Fuzzy Entropy Analysis of the Electroencephalogram in Patients with Alzheimer’s Disease: Is the Method Superior to Sample Entropy?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and EEG Recording
2.2. Fuzzy Entropy
- For 1 ≤ i ≤ N − m + 1, form m-vectors Xm(1) … Xm(N − m + 1) defined as:These vectors represent m consecutive x values, commencing with the ith point, with the baseline () removed.
- Define the distance between vectors Xm(i) and Xm(j), , as the maximum absolute difference between their scalar components.
- Given n and r, calculate the similarity degree of the vectors Xm(i) and Xm(j) with a fuzzy function:
- Define the function as:
- We increase the dimension to m + 1, form vectors Xm+1(i), and, subsequently, obtain the function repeating steps 2 to 4.
- For time series with a finite number of samples N, FuzzyEn can be estimated with the following equation [13]:
2.3. Statistical Analysis
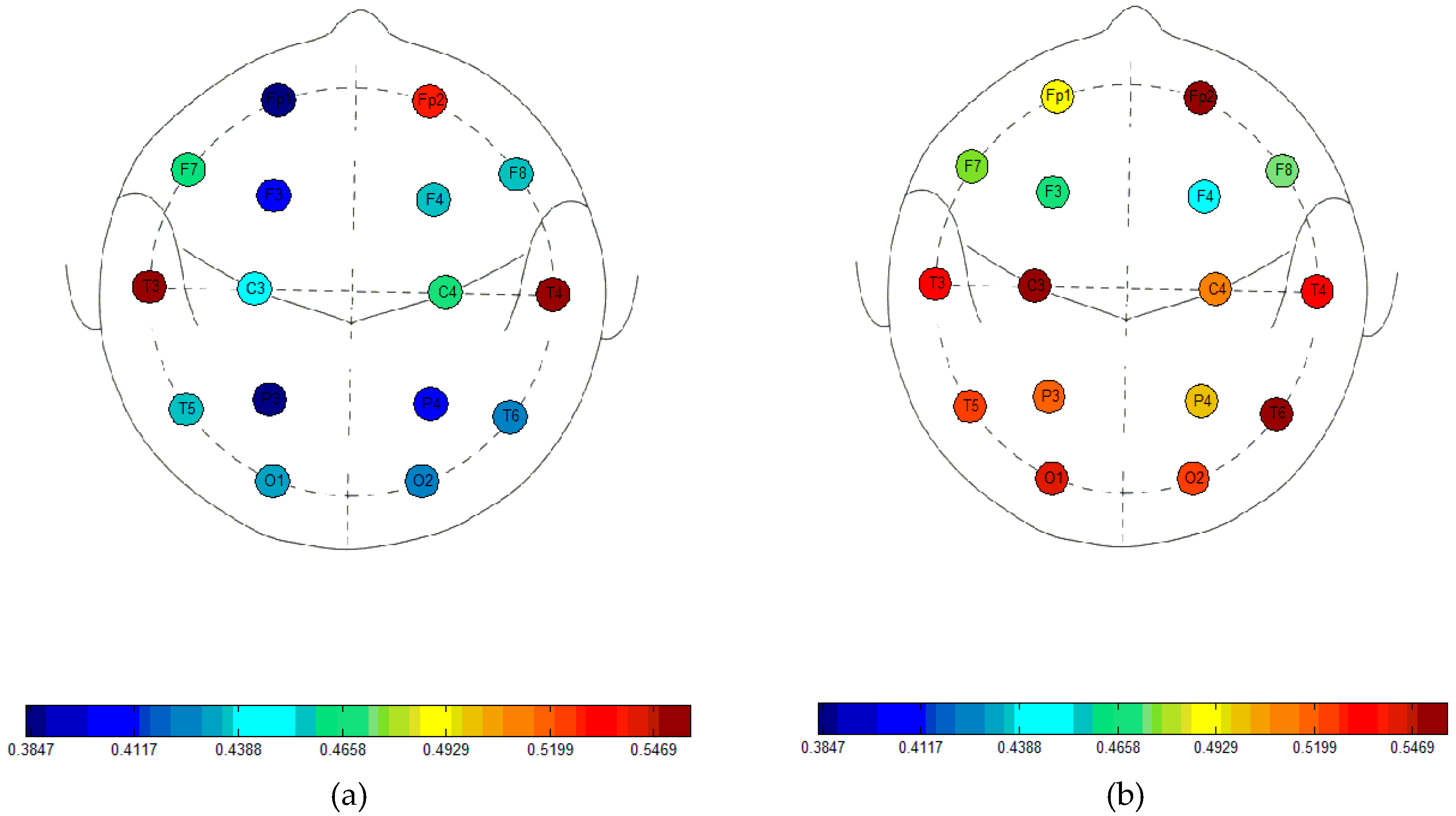
3. Results
4. Discussion and Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jeong, J. EEG dynamics in patients with Alzheimer’s disease. Clin. Neurophysiol. 2004, 115, 1490–1505. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef]
- Bird, T.D. Alzheimer’s disease and other primary dementias. In Harrison’s Principles of Internal Medicine, 15th ed.; Braunwald, E., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Jameson, J.L., Eds.; McGraw-Hill: New York, NY, USA, 2001; pp. 2391–2399. [Google Scholar]
- Kalaria, R.N.; Maestre, G.E.; Arizaga, R.; Friedland, R.P.; Galasko, D.; Hall, K.; Luchsinger, J.A.; Ogunniyi, A.; Perry, E.K.; Potocnik, F.; et al. Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 2008, 7, 812–826. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical-diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Dauwels, J.; Vialatte, F.; Cichocki, A. Diagnosis of Alzheimer’s disease from EEG signals: Where are we standing? Curr. Alzheimer Res. 2010, 7, 487–505. [Google Scholar] [CrossRef] [PubMed]
- Markand, O.N. Organic brain syndromes and dementias. In Current Practice of Clinical Electroencephalography; Daly, D.D., Pedley, T.A., Eds.; Raven Press: New York, NY, USA, 1990; pp. 401–423. [Google Scholar]
- Sleigh, J.W.; Steyn-Ross, D.A.; Grant, C.; Ludbrook, G. Cortical entropy changes with general anaesthesia: Theory and experiment. Physiol. Meas. 2004, 25, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; Gnecchi-Ruscone, T.; Tobaldini, E.; Guzzetti, S.; Furlan, R.; Montano, N. Progressive decrease of heart period variability entropy-based complexity during graded head-up tilt. J. Appl. Physiol. 2007, 103, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. (Heart Circ. Physiol.) 2000, 274, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Lake, D.E. Improved entropy rate estimation in physiological data. In Proceedings of the 33rd Annual International Conference of the IEEE EMBS, Boston, MA, USA, 30 August–3 September 2011; pp. 1463–1466. [Google Scholar]
