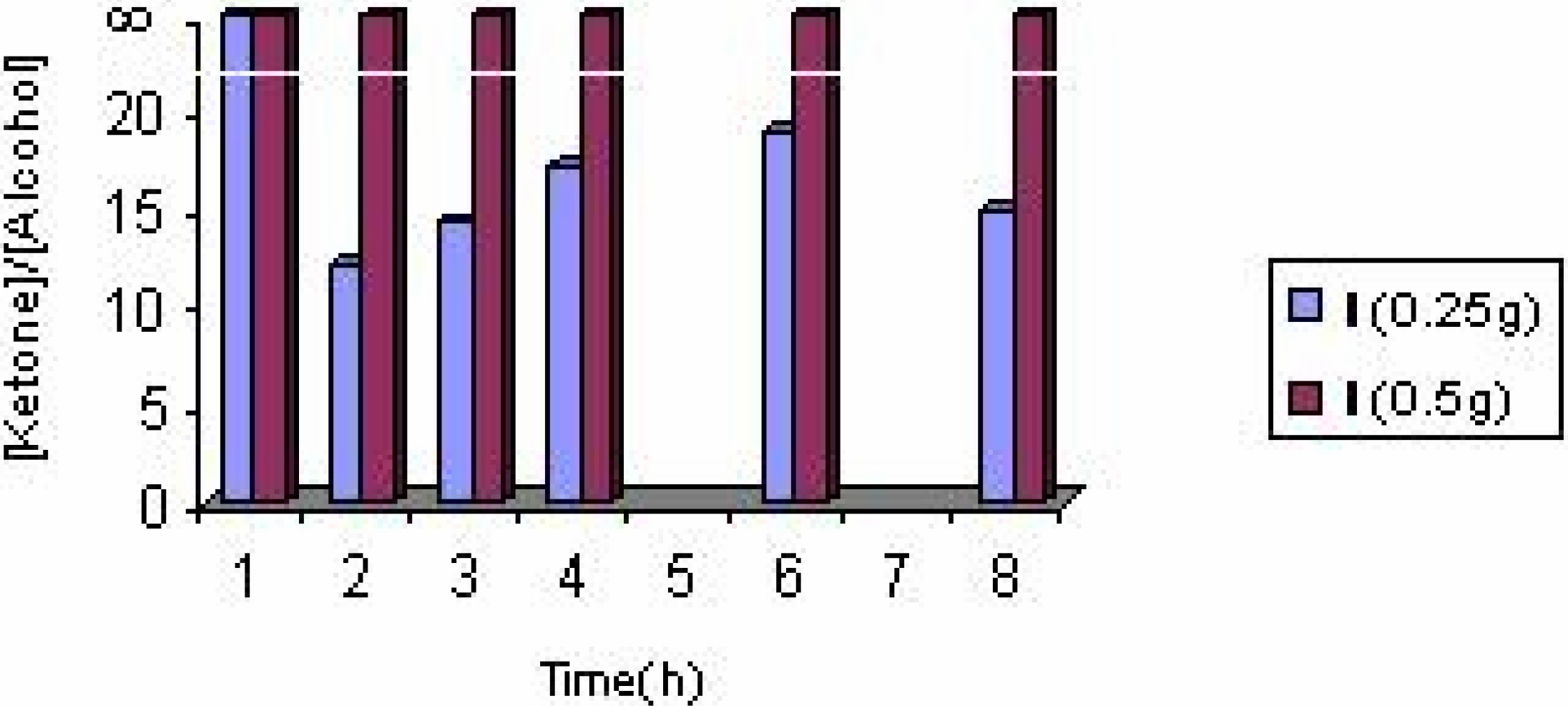
| Experiment No. | Catalyst | Substrate (ml) | Solvent | Products(%) | Styrene conversion (%) | Turnover number | [ketone] / [alcohol] | |
|---|---|---|---|---|---|---|---|---|
