Entropy Generation Analysis of Laminar Flows of Water-Based Nanofluids in Horizontal Minitubes under Constant Heat Flux Conditions
Abstract
:1. Introduction
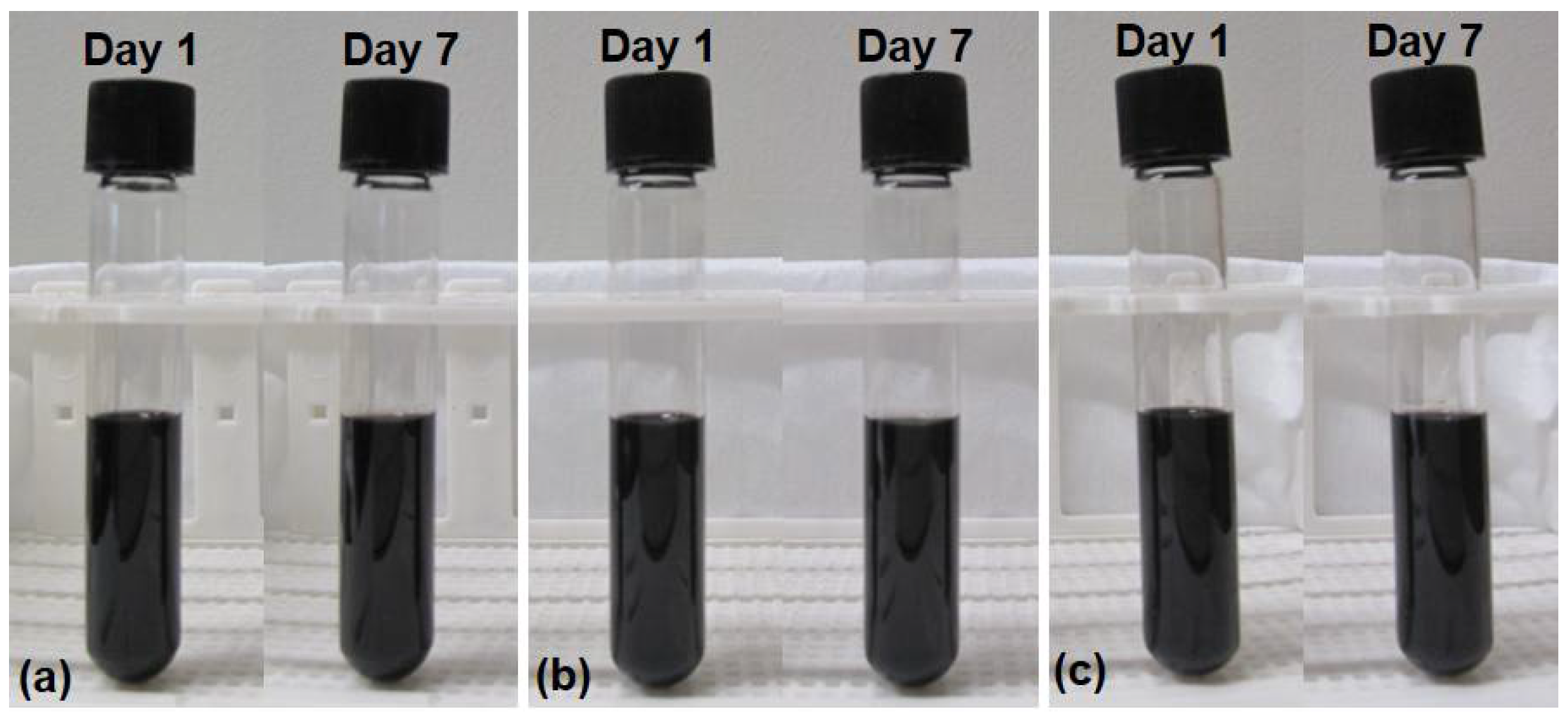
2. Nanofluid Preparation, Characterization and Thermophysical Properties
3. Experimental Setup and Procedure
4. Data Reduction
5. Results and Discussions
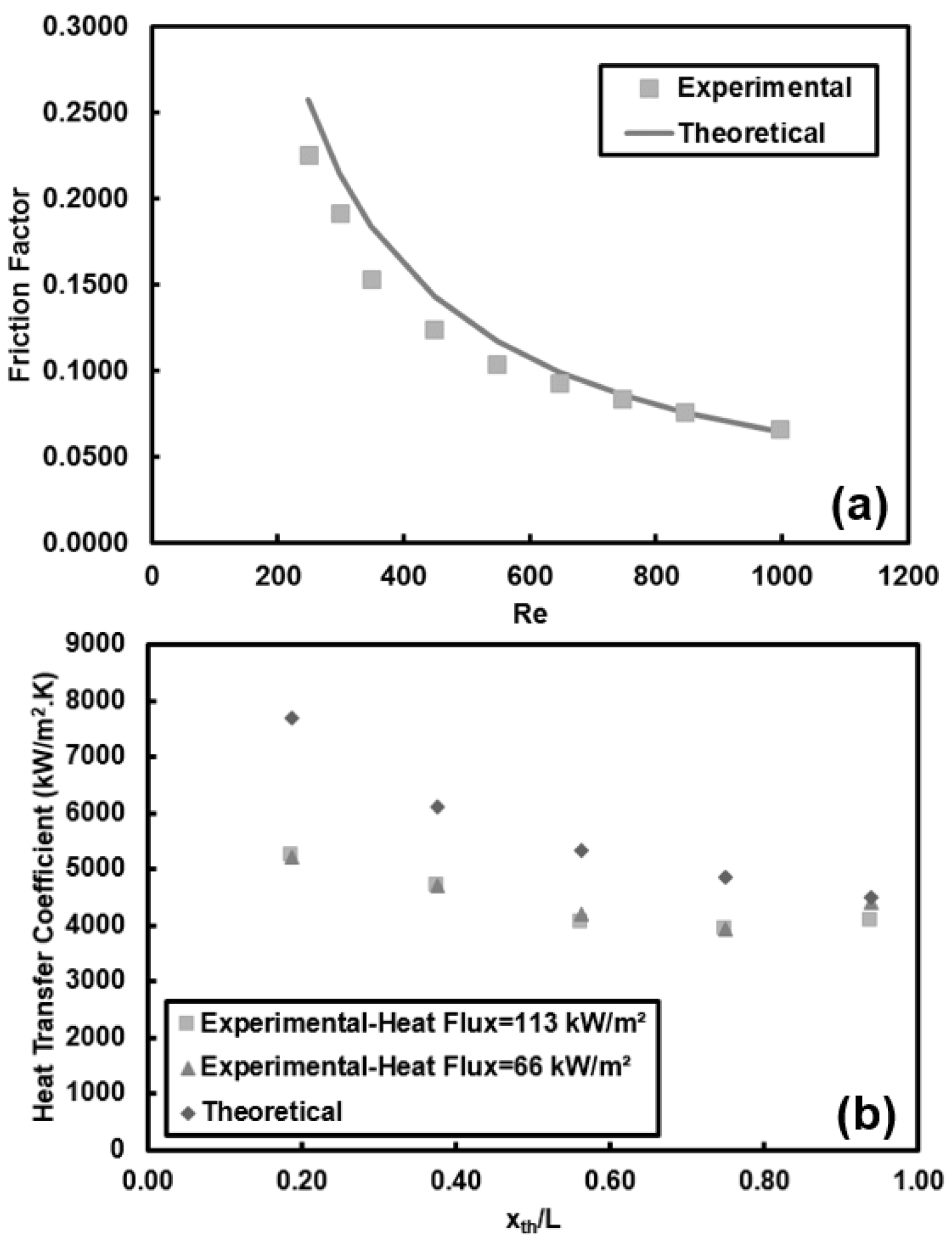