- Chen, W.; Wang, Z.; Xie, H.; Yu, W. Characterization of surface EMG signal based on fuzzy entropy. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Chen, W.; Zhuang, J.; Yu, W.; Wang, Z. Measuring complexity using FuzzyEn, ApEn and SampEn. Med. Eng. Phys. 2009, 31, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.B.; Chen, W.-T.; He, W.-X.; Liu, H. Complexity analysis of the biomedical signal using fuzzy entropy measurement. Appl. Soft Comput. 2011, 11, 2871–2879. [Google Scholar] [CrossRef]
- Fu, A.; Wang, C.; Qi, H.; Li, F.; Wang, Z.; He, F.; Zhou, P.; Chen, S.; Ming, D. Electromyography-based analysis of human upper limbs during 45-day head-down bed-rest. Acta Astronaut. 2016, 120, 260–269. [Google Scholar] [CrossRef]
- Cao, Y.; Cai, L.; Wang, J.; Wang, R.; Yu, H.; Cao, Y.; Liu, J. Characterization of complexity in the electroencephalogram activity of Alzheimer’s disease based on fuzzy entropy. Chaos 2015, 25, 083136. [Google Scholar] [CrossRef] [PubMed]
- Cirugeda-Roldan, E.; Cuesta-Frau, D.; Miro-Martinez, P.; Oltra-Crespo, S. Comparative study of entropy sensitivity to missing biosignal data. Entropy 2014, 16, 5901–5918. [Google Scholar] [CrossRef]
- Liu, ].C.; Li, K.; Zhao, L.; Liu, F.; Zheng, D.; Liu, C.; Liu, S. Analysis of heart rate variability using fuzzy measure entropy. Comput. Biol. Med. 2013, 43, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Abásolo, D.; Hornero, R.; Gómez, C.; García, M.; López, M. Analysis of EEG background activity in Alzheimer’s disease patients with Lempel-Ziv complexity and Central Tendency Measure. Med. Eng. Phys. 2006, 28, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abásolo, D.; Escudero, J.; Hornero, R.; Gómez, C.; Espino, P. Approximate entropy and auto mutual information analysis of the electroencephalogram in Alzheimer’s disease patients. Med. Biol. Eng. Comput. 2008, 46, 1019–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escudero, J.; Abásolo, D.; Hornero, R.; Espino, P.; López, M. Analysis of electroencephalograms in Alzheimer’s disease patients with multiscale entropy. Physiol. Meas. 2006, 27, 1091–1106. [Google Scholar] [CrossRef] [PubMed]
- Abásolo, D.; Hornero, R.; Espino, P.; Álvarez, D.; Poza, J. Entropy analysis of the EEG background activity in Alzheimer’s disease patients. Physiol. Meas. 2006, 27, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Simons, S.; Abásolo, D.; Escudero, J. Classification of Alzheimer’s disease from Quadratic Sample Entropy of the EEG. IET Healthc. Technol. Lett. 2015, 2, 70–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azami, H.; Abásolo, D.; Simons, S.; Escudero, J. Univariate and multivariate generalized multiscale entropy to characterise EEG signals in Alzheimer’s Disease. Entropy 2017, 19, 31. [Google Scholar] [CrossRef]