| Ketone | Alcohol | |||||||
| 1 | II(0.25g) | 3 | EtOH | 81.78 | 18.22 | 19.75 | 11.94 | 4.49 |
| 2 | II(0.25g) | 3 | 1-PrOH | 93.72 | 6.28 | 13.54 | 8.19 | 14.93 |
| 3 | II(0.25g) | 3 | 2-PrOH | 61.40 | 35.90 | 2.53 | 1.53 | 1.78 |
| 4 | II(0.25g) | 6 | 1-PrOH | 84.90 | 15.10 | 5.73 | 5.12 | 5.63 |
| 5 | II(0.5g) | 3 | 1-PrOH | 100.00 | __ | 5.80 | 1.75 | ∞ |
| 6 | II(0.25g) | 6 | EtOH | 54.12 | 45.88 | 4.18 | 5.06 | 1.18 |
| 7 | II(0. 5g) | 3 | EtOH | 100.00 | __ | 2.69 | 0.81 | ∞ |
| 8 | I(0.25g) | 3 | EtOH | 84.13 | 15.87 | 10.42 | 5.78 | 5.30 |
| 9 | I(0.25g) | 3 | 1-PrOH | 83.10 | 16.90 | 2.66 | 1.47 | 5.00 |
| 10 | I(0.25g) | 3 | 2-PrOH | 86.82 | 13.18 | 12.14 | 6.73 | 6.59 |