5.1. Convective Heat Transfer Analysis
5.2. Second Law Analysis
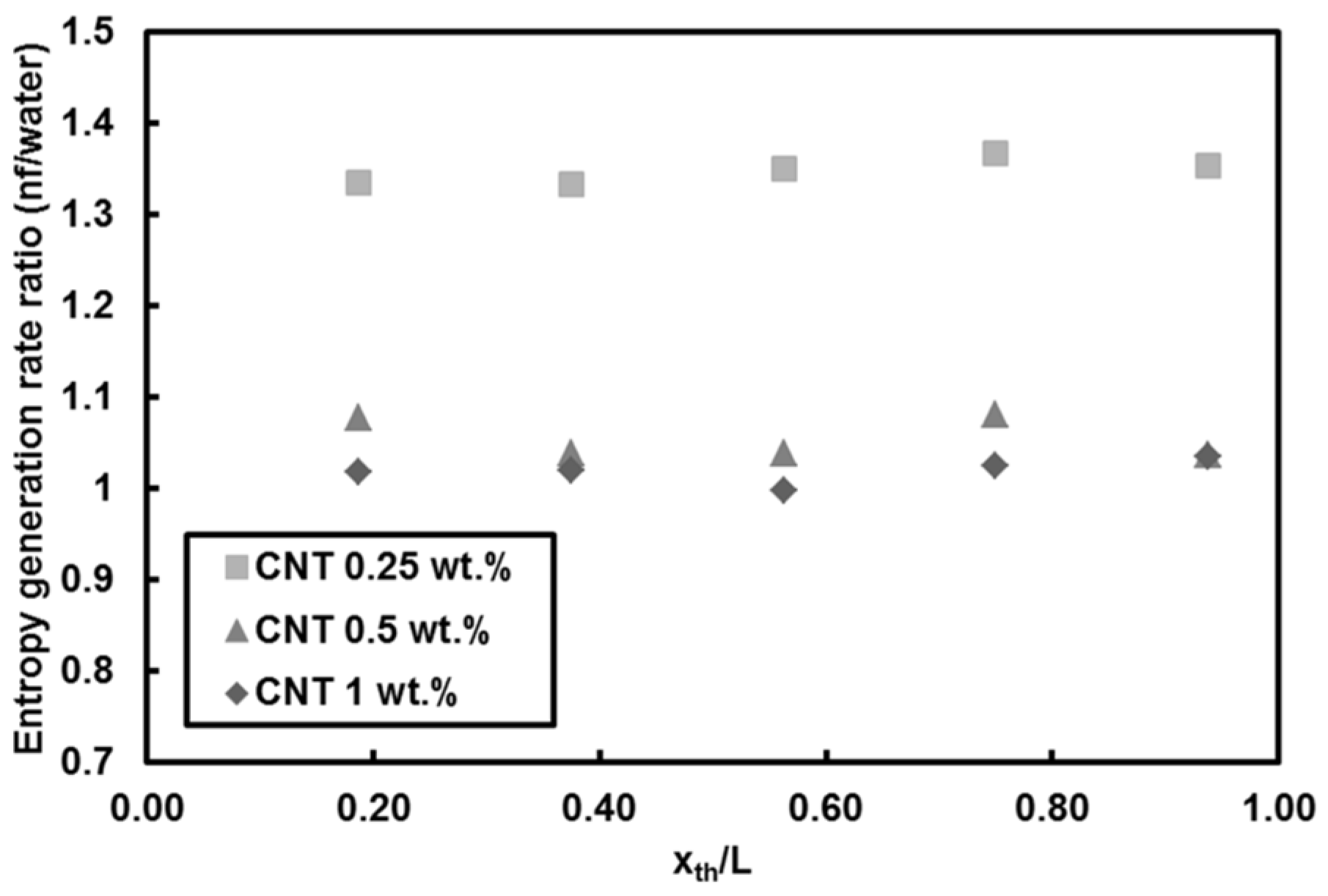
5.2.1. Entropy Generation of CNTs/Water Nanofluids Flows
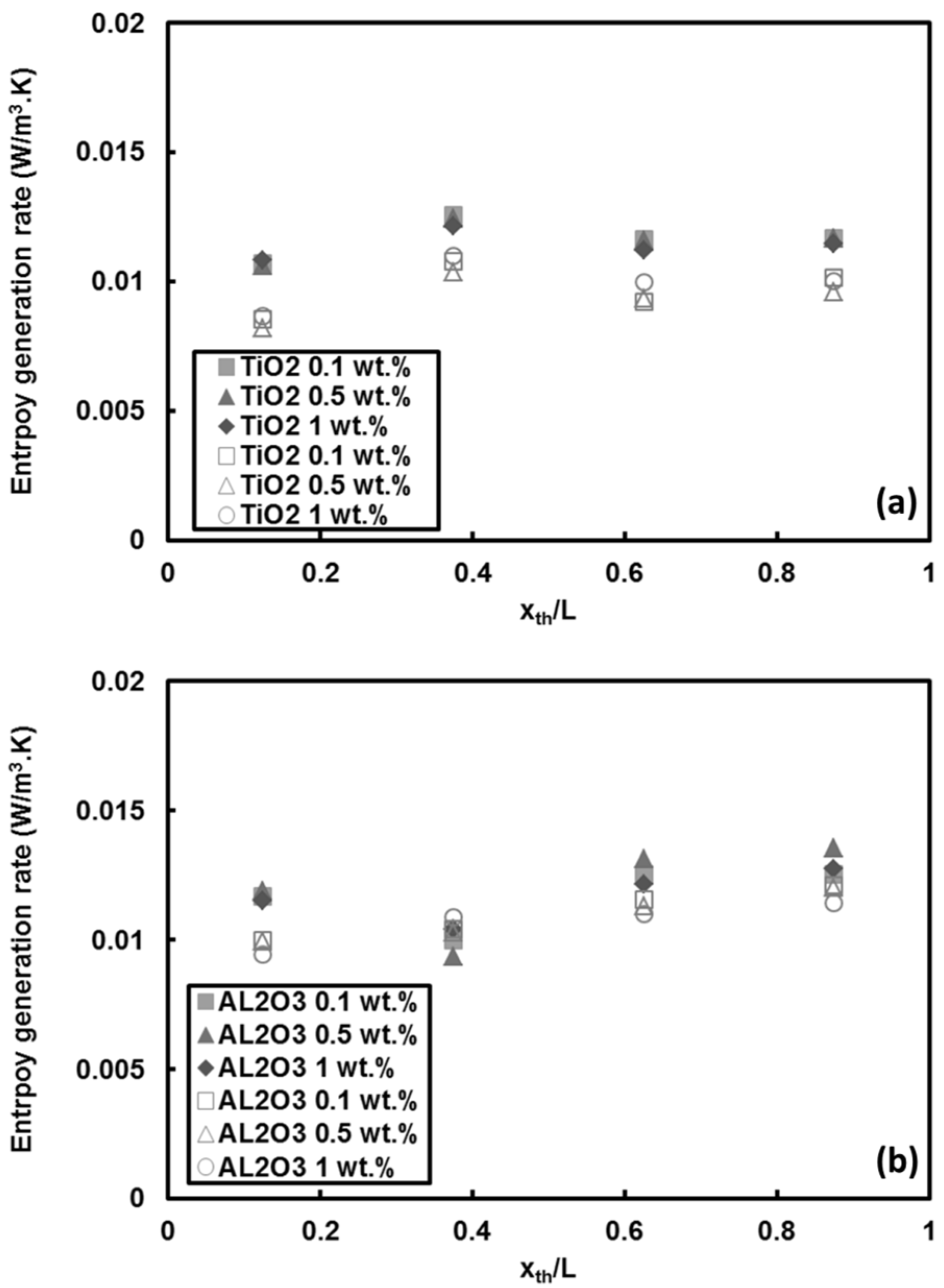
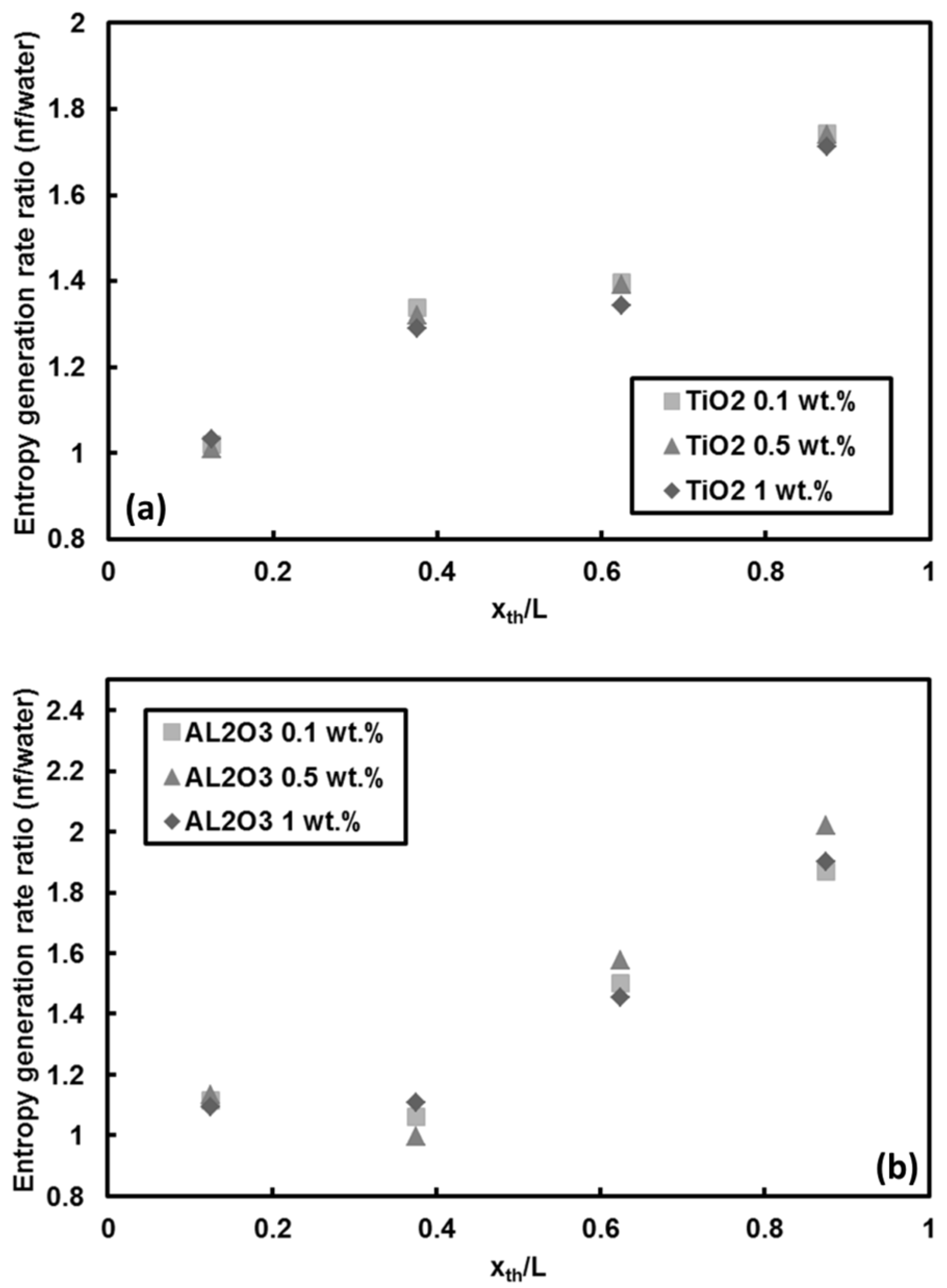
5.2.2. Entropy Generations in TiO2 and Al2O3 Nanoparticle/Water Nanofluids
6. Conclusions
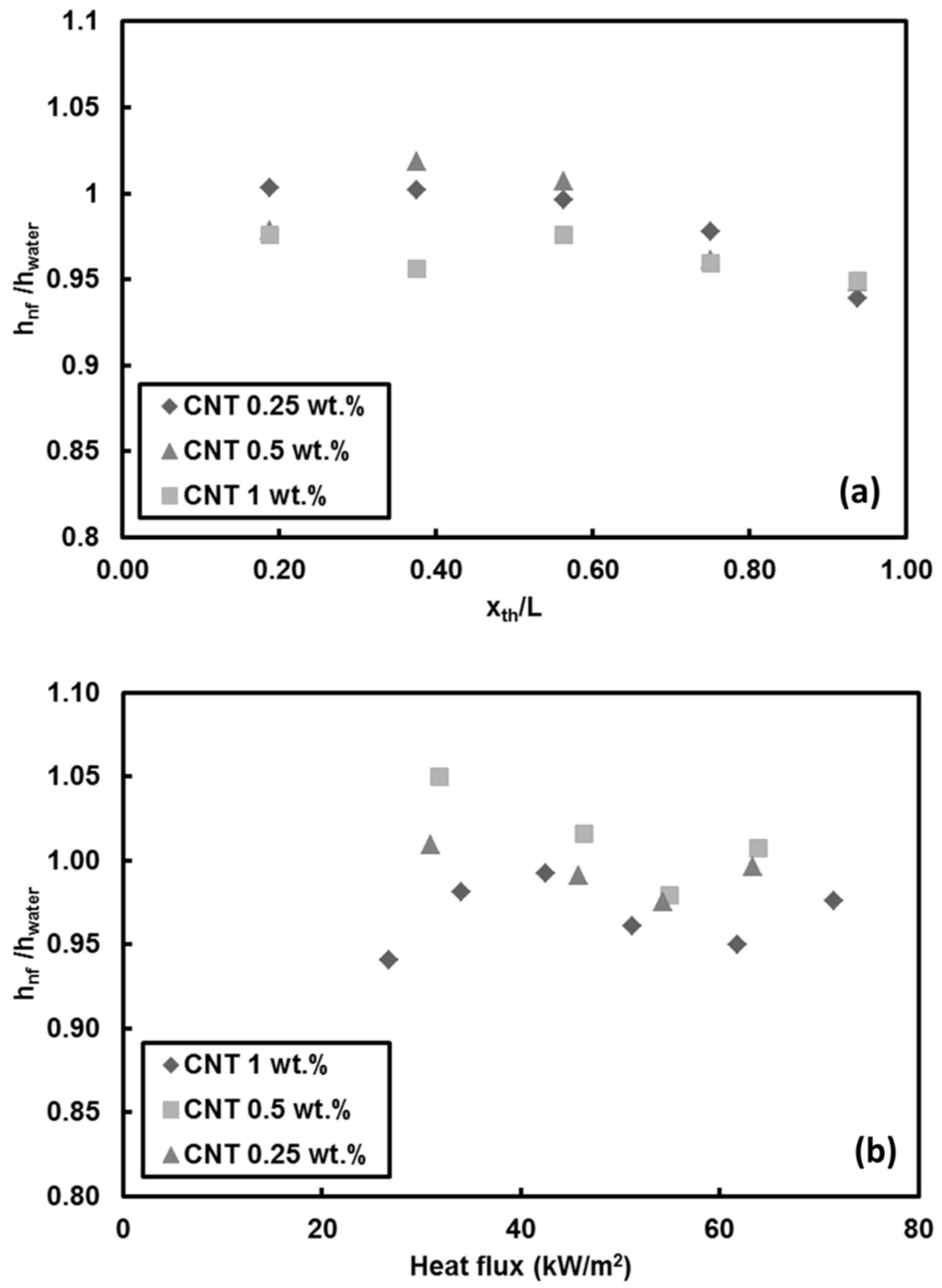
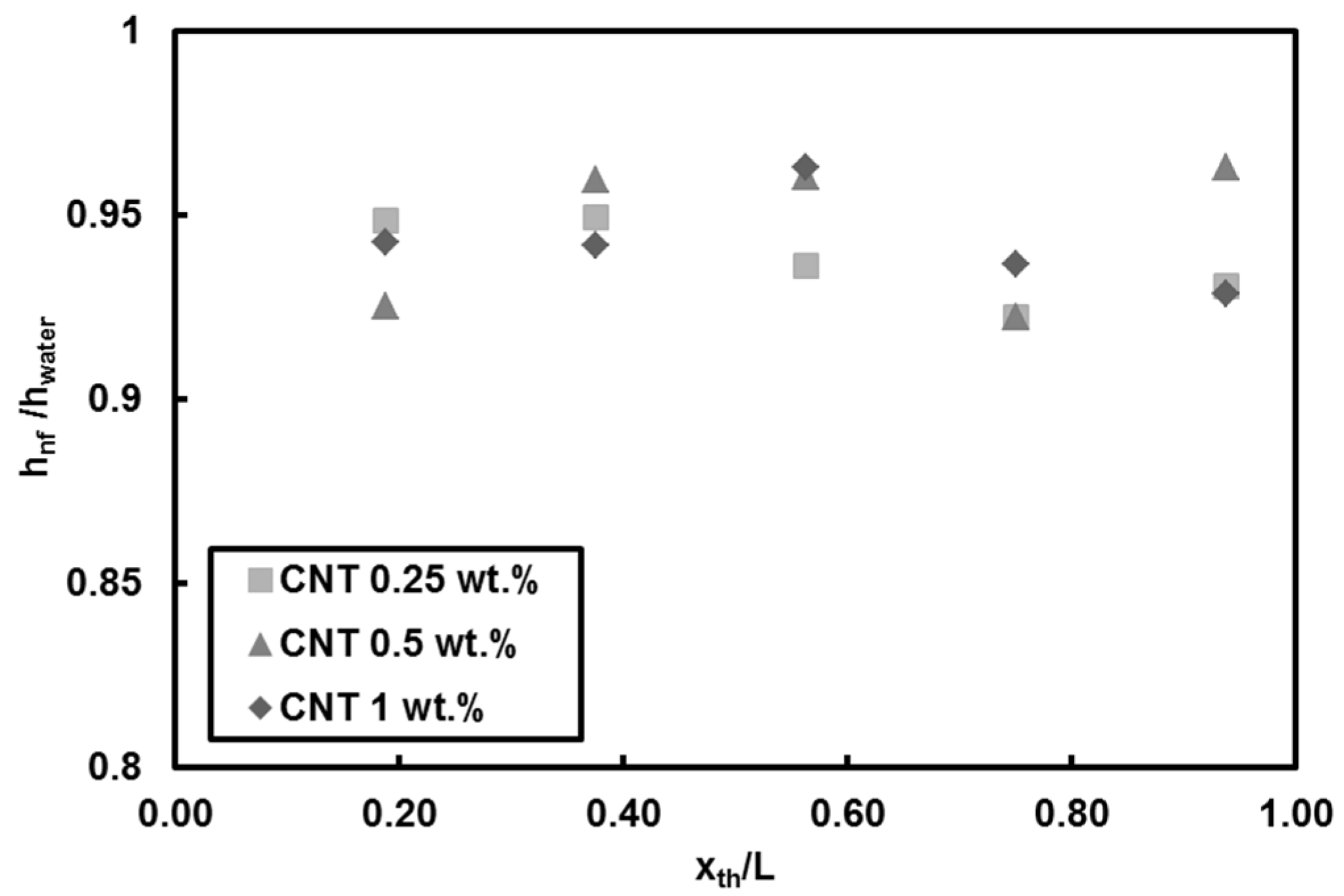