- Delbeuck, X.; Van der Linden, M.; Collette, F. Alzheimer’s disease as a disconnection syndrome? Neuropsychol. Rev. 2003, 13, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.H.; Scherr, S.; Lewis, D.A.; Campbell, M.J.; Bloom, F.E.; Rogers, L.; Benoit, R. The laminar and regional distribution of neocortical somatostatin and neuritic plaques: Implications for Alzheimer’s disease as a global neocortical disconnection syndrome. In The Biological Substrates of Alzheimer’s Disease; Scheibel, A.B., Wechsler, A.F., Eds.; Academic Press: Orlando, FA, USA, 1986; pp. 115–131. [Google Scholar]
- Burioka, N.; Cornélissen, G.; Halberg, F.; Kaplan, D.T.; Suyama, H.; Sako, T.; Shimizu, E. Approximate entropy of human respiratory movement during eye-closed waking and different sleep stages. Chest 2003, 123, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Roy, R.J. Derived fuzzy knowledge model for estimating the depth of anesthesia. IEEE Trans. Biomed. Eng. 2001, 48, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Babloyantz, A.; Destexhe, A. The Creutzfeldt-Jakob disease in the hierarchy of chaotic attractors. In From Chemical to Biological Organization; Markus, M., Müller, S.C., Nicolis, G., Eds.; Springer: Berlin, Germany, 1988; pp. 307–316. [Google Scholar]
- Jeong, J.; Kim, S.J.; Han, S.H. Non-linear dynamical analysis of the EEG in Alzheimer’s disease with optimal embedding dimension. Electroenceph. Clin. Neurophysiol. 1998, 106, 220–228. [Google Scholar] [CrossRef]
- Röschke, J.; Fell, J.; Beckmann, P. Non-linear analysis of sleep EEG data in schizophrenia: Calculation of the principal Lyapunov exponent. Psychiatr. Res. 1995, 56, 257–269. [Google Scholar] [CrossRef]
- Stam, C.J.; Jelles, B.; Achtereekte, H.A.M.; Rombouts, S.A.R.B.; Slaets, J.P.J.; Keunen, R.W.M. Investigation of EEG nonlinearity in dementia and Parkinson’s disease. Electroenceph. Clin. Neurophysiol. 1995, 95, 309–317. [Google Scholar] [CrossRef]
- Azami, H.; Rostaghi, M.; Abásolo, D.; Escudero, J. Refined composite multiscale dispersion entropy and its application to biomedical signals. IEEE Trans. Biomed. Eng. 2017, 64, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Azami, H.; Fernández, A.; Escudero, J. Refined multiscale fuzzy entropy based on standard deviation for biomedical signal analysis. Med. Biol. Eng. Comput. 2017, 55, 2037–2052. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; De Maria, B.; Bari, V.; Marchi, A.; Faes, L. Are nonlinear model-free conditional entropy approaches for the assessment of cardiac control complexity superior to the linear model-based one? IEEE Trans. Biomed. Eng. 2017, 64, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Bandt, C.; Pompe, B. Permutation entropy: A natural complexity measure for time series. Phys. Rev. Lett. 2002, 88, 174102. [Google Scholar] [CrossRef] [PubMed]