| 11 | I(0.25g) | 6 | 2-PrOH | 80.79 | 19.21 | 1.07 | 1.19 | 4.58 |
| 12 | I(0.5g) | 3 | 2-PrOH | 82.00 | 18.00 | 2.92 | 0.81 | 4.55 |
The effect of the solvent
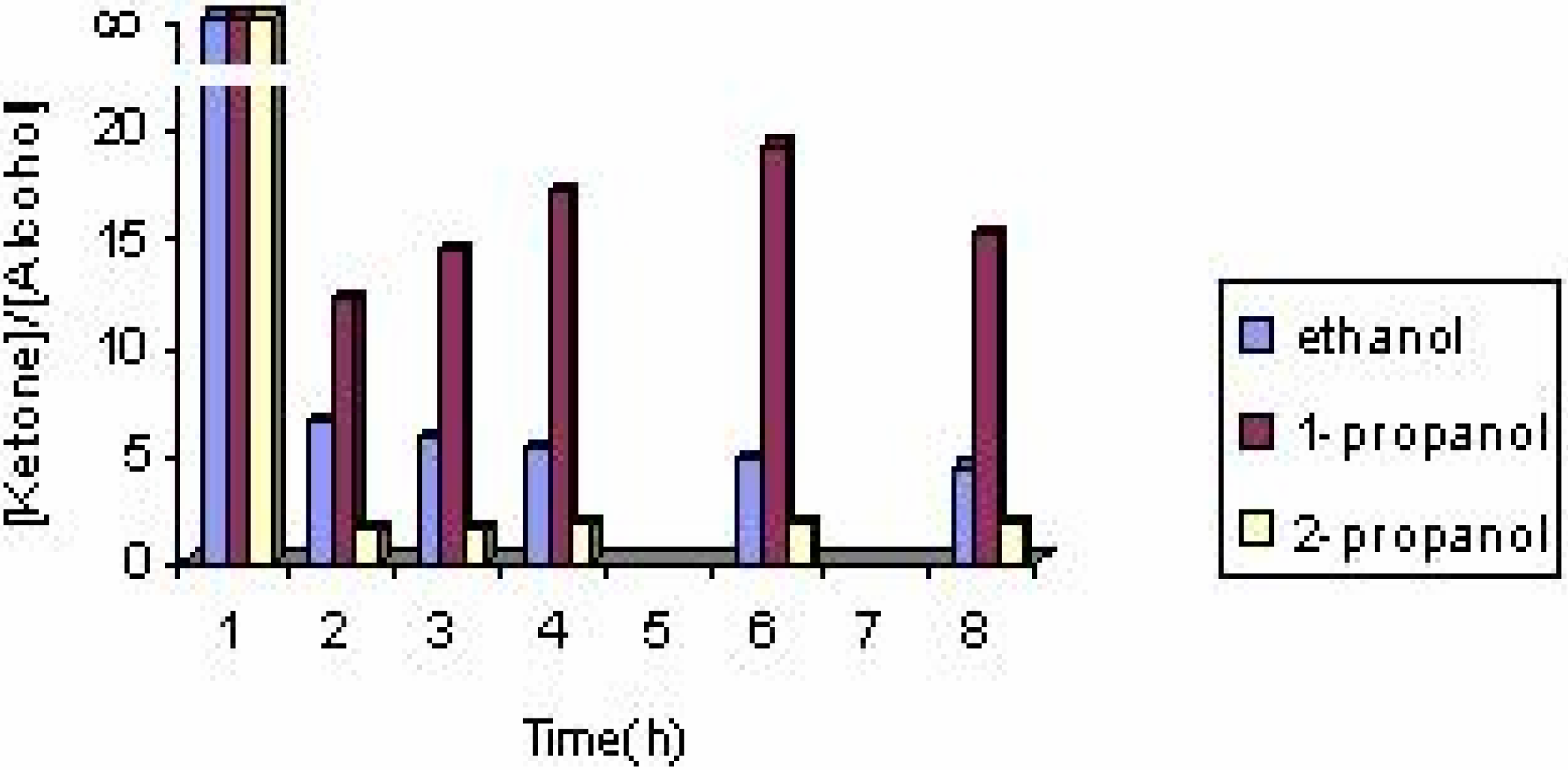
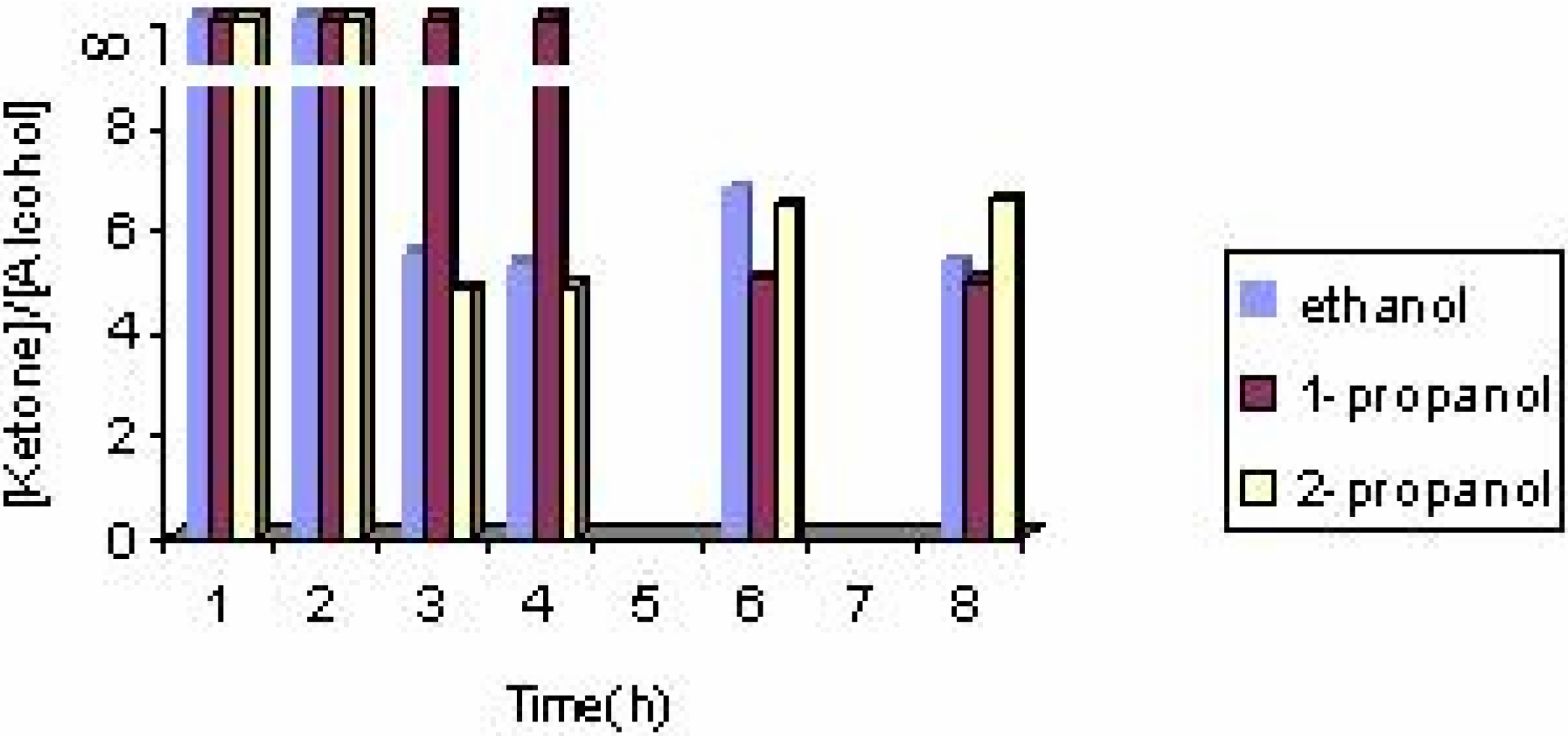