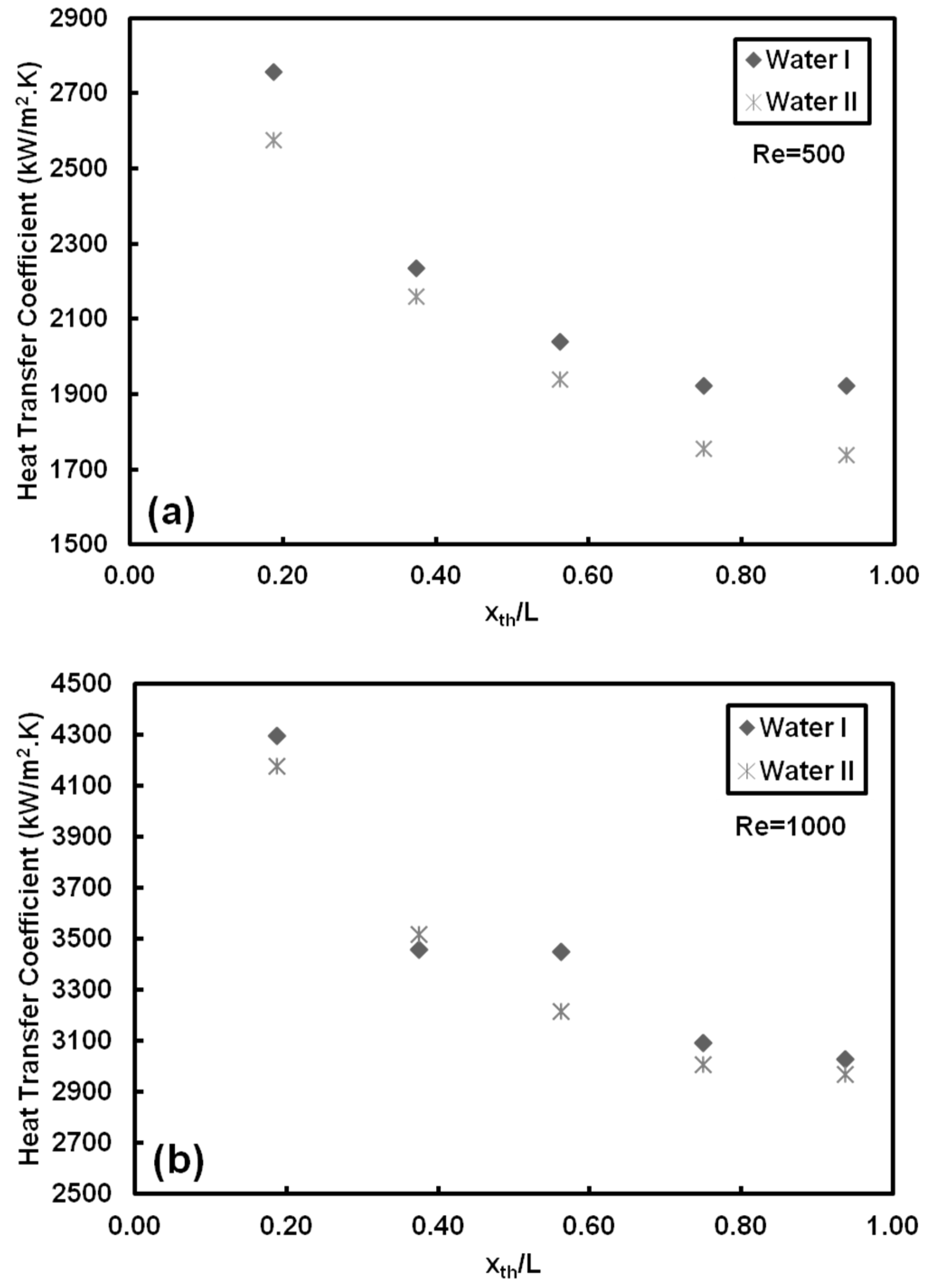
- Adding CNT to pure water has no considerable effect on heat transfer coefficient for low Reynolds number (Re = 500). However, degradation in heat transfer was observed (by less than 10%) for high Reynolds number (Re = 1000). Deposition of CNT on the surface toward the end of the test section is the reason behind the decrease in heat transfer coefficient