| m | r | Electrode | Threshold | Sensitivity | Specificity | Accuracy | Area Under Curve |
|---|---|---|---|---|---|---|---|
| 1 | 0.1 | Fp1 | 1.1169 | 63.64 | 81.82 | 72.73 | 0.7934 |
| T6 | 1.3916 | 81.82 | 81.82 | 81.82 | 0.8182 | ||
| P3 | 1.1755 | 81.82 | 81.82 | 81.82 | 0.8595 | ||
| O2 | 1.2918 | 81.82 | 90.91 | 86.36 | 0.8595 | ||
| 1 | 0.15 | Fp1 | 0.8015 | 63.64 | 81.82 | 72.73 | 0.7934 |
| T6 | 1.0217 | 81.82 | 81.82 | 81.82 | 0.8182 | ||
| P3 | 0.8516 | 81.82 | 90.91 | 86.36 | 0.8678 | ||
| O2 | 0.9393 | 81.82 | 90.91 | 86.36 | 0.8678 | ||
| 1 | 0.2 | Fp1 | 0.6248 | 63.64 | 81.82 | 72.73 | 0.7934 |
| T6 | 0.8040 | 81.82 | 81.82 | 81.82 | 0.8182 | ||
| P3 | 0.6669 | 81.82 | 90.91 | 86.36 | 0.8678 | ||
| O2 | 0.7362 | 81.82 | 81.82 | 81.82 | 0.8554 | ||
| 1 | 0.25 | Fp1 | 0.5105 | 63.64 | 81.82 | 72.73 | 0.7934 |
| T6 | 0.6662 | 81.82 | 81.82 | 81.82 | 0.8182 | ||
| P3 | 0.5473 | 81.82 | 90.91 | 86.36 | 0.8678 | ||
| O2 | 0.6054 | 81.82 | 81.82 | 81.82 | 0.8512 | ||
| 2 | 0.1 | T6 | 0.8755 | 81.82 | 81.82 | 81.82 | 0.8182 |
| P3 | 0.7847 | 81.82 | 81.82 | 81.82 | 0.9091 | ||
| P4 | 0.7380 | 72.73 | 81.82 | 77.27 | 0.8099 | ||
| O1 | 0.8414 | 81.82 | 72.73 | 77.27 | 0.8264 | ||
| O2 | 0.8197 | 90.91 | 81.82 | 86.36 | 0.8512 | ||
| 2 | 0.15 | T6 | 0.7127 | 81.82 | 81.82 | 81.82 | 0.8182 |
| P3 | 0.6295 | 81.82 | 81.82 | 81.82 | 0.8843 | ||
| P4 | 0.5885 | 63.64 | 90.91 | 77.27 | 0.8099 | ||
| O1 | 0.6879 | 81.82 | 72.73 | 77.27 | 0.8182 | ||
| O2 | 0.6617 | 90.91 | 81.82 | 86.36 | 0.8678 | ||
| 2 | 0.2 | T6 | 0.6018 | 81.82 | 81.82 | 81.82 | 0.8264 |
| P3 | 0.5301 | 81.82 | 81.82 | 81.82 | 0.8926 | ||
| P4 | 0.4895 | 63.64 | 100 | 81.82 | 0.8182 | ||
| O1 | 0.5743 | 81.82 | 72.73 | 77.27 | 0.8099 | ||
| O2 | 0.5523 | 90.91 | 81.82 | 86.36 | 0.8595 | ||
| 2 | 0.25 | T6 | 0.5206 | 81.82 | 81.82 | 81.82 | 0.8264 |
| P3 | 0.4564 | 81.82 | 81.82 | 81.82 | 0.9008 | ||
| P4 | 0.4182 | 63.64 | 100 | 81.82 | 0.8182 | ||
| O1 | 0.4926 | 81.82 | 72.73 | 77.27 | 0.8099 | ||
| O2 | 0.4727 | 90.91 | 81.82 | 86.36 | 0.8595 |
| m | r | Electrode | Threshold | Sensitivity | Specificity | Accuracy | Area Under Curve |
|---|---|---|---|---|---|---|---|
| 1 | 0.1 | P3 | 1.0782 | 81.82 | 81.82 | 81.82 | 0.8347 |
| O2 | 1.4320 | 72.73 | 81.82 | 77.27 | 0.8512 | ||
| 1 | 0.15 | P3 | 0.8648 | 81.82 | 81.82 | 81.82 | 0.8264 |
| O2 | 1.1963 | 72.73 | 81.82 | 77.27 | 0.8430 | ||
| 1 | 0.2 | P3 | 0.7279 | 81.82 | 81.82 | 81.82 | 0.8264 |
| O2 | 1.0326 | 72.73 | 81.82 | 77.27 | 0.8430 | ||
| 1 | 0.25 | P3 | 0.6377 | 81.82 | 81.82 | 81.82 | 0.8264 |
| 2 | 0.15 | P3 | 0.7627 | 81.82 | 81.82 | 81.82 | 0.8843 |
| 2 | 0.2 | P3 | 0.7231 | 81.82 | 81.82 | 81.82 | 0.8843 |