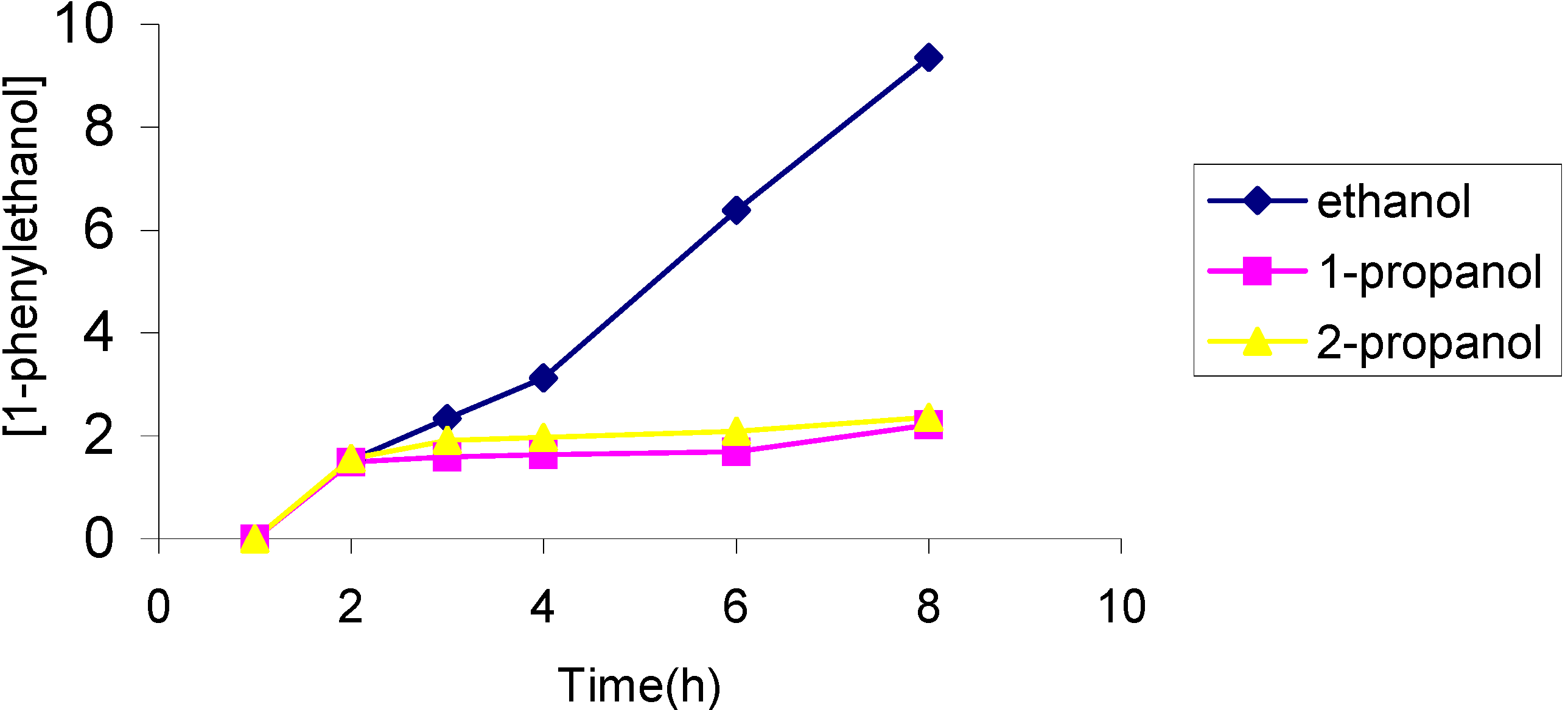
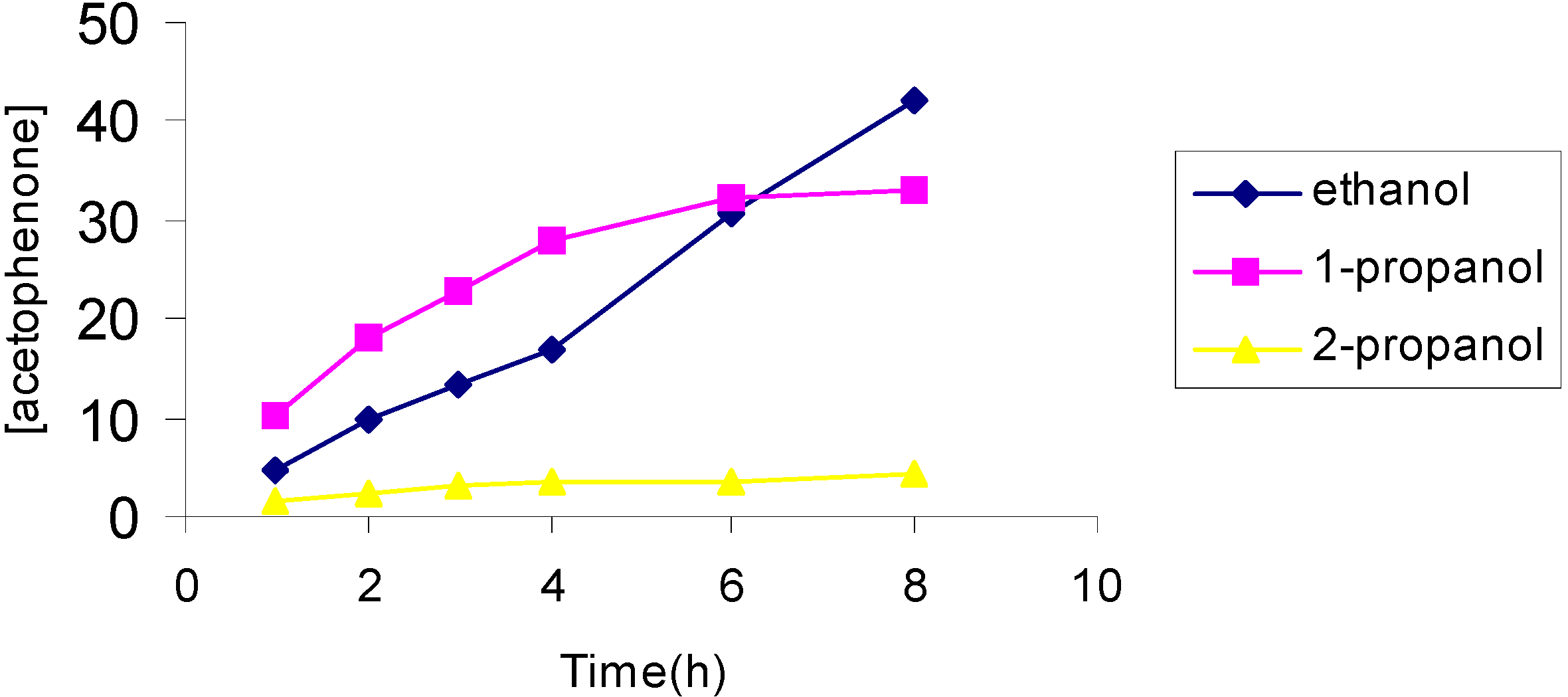
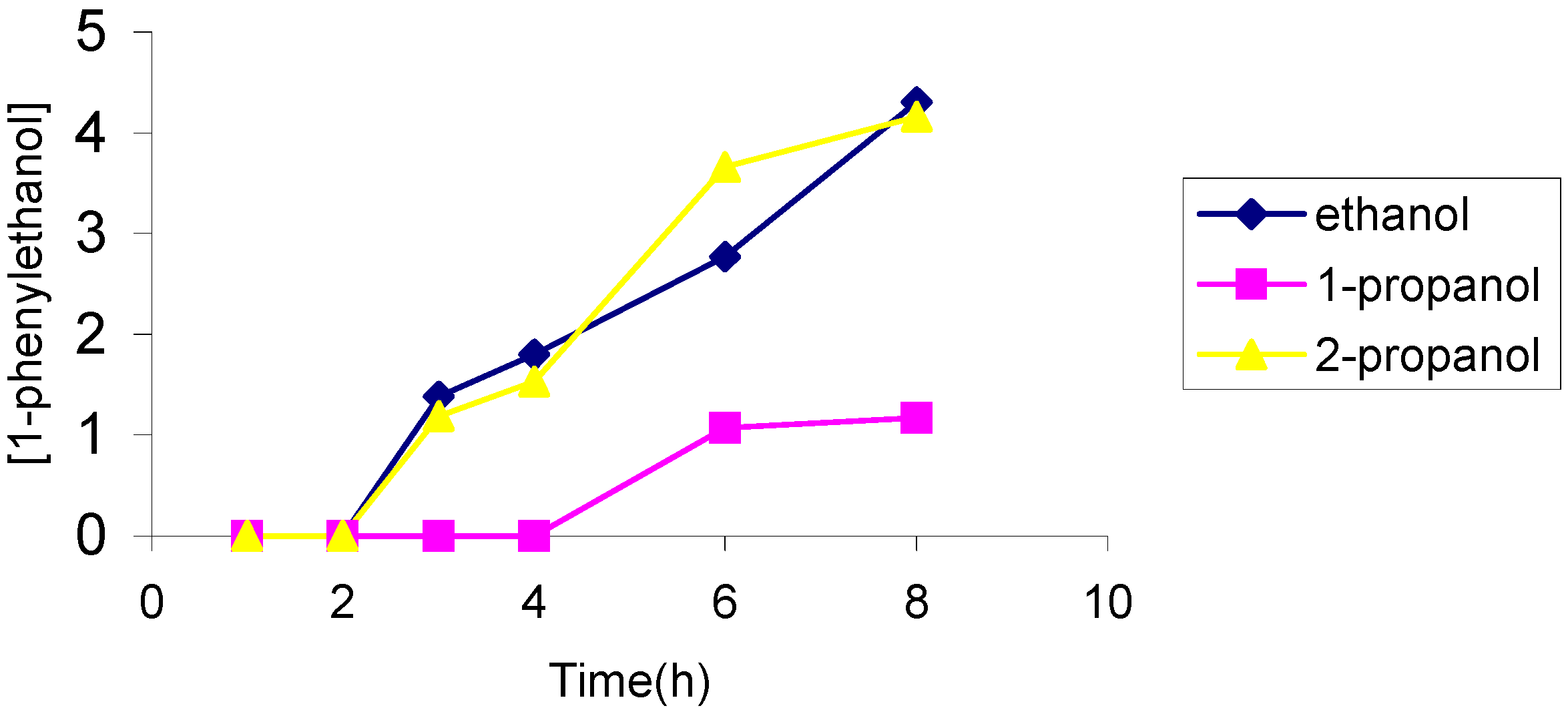
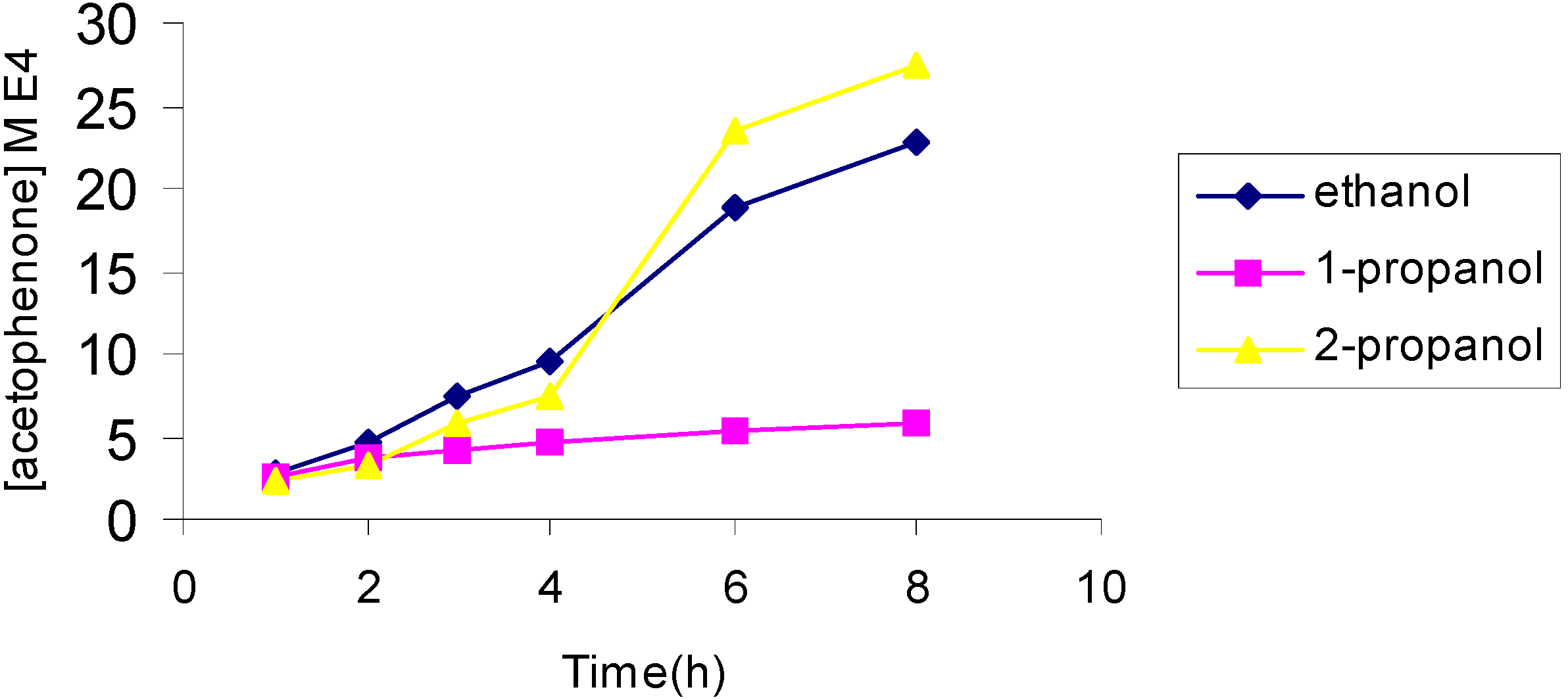
The effect of the catalyst
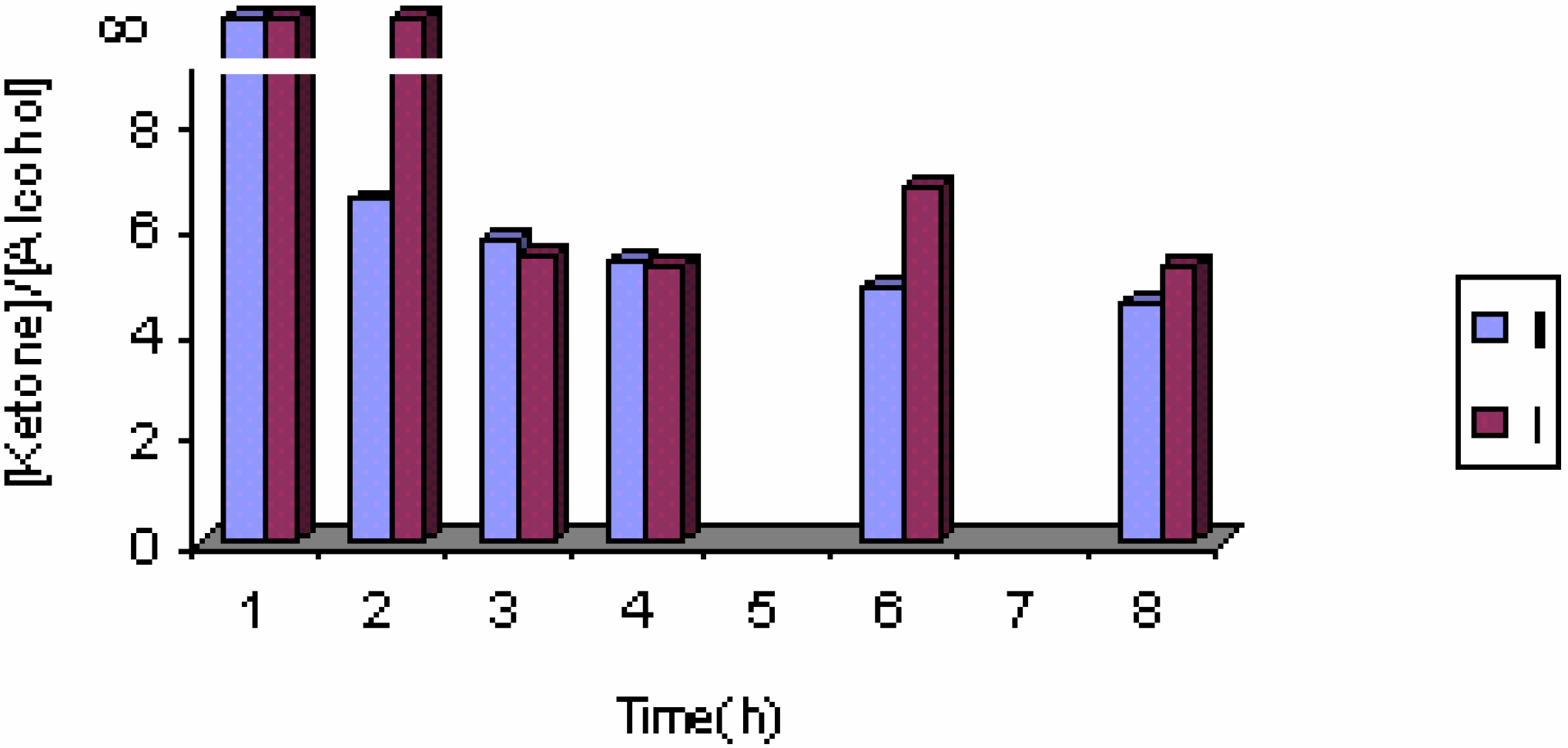