and slight deterioration in heat transfer near the exit. Soret effect and corresponding mass diffusion at the thermally developing flows play a major role on particle distribution and heat transfer due to higher temperature gradient especially near the inlet of the tube.
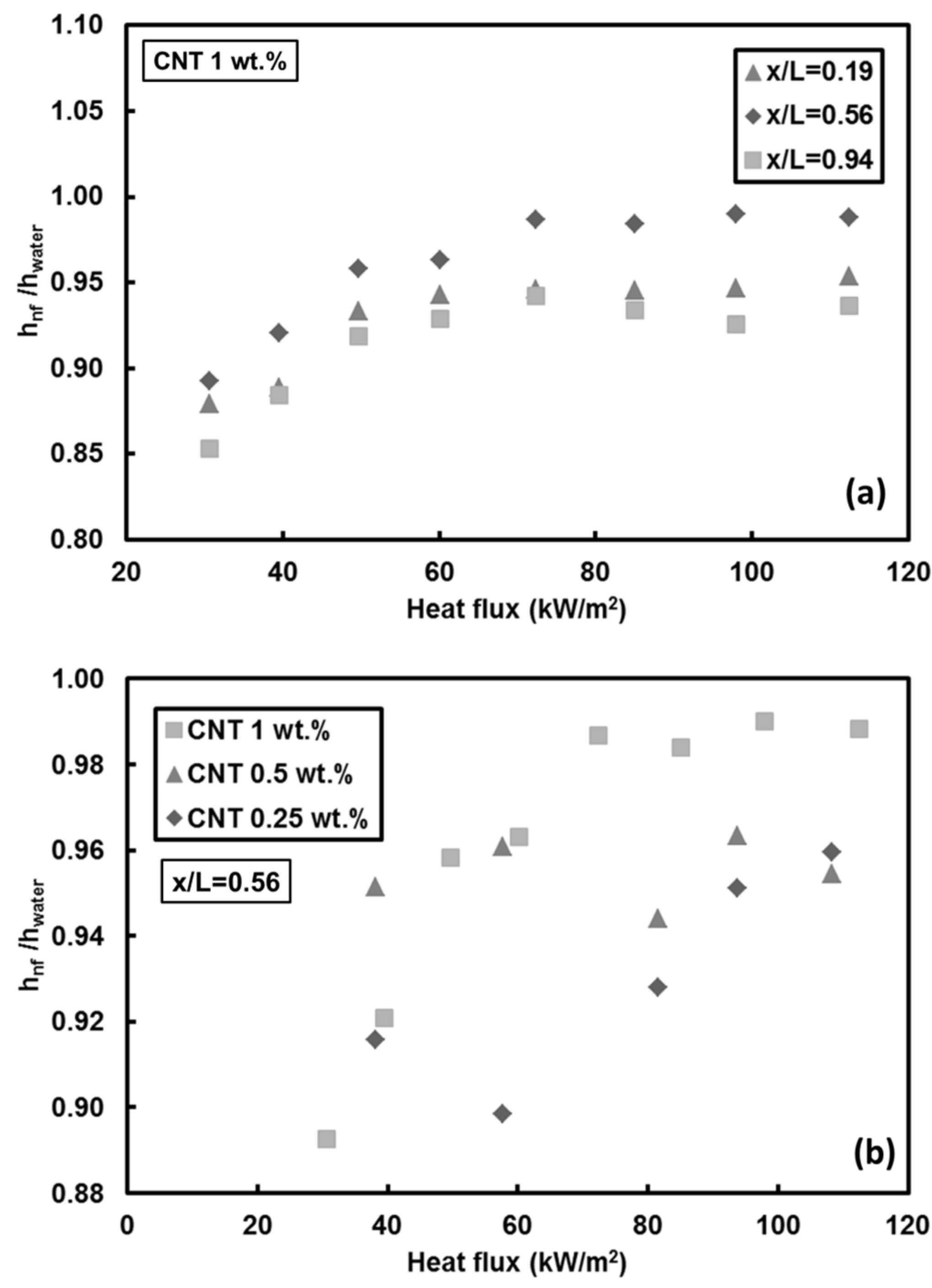
- Adding CNTs to the base fluid increases the thermal conductivity of the nanofluid. However, the results showed that mass fraction has no considerable effect on heat transfer at low Reynolds number because of short heating length. Small hydraulic diameter and small heating length intensify the Soret effect, resulting in non-uniform nanoparticle dispersion in the working fluid. This could be a possible reason for the low heat transfer coefficient ratio between the nanofluid and pure water.