| O2 | 0.7832 | 72.73 | 81.82 | 77.27 | 0.8264 | ||
| 2 | 0.25 | P3 | 0.6875 | 81.82 | 81.82 | 81.82 | 0.8843 |
| O2 | 0.7493 | 72.73 | 81.82 | 77.27 | 0.8347 |
| m | r | Electrode | Threshold | Sensitivity | Specificity | Accuracy | Area Under Curve |
|---|---|---|---|---|---|---|---|
| 1 | 0.1 | O2 | 1.4739 | 72.73 | 81.82 | 77.27 | 0.8430 |
| 1 | 0.15 | O2 | 1.3153 | 72.73 | 81.82 | 77.27 | 0.8554 |
| 1 | 0.2 | O2 | 1.2030 | 72.73 | 81.82 | 77.27 | 0.8678 |
| 1 | 0.25 | O2 | 1.1147 | 72.73 | 81.82 | 77.27 | 0.8512 |
| 2 | 0.2 | P3 | 0.7747 | 81.82 | 81.82 | 81.82 | 0.8306 |
| 2 | 0.25 | P3 | 0.7571 | 81.82 | 81.82 | 81.82 | 0.8347 |
| Method | Electrode | ROC Classification Results | ||
|---|---|---|---|---|
| Sensitivity | Specificity | Accuracy | ||
| LZC (3 symbol conversion) [22] | T5 | 72.73 | 72.73 | 72.73 |
| P3 | 81.82 | 81.82 | 81.82 | |
| P4 | 72.73 | 90.91 | 81.82 | |
| O1 | 90.91 | 72.73 | 81.82 | |
| Slope of MSE (m = 1, r = 0.25, 12 scales) for large time scales [24] | F3 | 81.82 | 81.82 | 81.82 |
| F7 | 81.82 | 72.73 | 77.27 | |
| Fp1 | 90.91 | 90.91 | 90.91 | |
| Fp2 | 100 | 72.73 | 86.36 | |
| T5 | 90.91 | 81.82 | 86.36 | |
| T6 | 81.82 | 81.82 | 81.82 | |
| P3 | 81.82 | 90.91 | 86.36 | |
| P4 | 72.73 | 90.91 | 81.82 | |
| O1 | 81.82 | 90.91 | 86.36 | |
| O2 | 81.82 | 81.82 | 81.82 | |
| ApEn (m = 1, r = 0.25) [23] | P3 | 72.73 | 81.82 | 77.27 |
| P4 | 63.64 | 81.82 | 72.73 | |
| O1 | 81.82 | 72.73 | 77.27 | |
| O2 | 90.91 | 63.64 | 77.27 | |
| AMI rate of decrease [23] | T5 | 90.91 | 72.73 | 81.82 |
| T6 | 81.82 | 81.82 | 81.82 | |
| P3 | 100 | 81.82 | 90.91 | |
| P4 | 81.82 | 81.82 | 81.82 | |
| O1 | 81.82 | 81.82 | 81.82 | |
| O2 | 81.82 | 81.82 | 81.82 | |
| SampEn (m = 1, r = 0.25) [25] | P3 | 72.73 | 81.82 | 77.27 |
| P4 | 63.64 | 90.91 | 77.27 | |
| O1 | 81.82 | 72.73 | 77.27 | |
| O2 | 90.91 | 63.64 | 77.27 | |
| * QSE (m = 1 and m = 2, different values of r) [26] | P3 | NR | NR | 77.27 |
| P4 | NR | NR | 77.27 | |
| O1 | NR | NR | 77.27 | |
| O2 | NR | NR | 77.27 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simons, S.; Espino, P.; Abásolo, D. Fuzzy Entropy Analysis of the Electroencephalogram in Patients with Alzheimer’s Disease: Is the Method Superior to Sample Entropy? Entropy 2018, 20, 21. https://doi.org/10.3390/e20010021
Simons S, Espino P, Abásolo D. Fuzzy Entropy Analysis of the Electroencephalogram in Patients with Alzheimer’s Disease: Is the Method Superior to Sample Entropy? Entropy. 2018; 20(1):21. https://doi.org/10.3390/e20010021
Chicago/Turabian StyleSimons, Samantha, Pedro Espino, and Daniel Abásolo. 2018. "Fuzzy Entropy Analysis of the Electroencephalogram in Patients with Alzheimer’s Disease: Is the Method Superior to Sample Entropy?" Entropy 20, no. 1: 21. https://doi.org/10.3390/e20010021