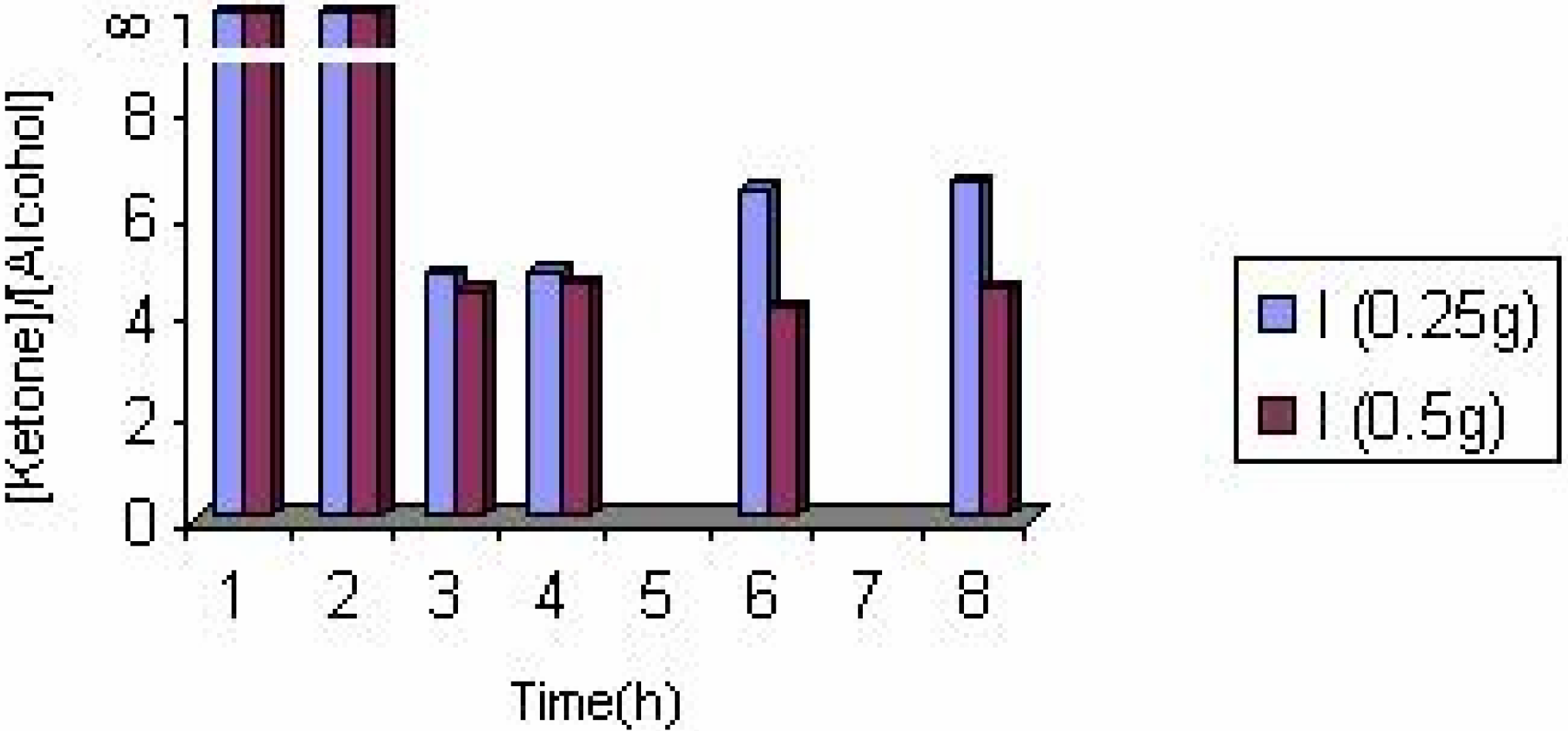
The effect of catalyst concentration
Conclusions
Acknowledgements
Experimental
General
Complexes
Oxidation Reactions
References
- Werner, A.; Mylius, A.Z. Anorg. Allg. Chem. 1898, 16, 245.