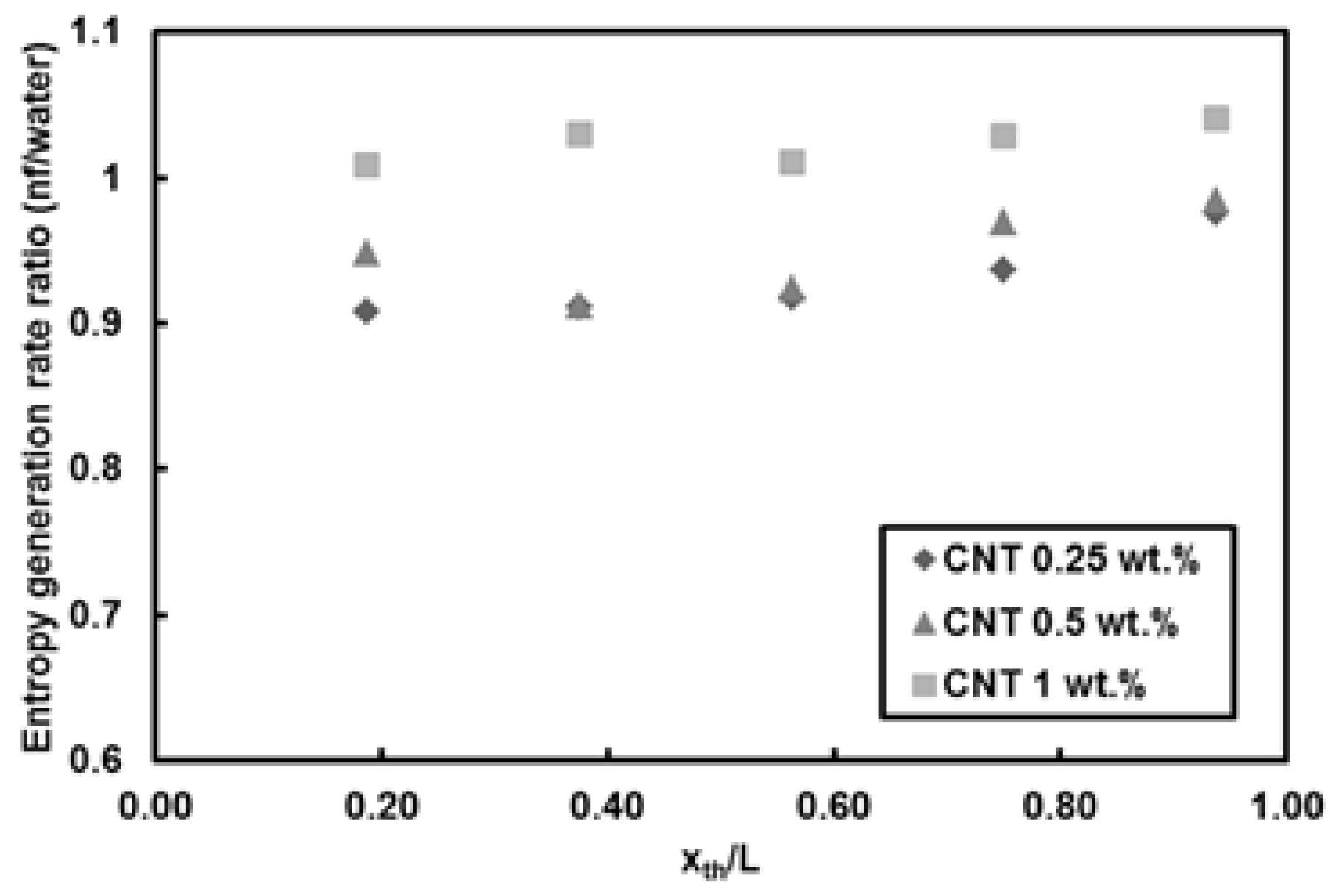
- For CNTs/water nanofluids, the entropy generation rate remains almost unchanged at mass fraction of 1 wt.% (high nanofluid mass fraction). A reduction in the entropy generation rate (down to 10%) compared to pure water was observed at Re = 500 for mass fractions of 0.25 wt.% and 0.5 wt.% at Re = 1000. The only considerable increase in the entropy generation rate (about 35%) relative to pure water was seen for the lowest mass fraction of CNTs. In addition, heat flux did not exhibit any remarkable change in the entropy generation rate.
- For the other traditional nanofluids, the entropy generation rate pertinent to TiO2 and Al2O3 nanoparticles cases demonstrated that the entropy generation rate raised up to 100% at downstream locations. However, the increase in mass fraction of these nanoparticles (i.e., TiO2 and Al2O3) did not significantly change the entropy generation rate.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roberts, N.A.; Walker, D.G. Convective Performance of Nanofluids in Commercial Electronics Cooling Systems. Appl. Therm. Eng. 2010, 30, 2499–2504. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Chein, R. Performance analysis of nanofluid-cooled microchannel heat sinks. Int. J. Heat Fluid Flow 2007, 28, 1013–1026. [Google Scholar] [CrossRef]
- Bianco, V.; Scarpa, F.; Tagliafico, L.A. Numerical analysis of the Al2O3-water nanofluid forced laminar convection in an asymmetric heated channel for application in flat plate PV/T collector. Renew. Energy 2018, 116, 9–21. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. In Proceedings of the 1995 International mechanical engineering congress and exhibition, San Francisco, CA, USA, 12–17 November 1995. [Google Scholar]
- Gurav, P.; Naik, S.S.; Ansari, K.; Srinath, S.; Kishore, K.A.; Setty, Y.P.; Sonawane, S. Stable colloidal copper nanoparticles for a nanofluid: Production and application. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 589–597. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K. A review and analysis on influence of temperature and concentration of nanofluids on thermophysical properties, heat transfer and pumping power. Int. J. Heat Mass Transf. 2012, 55, 4063–4078. [Google Scholar] [CrossRef]
- Suresh, S.; Venkitaraj, K.P.P.; Selvakumar, P.; Chandrasekar, M. Effect of Al2O3–Cu/water hybrid nanofluid in heat transfer. Exp. Therm. Fluid Sci. 2012, 38, 54–60. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Routbort, J.L.; Singh, D. Particle shape effects on thermophysical properties of alumina nanofluids. J. Appl. Phys. 2009, 106, 14304. [Google Scholar] [CrossRef]
- Kandlikar, S.G.; Colin, S.; Peles, Y.; Garimella, S.; Pease, R.F.; Brandner, J.J.; Tuckerman, D.B. Heat transfer in microchannels—2012 Status and research needs. J. Heat Transf. 2013, 135, 91001. [Google Scholar] [CrossRef]
- Lee, J.; Mudawar, I. Assessment of the effectiveness of nanofluids for single-phase and two-phase heat transfer in micro-channels. Int. J. Heat Mass Transf. 2007, 50, 452–463. [Google Scholar] [CrossRef]
- Kim, S.J.; McKrell, T.; Buongiorno, J.; Hu, L. Subcooled flow boiling heat transfer of dilute alumina, zinc oxide, and diamond nanofluids at atmospheric pressure. Nucl. Eng. Des. 2010, 240, 1186–1194. [Google Scholar] [CrossRef]
- Kim, T., II; Jeong, Y.H.; Chang, S.H. An experimental study on CHF enhancement in flow boiling using Al2O3 nano-fluid. Int. J. Heat Mass Transf. 2010, 53, 1015–1022. [Google Scholar] [CrossRef]
- Ahn, H.S.; Kim, H.; Jo, H.; Kang, S.; Chang, W.; Kim, M.H. Experimental study of critical heat flux enhancement during forced convective flow boiling of nanofluid on a short heated surface. Int. J. Multiph. Flow 2010, 36, 375–384. [Google Scholar] [CrossRef]
- Karimzadehkhouei, M.; Yalcin, S.E.; Şendur, K.; Pınar Mengüç, M.; Koşar, A. Pressure drop and heat transfer characteristics of nanofluids in horizontal microtubes under thermally developing flow conditions. Exp. Therm. Fluid Sci. 2015, 67, 37–47. [Google Scholar] [CrossRef]
- Peng, H.; Ding, G.; Jiang, W.; Hu, H.; Gao, Y. Heat transfer characteristics of refrigerant-based nanofluid flow boiling inside a horizontal smooth tube. Int. J. Refrig. 2009, 32, 1259–1270. [Google Scholar] [CrossRef]
- Peng, H.; Ding, G.; Jiang, W.; Hu, H.; Gao, Y. Measurement and correlation of frictional pressure drop of refrigerant-based nanofluid flow boiling inside a horizontal smooth tube. Int. J. Refrig. 2009, 32, 1756–1764. [Google Scholar] [CrossRef]
- Henderson, K.; Park, Y.-G.; Liu, L.; Jacobi, A.M. Flow-boiling heat transfer of R-134a-based nanofluids in a horizontal tube. Int. J. Heat Mass Transf. 2010, 53, 944–951. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L.; Sun, J. Production of aqueous colloidal dispersions of carbon nanotubes. J. Colloid Interface Sci. 2003, 260, 89–94. [Google Scholar] [CrossRef]
- Liu, M.-S.; Lin, M.C.-C.; Huang, I.-T.; Wang, C.-C. Enhancement of thermal conductivity with carbon nanotube for nanofluids. Int. Commun. Heat Mass Transf. 2005, 32, 1202–1210. [Google Scholar] [CrossRef]
- Kumaresan, V.; Mohaideen Abdul Khader, S.; Karthikeyan, S.; Velraj, R. Convective heat transfer characteristics of CNT nanofluids in a tubular heat exchanger of various lengths for energy efficient cooling/heating system. Int. J. Heat Mass Transf. 2013, 60, 413–421. [Google Scholar] [CrossRef]
- Ding, Y.; Alias, H.; Wen, D.; Williams, R.A. Heat transfer of aqueous suspensions of carbon nanotubes (CNT nanofluids). Int. J. Heat Mass Transf. 2006, 49, 240–250. [Google Scholar] [CrossRef]
- Meyer, J.P.; McKrell, T.J.; Grote, K. The influence of multi-walled carbon nanotubes on single-phase heat transfer and pressure drop characteristics in the transitional flow regime of smooth tubes. Int. J. Heat Mass Transf. 2013, 58, 597–609. [Google Scholar] [CrossRef]
- Abreu, B.; Lamas, B.; Fonseca, A.; Martins, N.; Oliveira, M.S.A. Experimental characterization of convective heat transfer with MWCNT based nanofluids under laminar flow conditions. Heat Mass Transf. 2014, 50, 65–74. [Google Scholar] [CrossRef]
- Nazari, M.; Karami, M.; Ashouri, M. Comparing the thermal performance of water, Ethylene Glycol, Alumina and CNT nanofluids in CPU cooling: Experimental study. Exp. Therm. Fluid Sci. 2014, 57, 371–377. [Google Scholar] [CrossRef]
- Elnajjar, E.; Haik, Y.; Hamdan, M.O.; Khashan, S. Heat transfer characteristics of multi-walled carbon nanotubes suspension in a developing channel flow. Heat Mass Transf. 2013, 49, 1681–1687. [Google Scholar] [CrossRef]
- Utomo, A.T.T.; Haghighi, E.B.B.; Zavareh, A.I.T.; Ghanbarpourgeravi, M.; Poth, H.; Khodabandeh, R.; Palm, B.B.; Pacek, A.W.W. The effect of nanoparticles on laminar heat transfer in a horizontal tube. Int. J. Heat Mass Transf. 2014, 69, 77–91. [Google Scholar] [CrossRef]
- Ashtiani, D.; Akhavan-Behabadi, M.A.; Pakdaman, M.F. An experimental investigation on heat transfer characteristics of multi-walled CNT-heat transfer oil nanofluid flow inside flattened tubes under uniform wall temperature condition. Int. Commun. Heat Mass Transf. 2012, 39, 1404–1409. [Google Scholar] [CrossRef]