- Tsumaki, T. Bull. Chem. Soc. Jpn. 1938, 13, 252.
- Busch, D.H.; Alcock, N.W. Chem. Rev. 1994, 94, 585.
- Niederhoffer, E.C.; Timmons, J.H.; Martell, A.E. Chem. Rev. 1984, 84, 137.
- Jones, R.D.; Summerville, D.A.; Basolo, F. Chem. Rev. 1979, 79, 139.
- Mimoun, H. J. Mol. Cat. 1980, 7, 1.
- Ebner, J.; Riley, D.; Foote, C.S.; Valentine, J.S.; Greenberg, A.; Liebman, J.F. (Eds.) Active Oxygen in Chemistry, SEARCH Series, Vol. 2; Blackie Academic and Professional Publishers: Glasgow, U.K, 1995; Chap. 6; p. 205.
- Cui, Y.; Patt, R.; Chen, R.; Gratzl, J. J. Mol. Catal. A 1999, 144, 411.
- Hage, R.; Iburg, J.E.; Koek, J.H.; Martens, R.; Kerschner, J.; Lempers, E.L.M.; Martens, R.J.; Racheria, U.S.; Russell, S.W.; Swaryhoff, T.; van Vliet, M.R.P.; Warnaar, J.B.; van der Wolf, L.; Krijnen, B. Nature 1994, 369, 637.
- Bozell, J.; Hames, B.R.; Dimmel, D.R. J. Org. Chem. 1995, 60, 2398.
- Nishinaga, A.; Tsutsui, T.; Moriyama, H.; Wazaki, T.; Mashino, T.; Fujii, Y. J. Mol. Catal. 1993, 83, 117.
- Vogt Jr, L.H.; Faigenbaum, H.M.; Wiberley, S.E. Chem. Rev. 1963, 63, 269.
- Kervinen, K.; Lahtinen, P.; Repo, T.; Svahn, M.; Leskela, M. Catal. Today 2002, 75, 183.
- Sheldon, R.A.; Kochi, J.K. Metal-Catalyzed Oxidation of Organic Compounds; Academic Press: New York, 1981; p. 97. [Google Scholar]
- Drago, R.S.; Corden, B.B. Acc. Chem. Res. 1980, 13, 353.
- Corden, B.B.; Drago, R.S.; Perito, R.P. J. Am. Chem. Soc. 1985, 107, 2903.
- Martell, A.E. Oxygen Complexes and Oxygen Activation by Transition Metals; Plenum Press: New York, 1987; p. 87. [Google Scholar]
- McLendon, G.; Martell, A.E. Coord. Chem. Rev. 1976, 19, 1.
- Sasaki, I.; Pujol, D.; Gaudemer, A. New J. Chem. 1989, 13, 843.
- Jager, E.G.; Knaudt, J.; Rudolph, M.; Rost, M. Chem. Ber. 1996, 129, 1041.
- Lippard, S.; Feig, A. Chem. Rev. 1994, 94, 759.
- Henson, N.J.; Hay, P.J.; Redondo, A. Inorg. Chem. 1999, 38, 1618.
- Chen, D.; Martell, A.E. Inorg. Chem. 1987, 26, 1026.
- Chen, D.; Martell, A.E.; Sun, Y. Inorg. Chem. 1989, 28, 2647.
- Nakamoto, K.; Nonaka, Y.; Ishiguro, T.; Urban, W.M.; Suzuki, M.; Kozuka, M.; Nishida, Y.; Kida, S. J. Am. Chem. Soc. 1982, 104, 3386.
- Stynes, D.V.; Stynes, H.C.; Ibers, J.A.; James, B.R. J. Am. Chem. Soc. 1973, 95, 1142.
- Khandar, A.A.; Nejati, K. Polyhedron 2000, 19, 607.
- Sample availability: Available from the authors.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Khandar, A.A.; Nejati, K.; Rezvani, Z. Syntheses, Characterization and Study of the Use of Cobalt (II) Schiff–Base Complexes as Catalysts for the Oxidation of Styrene by Molecular Oxygen. Molecules 2005, 10, 302-311. https://doi.org/10.3390/10010302
Khandar AA, Nejati K, Rezvani Z. Syntheses, Characterization and Study of the Use of Cobalt (II) Schiff–Base Complexes as Catalysts for the Oxidation of Styrene by Molecular Oxygen. Molecules. 2005; 10(1):302-311. https://doi.org/10.3390/10010302
Chicago/Turabian StyleKhandar, Ali Akbar, Kamellia Nejati, and Zolfaghar Rezvani. 2005. "Syntheses, Characterization and Study of the Use of Cobalt (II) Schiff–Base Complexes as Catalysts for the Oxidation of Styrene by Molecular Oxygen" Molecules 10, no. 1: 302-311. https://doi.org/10.3390/10010302