- Ting, T.W.; Hung, Y.M.; Guo, N. Entropy generation of nanofluid flow with streamwise conduction inmicrochannels. Energy 2014, 64, 979–990. [Google Scholar] [CrossRef]
- Siavashi, M.; Talesh Bahrami, H.R.; Saffari, H. Numerical investigation of flow characteristics, heat transfer and entropy generation of nanofluid flow inside an annular pipe partially or completely filled with porous media using two-phase mixture model. Energy 2015, 93, 2451–2466. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Rikhtegar, F.; Sabaghan, A.; Roohi, E. Heat transfer and entropy generation in a microchannel with longitudinal vortex generators using nanofluids. Energy 2016, 101, 190–201. [Google Scholar] [CrossRef]
- Ibáñez, G.; López, A.; Pantoja, J.; Moreira, J. Entropy generation analysis of a nanofluid flow in MHD porous microchannel with hydrodynamic slip and thermal radiation. Int. J. Heat Mass Transf. 2016, 100, 89–97. [Google Scholar] [CrossRef]
- Mahian, O.; Kianifar, A.; Sahin, A.Z.; Wongwises, S. Entropy generation during Al2O3/water nanofluid flow in a solar collector: Effects of tube roughness, nanoparticle size, and different thermophysical models. Int. J. Heat Mass Transf. 2014, 78, 64–75. [Google Scholar] [CrossRef]
- Bianco, V.; Manca, O.; Nardini, S. Entropy generation analysis of turbulent convection flow of Al2O3–water nanofluid in a circular tube subjected to constant wall heat flux. Energy Convers. Manag. 2014, 77, 306–314. [Google Scholar] [CrossRef]
- Singh, P.K.; Anoop, K.B.B.; Sundararajan, T.; Das, S.K. Entropy generation due to flow and heat transfer in nanofluids. Int. J. Heat Mass Transf. 2010, 53, 4757–4767. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, H.-C.; Yang, X.; Shi, L. Entropy Generation Analysis and Performance Evaluation of Turbulent Forced Convective Heat Transfer to Nanofluids. Entropy 2017, 19, 108. [Google Scholar] [CrossRef]
- Li, J.; Kleinstreuer, C. Entropy Generation Analysis for Nanofluid Flow in Microchannels. J. Heat Transf. 2010, 132, 122401. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Ganji, D.D. Entropy generation of nanofluid in presence of magnetic field using Lattice Boltzmann Method. Phys. A Stat. Mech. Appl. 2014, 417, 273–286. [Google Scholar] [CrossRef]
- Ellahi, R.; Hassan, M.; Zeeshan, A. Shape effects of nanosize particles in Cu-H2O nanofluid on entropy generation. Int. J. Heat Mass Transf. 2015, 81, 449–456. [Google Scholar] [CrossRef]
- Shahi, M.; Mahmoudi, A.H.; Raouf, A.H. Entropy generation due to natural convection cooling of a nanofluid. Int. Commun. Heat Mass Transf. 2011, 38, 972–983. [Google Scholar] [CrossRef]
- Mahian, O.; Mahmud, S.; Heris, S.Z. Analysis of entropy generation between co-rotating cylinders using nanofluids. Energy 2012, 44, 438–446. [Google Scholar] [CrossRef]
- Helvaci, H.U.; Khan, Z.A. Heat transfer and entropy generation analysis of HFE 7000 based nanorefrigerants. Int. J. Heat Mass Transf. 2017, 104, 318–327. [Google Scholar] [CrossRef]
- Mahian, O.; Kianifar, A.; Kleinstreuer, C.; Al-Nimr, M.A.; Pop, I.; Sahin, A.Z.; Wongwises, S. A review of entropy generation in nanofluid flow. Int. J. Heat Mass Transf. 2013, 65, 514–532. [Google Scholar] [CrossRef]
- Singh, P.K.; Ahmed, N.Z.; Das, S.K.; Shatilla, Y. Exergy analysis of nanofluids in microchannel. In Proceedings of the ASME 2011 9th International Conference on Nanochannels, Microchannels, and Minichannels, Edmonton, AB, Canada, 19–22 June 2011. [Google Scholar]
- Yu, W.; Xie, H. A review on nanofluids: Preparation, stability mechanisms, and applications. J. Nanomater. 2012, 2012, 1–17. [Google Scholar] [CrossRef]
- Khanafer, K.; Vafai, K.; Lightstone, M. Buoyancy-driven heat transfer enhancement in a two-dimensional enclosure utilizing nanofluids. Int. J. Heat Mass Transf. 2003, 46, 3639–3653. [Google Scholar] [CrossRef]
- Garg, P.; Alvarado, J.L.; Marsh, C.; Carlson, T.A.; Kessler, D.A.; Annamalai, K. An experimental study on the effect of ultrasonication on viscosity and heat transfer performance of multi-wall carbon nanotube-based aqueous nanofluids. Int. J. Heat Mass Transf. 2009, 52, 5090–5101. [Google Scholar] [CrossRef]
- Selvakumar, P.; Suresh, S. Convective performance of CuO/water nanofluid in an electronic heat sink. Exp. Therm. Fluid Sci. 2012, 40, 57–63. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Maeda, Y.; Kanetsuna, H. Measurement of Specific Heat of Polyethylene. Sen’i Gakkaishi 1970, 26, 61–65. [Google Scholar] [CrossRef]
- Kline, S.; McClintock, F. Describing uncertainties in single-sample experiments. Mech. Eng. 1953, 75, 3–8. [Google Scholar]
- Bejan, A. Entropy Generation through Heat and Fluid Flow; Wiley: Hoboken, NJ, USA, 1982. [Google Scholar]
- Shah, R.K.; Alexander, L.L. Laminar Flow Forced Convection Heat Transfer and Flow Friction in Straight and Curved Ducts-A Summary of Analytical Solutions; No. TR-75; Stanford University: Stanford, CA, USA, 1971. [Google Scholar]
- Sadaghiani, A.K.; Yildiz, M.; Koşar, A. Numerical modeling of convective heat transfer of thermally developing nanofluid flows in a horizontal microtube. Int. J. Therm. Sci. 2016, 109, 54–69. [Google Scholar] [CrossRef]
- Bejan, A. Entropy Generation Minimization: The Method of Thermodynamic Optimization of Finite-Size Systems and Finite-Time Processes; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Sellens, R.W.; Brzustowski, T.A. A prediction of the drop size distribution in a spray from first principles. At. Spray Technol. 1985, 1, 89–102. [Google Scholar]
- Li, X.-G.; Tankin, R.S.; Sztal, B.; Most, J.-M. Derivation of droplet size distribution in sprays by using information theory. Combust. Sci. Technol. 1988, 60, 345–357. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimzadehkhouei, M.; Shojaeian, M.; Sadaghiani, A.K.; Şendur, K.; Mengüç, M.P.; Koşar, A. Entropy Generation Analysis of Laminar Flows of Water-Based Nanofluids in Horizontal Minitubes under Constant Heat Flux Conditions. Entropy 2018, 20, 242. https://doi.org/10.3390/e20040242
Karimzadehkhouei M, Shojaeian M, Sadaghiani AK, Şendur K, Mengüç MP, Koşar A. Entropy Generation Analysis of Laminar Flows of Water-Based Nanofluids in Horizontal Minitubes under Constant Heat Flux Conditions. Entropy. 2018; 20(4):242. https://doi.org/10.3390/e20040242
Chicago/Turabian StyleKarimzadehkhouei, Mehrdad, Mostafa Shojaeian, Abdolali Khalili Sadaghiani, Kürşat Şendur, M. Pinar Mengüç, and Ali Koşar. 2018. "Entropy Generation Analysis of Laminar Flows of Water-Based Nanofluids in Horizontal Minitubes under Constant Heat Flux Conditions" Entropy 20, no. 4: 242. https://doi.org/10.3390/e20040242






